Abstract
The swine industry across the globe is recently facing a devastating situation imparted by a highly contagious and deadly viral disease, African swine fever. The disease is caused by a DNA virus, the African swine fever virus (ASFV) of the genus Asfivirus. ASFV affects both wild boars and domestic pigs resulting in an acute form of hemorrhagic fever. Since the first report in 1921, the disease remains endemic in some of the African countries. However, the recent occurrence of ASF outbreaks in Asia led to a fresh and formidable challenge to the global swine production industry. Culling of the infected animals along with the implementation of strict sanitary measures remains the only options to control this devastating disease. Efforts to develop an effective and safe vaccine against ASF began as early as in the mid-1960s. Different approaches have been employed for the development of effective ASF vaccines including inactivated vaccines, subunit vaccines, DNA vaccines, virus-vectored vaccines, and live attenuated vaccines (LAVs). Inactivated vaccines are a non-feasible strategy against ASF due to their inability to generate a complete cellular immune response. However genetically engineered vaccines, such as subunit vaccines, DNA vaccines, and virus vector vaccines, represent tailored approaches with minimal adverse effects and enhanced safety profiles. As per the available data, gene deleted LAVs appear to be the most potential vaccine candidates. Currently, a gene deleted LAV (ASFV-G-∆I177L), developed in Vietnam, stands as the sole commercially available vaccine against ASF. The major barrier to the goal of developing an effective vaccine is the critical gaps in the knowledge of ASFV biology and the immune response induced by ASFV infection. The precise contribution of various hosts, vectors, and environmental factors in the virus transmission must also be investigated in depth to unravel the disease epidemiology. In this review, we mainly focus on the recent progress in vaccine development against ASF and the major gaps associated with it.

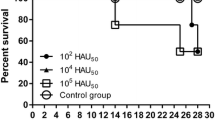
Adapted from Turlewicz-Podbielska et al. [15] under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
FAO (2011) looking ahead in world food and agriculture: perspectives to 2050. FAO, Rome
OIE (World Organization for Animal Health) (2021) Listed diseases 2021. https://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2021/
Montgomery RE (1921) On a form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol 34:159–191
Costard S, Mur L, Lubroth J, Sanchez-Vizcaino JM, Pfeiffer DU (2013) Epidemiology of African swine fever virus. Virus Res 173(1):191–197
Penrith ML, Vosloo W (2009) Review of African swine fever: transmission, spread and control. J S Afr Vet Assoc 80(2):58–62
Costard S, Randriamparany T, Humbert C, Franco S, Rasamoelina H, Rakotoharinome M, Rakotondrahanta S, Albina E, Roger F, Pfeiffer DU (2009) Estimating the prevalence of African swine fever in Madagascar. In: 12th Symposium of the International Society for Veterinary Epidemiology and Economics (ISVEE), vol 12. ISVEE
Ito S, Bosch J, Martínez-Avilés M, Sánchez-Vizcaíno JM (2022) The evolution of African swine fever in China: A global threat Front. Vet Sci 9:828498
Rajukumar K, Senthilkumar D, Venkatesh G, Singh F, Patil VP, Kombiah S, Tosh C, Dubey CK, Sen A, Barman NN (2021) Genetic characterization of African swine fever virus from domestic pigs in India. Transbound Emerg Dis 68(5):2687–2692
Goonewardene KB, Onyilagha C, Goolia M, Le VP, Blome S, Ambagala A (2022) Superficial inguinal lymph nodes for screening dead pigs for African swine fever. Viruses 14:83
African Swine Fever (ASF)—Situation report 4. Available online: https://www.oie.int/app/uploads/2022/01/asf-situationreport-4.pdf. Accessed 18 Jan 2022
OIE (2019) African swine fever: aetiology epidemiology diagnosis prevention and control references. Technical Disease Cards. http://www.oie.int/wahis/public.php?page=home
Plowright W, Perry CT, Greig A (1974) Sexual transmission of African swine fever virus in the tick, Ornithodoros moubata porcinus. Walton Res Vet Sci 17:106–113
Chenais E, Stahl K, Guberti V, Depner K (2018) Identification of wild boar–habitat epidemiologic cycle in African swine fever epizootic. Emerg Infect Dis 24:810
EFSA (2014) Scientific Opinion on African swine fever. EFSA J 12:3628
Turlewicz-Podbielska H, Kuriga A, Niemyjski R, Tarasiuk G, Pomorska-Mól M (2021) African swine fever virus as a difficult opponent in the fight for a vaccine -Current data. Viruses 13(7):1212
Blome S, Gabriel C, Beer M (2013) Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res 173(1):122–130
Gallardo C, Soler A, Nieto R, Sánchez MA, Martins C, Pelayo V, Carrascosa A, Revilla Y, Simón A, Briones V, Sánchez-Vizcaíno JM (2015) Experimental transmission of African swine fever (ASF) low virulent isolate NH/P68 by surviving pigs. Transbound Emerg Dis 62(6):612–622
Sánchez-Vizcaíno JM, Mur L, Martínez-López B (2012) African swine fever: an epidemiological update. Transbound Emerg Dis 59:27–35
Matsuyama T, Takano T, Nishik I, Fujiwara A, Kiryu I, Inada M, Sakai T, Terashima S, Matsuura Y, Isowa K (2020) A novel Asfarvirus-like virus identified as a potential cause of mass mortality of abalone. Sci Rep 10:1–12
Galindo I, Alonso C (2017) African swine fever virus: a review. Viruses 9:103
Andrés G, Simón-Mateo C, Vinuela E (1997) Assembly of African swine fever virus: role of polyprotein. J Virol 71:2331–2341
Salas ML, Andrés G (2013) African swine fever virus morphogenesis. Virus Res 173:29–41
Tulman ER, Delhon GA, Ku BK, Rock DL (2009) African swine fever virus. Curr Top Microbiol Immunol 328:43–87
Dixon LK, Chapman DA, Netherton CL, Upton C (2013) African swine fever virus replication and genomics. Virus Res 173:3–14
Chapman DA, Tcherepanov V, Upton C, Dixon LK (2008) Comparison of the genome sequences of non-pathogenic and pathogenic African swine fever virus isolates. J Gen Virol 9:397–408
Bastos ADS, Penrith ML, Cruciere C, Edrich JL, Hutchings G, Roger F, Couacy-Hymann E, Thomson GR (2003) Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch Virol 148:693–706
Reis AL, Goatley LC, Jabbar T, Sanchez-Cordon PJ, Netherton CL, Chapman DA, Dixon LK (2017) Deletion of the African swine fever virus gene DP148R does not reduce virus replication in culture but reduces virus virulence in pigs and induces high levels of protection against challenge. J Virol 91(24):e01428-e1517
Plowright W, Parker J (1967) The stability of African swine fever virus with particular reference to heat and pH inactivation. Arch Gesamte Virusforsch 21:383–402
Mebus C, Arias M, Pineda JM, Tapiador J, House C, Sánchez-Vizcaíno JM (1997) Survival of several porcine viruses indifferent Spanish dry-cured meat products. Food Chem 59:555–559
Sindryakova IP, Morgunov YP, Chichikin AY, Gazaev IK, Kudryashov DA, Tsybanov SZ (2016) The influence of temperature on the Russian isolate of African swine fever virus in pork products and feed with extrapolation to natural conditions. Sel’skokhozyaistvennaya Biol 51:467–474
Ivashkiv LB, Donlin LT (2014) Regulation of type I interferon responses. Nat Rev Immunol 14:36–49
Takeuchi O, Akira S (2009) Innate immunity to virus infection. Immunol Rev 227:75–86
Dixon LK, Abrams CC, Bowick G, Goatley LC, Kay-Jackson PC, Chapman D, Zhang F (2004) African swine fever virus proteins involved in evading host defence systems. Vet Immunol Immunopathol 100:117–134
Hernaez B, Alonso C (2010) Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J Virol 84:2100–2109
Correia S, Ventura S (2013) Goodbourn RME Parkhouse, M. ASFV includes several mechanisms for the manipulation of IFN responses. Cytokine 63:256
Wang X, Wu J, Wu Y, Chen H, Zhang S, Li J, Xin T, Jia H, Hou S, Jiang Y (2018) Inhibition of cGAS-STING-TBK1 signalling pathway by DP96R of ASFV China 2018/1. Biochem Biophys Res Commun 506:437–443
Zhuo Y, Guo Z, Ba T, Zhang C, He L, Zeng C, Dai H (2020) African swine fever virus MGF360-12L inhibits type I interferon production by blocking the interaction of importin alpha and NF-kappa B signalling pathway. Virol Sin 36:176–186
Wang Z, Ai Q, Huang S, Ou Y, Gao Y, Tong T, Fan H (2022) Immune escape mechanism and vaccine research progress of African swine fever virus. Vaccines 10(3):344
Liu H, Shi K, Sun W, Zhao J, Yin Y, Si H, Qu S, Lu W (2021) Development a multiplex RT-PCR assay for simultaneous detection of African swine fever virus, classical swine fever virus and atypical porcine pestivirus. J Virol Methods 287:114006
Yang J, Li S, Feng T, Zhang X, Yang F, Cao W (2021) African swine fever virus F317L protein inhibits NF-κ B activation to evade host immune response and promote viral replication. Sphere 6:e0065821
Zhang Y, Ke J, Zhang J, Yue H, Chen T, Li Q (2021) I267L is neither the virulence- nor the replication-related gene of African swine fever virus and its deletant is an ideal fluorescent-tagged virulence strain. Viruses 14:53
Ran Y, Li D, Xiong MG, Liu HN, Feng T, Shi ZW (2022) African swine fever virus I267L acts as an important virulence factor by inhibiting RNA polymerase III-RIG-I-mediated innate immunity. PLoS Pathog 18(1):e1010270
Zhang K, Yang B, Shen C, Zhang T, Hao Y, Zhang D (2022) MGF360-9L is a major virulence factor associated with the African swine fever virus by antagonizing the JAK/STAT signalling pathway. MBio 13(1):e0233021
Arias M, de la Torre A, Dixon L, Gallardo C, Jori F, Laddomada A, Sanchez-Vizcaino, (2017) Approaches and perspectives for development of African swine fever virus vaccines. Vaccine 5:35
Burmakina G, Malogolovkin A, Tulman ER, Zsak L, Delhon G, Diel DG, Shobogorov NM, Morgunov YP, Morgunov SY (2016) African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J Gen Virol 97:1670–1675
Oura CA, Denyer MS, Takamatsu H, Parkhouse RM (2005) In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol 86(9):2445–2450
Argilaguet JM, Pérez-Martin E, Nofrarías M, Gallardo C, Accensi F, Lacasta A, Mora M, Ballester M, Galindo-Cardiel I, Lopez-Soria S (2012) DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 7:e40942
Leitão A, Malur A, Cartaxeiro C, Vasco G, Cruz B, Cornelis P, Martins CL (2000) Bacterial lipoprotein-based expression vectors as tools for the characterisation of African swine fever virus (ASFV) antigens. Arch Virol 145:1639–1657
Alonso F, Domínguez J, Vinuela E, Revilla Y (1997) African swine fever virus-specific cytotoxic T lymphocytes recognize the 32 kDa immediate early protein (vp32). Virus Res 49:123–130
Tlaxca JL, Ellis S, Remmele R Jr (2015) Live attenuated and inactivated viral vaccine formulation and nasal delivery: potential and challenges. Adv Drug Deliv Rev 93:56–78
Stone SS, Hess WR (1967) Antibody response to inactivated preparations of African swine fever virus in pigs. Am J Vet Res 28:475–481
Forman AJ, Wardley RC, Wilkinson PJ (1982) The immunological response of pigs and guinea pigs to antigens of African swine fever virus. Arch Virol 74:91–100
Kihm U, Ackerman M, Mueller, H, Pool R (1987) Approaches to vaccination. In: Becker Y (ed) African swine fever. Martinus Nijhoff Publishing, Boston, MA, pp 127–144
Mebus CA (1988) African swine fever. Adv Virus Res 35:251–269
Sang H, Miller G, Lokhandwala S, Sangewar N, Waghela SD, Bishop RP, Mwangi W (2020) Progress toward development of effective and safe African swine fever virus vaccines. Front Vet Sci 7:84
Blome S, Gabriel C, Beer M (2014) Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 32:3879–3882
Walczak M, Juszkiewicz M, Szymankiewicz K, Szczotka-Bochniarz A, Woźniakowski G (2022) ASF -survivors’ sera do not inhibit African swine fever virus replication in vitro. J Vet Res 66:21–27
Alonso C, Borca M, Dixon L, Revilla Y, Rodriguez F, Escribano JM (2018) ICTV Report Consortium. ICTV virus taxonomy profile: Asfarviridae. J Gen Virol 99:613–614
Gómez-Puertas P, Rodríguez F, Oviedo JM, Ramiro-Ibanez F, Ruiz-Gonzalvo F, Alonso C, Escribano JM (1996) Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J Virol 70:5689–5694
Barderas MG, Rodríguez F, Gómez-Puertas P, Aviles M, Beitia F, Alonso C, Escribano JM (2001) Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch Virol 146:1681–1691
Ruiz-Gonzalvo F, Rodriguez F, Escribano JM (1996) Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 218:285–289
Jancovich JK, Chapman D, Hansen DT, Robida MD, Loskutov A, Craciunescu F, Borovkov A, Kibler K, Goatley L, King K (2018) Immunization of pigs by DNA prime and recombinant Vaccinia virus boost to identify and rank African swine fever virus immunogenic and protective proteins. J Virol 92:e02219-e2317
Freitas FB, Simões M, Frouco G, Martins C, Ferreira F (2019) Towards the generation of an ASFV-pA104R DISC mutant and a complementary cell line-a potential methodology for the production of a vaccine candidate. Vaccine 7:68
Urbano AC, Ferreira F (2022) African swine fever control and prevention: an update on vaccine development. Emerg Microbes Infect 11:2021–2033
Zhang H, Zhao S, Zhang H, Shen Y, Zhang P, Shan H (2023) Orally administered recombinant Lactobacillus expressing African swine fever virus antigens that induced immunity responses. Front Microbiol 13:1103327
Sunwoo SY, Pérez-Núñez D, Morozov I, Sánchez EG, Gaudreault NN, Trujillo JD, Mur L, Nogal M, Madden D, Urbaniak K, Kim IJ (2019) DNA-protein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines 7(1):12
Bosch-Camos L, López E, Rodriguez F (2020) African swine fever vaccines: a promising work still in progress. Porcine Health Manag 6(1):1–14
Lokhandwala S, Waghela SD, Bray J, Sangewar N, Charendoff C, Martin CL, Hassan WS, Koynarski T, Gabbert L, Burrage TG (2017) Adenovirus-vectored novel African swine fever virus antigens elicit robust immune responses in swine. PLoS ONE 12:e0177007
Goatley LC, Reis AL, Portugal R, Goldswain H, Shimmon GL, Hargreaves Z, Ho CS, Montoya M, Sánchez-Cordón PJ, Taylor G, Dixon LK (2020) A pool of eight virally vectored African swine fever antigens protects pigs against fatal disease. Vaccines 8(2):234
Kardani K, Bolhassani A, Shahbazi S (2016) Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 34:413–423
Minor PD (2015) Live attenuated vaccines: historical successes and current challenges. Virology 479:379–392
Petisca NJ (1965) Quelques aspects morphologiques des suites de la vaccination contre la peste porcine Africaine (virose L) au Portugal. Bull Off Int Epiz 63:199–237
Sered AD, Balyshe VM, Kazakova AS, Imatdinov AR, Kolbasov DV (2020) Protective properties of attenuated strains of African swine fever virus belonging to seroimmunotypes I-VIII. Pathogens 9(4):274
Gallardo C, Sánchez EG, Pérez-Núñez D, Nogal M, De León P, Carrascosa AL, Nieto R, Soler A, Arias ML, Revilla Y (2018) African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses. Vaccine 36:2694–2704
King K, Chapman D, Argilaguet JM, Fishbourne E, Hutet E, Cariolet R, Takamatsu HH (2011) Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine 29:4593–4600
Mulumba-Mfumu LK, Goatley LC, Saegerman C, Takamatsu HH, Dixon LK (2016) Immunization of African indigenous pigs with attenuated genotype I African swine fever virus OURT88/3 induces protection against challenge with virulent strains of genotype I. Transbound Emerg Dis 63:e323–e327
Gavier-Widén D, Ruiz Fons F, Iacolina L (2020) Four years of advances in African swine fever in Europe by the ASF-STOP COST Action.
Barasona JA, Cadenas-Fernández E, Kosowska A, Barroso-Arévalo S, Rivera B, Sánchez R (2021) Safety of African swine fever vaccine candidate Lv17/WB/ Rie1 in wild boar: overdose and repeated doses. Front Immunol 12:761753
Netherton CL, Goatley LC, Reis AL, Portugal R, Nash RH, Morgan SB, Gault L, Nieto R, Norlin V, Gallardo C, Ho CS (2019) Identification and immunogenicity of African swine fever virus antigens. Front Immunol 10:1318
Reis AL, Goatley LC, Jabbar T, Lopez E, Rathakrishnan A, Dixon LK (2020) Deletion of the gene for the Type I Interferon Inhibitor I329L from the attenuated African swine fever virus OURT88/3 strain reduces protection induced in pigs. Vaccines (Basel) 8:262
Manso Ribeiro J, Nunes-Petisca JL, Lopez-Frazao F, Sobral M (1963) Vaccination against ASF. Bull Off Int Epizoot 60:921–937
Sánchez BC (1963) Modificatión del virus de la peste porcina Africana en cultivos celulares. Bulletin de l’Office Int des Epizooties 60:901–919
Krug PW, Holinka LG, O’Donnell V, Reese B, Sanford B, Fernandez-Sainz I, Gladue DP, Arzt J, Rodriguez L, Risatti GR, Borca MV (2015) The progressive adaptation of a Georgian isolate of African swine fever virus to Vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J Virol 89(4):2324–2332
Borca MV, Rai A, Ramirez-Medina E, Silva E, Velazquez-Salinas L, Vuono E (2021) A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J Virol 95:e0012321
O’Donnell V, Holinka LG, Sanford B, Krug PW, Carlson J, Pacheco JM, Reese B, Risatti GR, Gladue DP, Borca MV (2016) African swine fever virus Georgia isolate harbouring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Res 221:8–14
O’Donnell V, Holinka LG, Gladue DP, Sanford B, Krug PW, Lu X, Arzt J, Reese B, Carrillo C, Risatti GR (2015) African swine fever virus Georgia isolate harbouring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J Virol 89:6048–6056
Reis AL, Abrams CC, Goatley LC, Netherton C, Chapman DG, Sanchez-Cordon P, Dixon LK (2016) Deletion of African swine fever virus interferon inhibitors from the genome of a virulent isolate reduces virulence in domestic pigs and induces a protective response. Vaccine 34(39):4698–4705
Monteagudo PL, Lacasta A, López E, Bosch L, Collado J, Pina-Pedrero S, Correa-Fiz F, Accensi F, Navas MJ, Vidal E (2017) BA71∆CD2: A new recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol 91(21):10–1128
Chen W, Zhao D, He X, Liu R, Wang Z, Zhang X, Li F, Shan D, Chen H, Zhang J (2020) A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci 63:623–634
Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Holinka LG, Velazquez-Salinas L, Zhu J, Gladue DP (2020) Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol 94(7):e02017-e2019
Zhang J, Zhang Y, Chen T, Yang J, Yue H, Wang L (2021) Deletion of the L7L–L11L genes attenuates ASFV and induces protection against homologous challenge. Viruses 13:255
Ramirez-Medina E, Vuono E, Rai A, Pruitt S, Espinoza N, Velazquez-Salinas L (2022) Deletion of E184L, a putative DIVA target from the pandemic strain of African swine fever virus, produces a reduction in virulence and protection against virulent challenge. J Virol 96:e0141921
Sanford B, Holinka LG, O’Donnell V, Krug PW, Carlson J, Alfano M (2016) Deletion of the thymidine kinase gene induces complete attenuation of the Georgia isolate of African swine fever virus. Virus Res 213:165–171
Li D, Wu P, Liu H, Feng T, Yang W, Ru Y (2022) A QP509L/QP383R-deleted African swine fever virus is highly attenuated in swine but does not confer protection against parental virus challenge. J Virol 96:e0150021
Ramirez-Medina E, Vuono E, Silva E, Rai A, Valladares A, Pruitt S (2022) Evaluation of the deletion of MGF110-5L-6L on swine virulence from the pandemic strain of African swine fever virus and use as a DIVA marker in vaccine candidate ASFV-G-ΔI177L. Virol J 96:e0059722
Tran XH, Phuong LTT, Huy NQ, Thuy DT, Nguyen VD, Quang PH (2022) Evaluation of the safety profile of the ASFV vaccine candidate ASFV-G-ΔI177L. Viruses 14:896
Tran XH, Le TTP, Nguyen QH, do TT, Nguyen VD, Gay CG, (2022) African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound Emerg Dis 69:e497–e504
Dixon LK, Sun H, Roberts H (2019) African swine fever. Antiviral Res 165:34–41
Rock DL (2017) Challenges for African swine fever vaccine development “perhaps the end of the beginning.” Vet Microbiol 206:52–58
Forth JH, Tignon M, Cay AB, Forth LF, Höper D, Beer BS, M, (2019) Comparative analysis of whole-genome sequence of African swine fever virus Belgium 2018/1. Emerg Infect Dis 25(6):1249
Guinat C, Gogin A, Blome S, Keil G, Pollin R, Pfeiffer DU, Dixon L (2016) Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet Rec 178(11):262–267
Blome S, Franzke K, Beer M (2020) African swine fever — a review of current knowledge. Virus Res 287:198099
Calzada-Nova G, Husmann RJ, Schnitzlein WM, Zuckermann FA (2012) Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 148:116–125
Carrascosa AL, Bustos MJ, de Leon P (2011) Methods for growing and titrating African swine fever virus: field and laboratory samples. Curr Protoc Cell Biol 53(1):26–14
Gallardo C (2013) In vivo testing of selected ASFV strains: studies with attenuated strains in different cell systems, and the use of adjuvants. In: Proceedings of the ASFORCE European Union project, Full Consortium Meeting (FCM). ASFORCE, Cagliari, Sardegna, Italy, pp 21–22
Wang T, Wang L, Han Y, Pan L, Yang J, Sun M (2021) Adaptation of African swine fever virus to HEK293T cells. Transbound Emerg Dis 68:2853–2866
Lacast A, Monteagud PL, Jiménez-Marín Á, Accensi F, Ballester M, Argilaguet J, Galindo-Cardiel I, Segalés J, Salas ML, Domínguez J, Moreno Á (2015) Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in viral pathogenesis and immune protection. Vet Res 46(1):1–16
Sánchez EG, Quintas A, Nogal M, Castelló A, Revilla Y (2013) African swine fever virus controls the host transcription and cellular machinery of protein synthesis. Virus Res 173(1):58–75
Ballesteros C, Garrido JM, Vicente J, Romero B, Galindo RC, Minguijón E, Villar M, Martín-Hernando MP, Sevilla I, Juste R, Aranaz A, de la Fuente J, Gortázar C (2009) First data on Eurasian wild boar response to oral immunization with BCG and challenge with a Mycobacterium bovis field strain. Vaccine 27(48):6668
Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Espinoza N, Velazquez-Salinas L, Gay CG, Gladue DP (2021) ASFV-G-∆ I177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses 13(5):765
Author information
Authors and Affiliations
Contributions
CMS and SSN conceptualized the idea. CMS, SSN, MSSC, and SB did the literature search. CMS, SSN, SS, KV, and PD wrote the manuscript. VKC and A edited the manuscript. SP assisted in revising the manuscript along with CMS and SSN.
Corresponding authors
Ethics declarations
Ethics approval
The authors confirm that no ethical approval was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Maria Aparecida Scatamburlo Moreira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandana, M.S., Nair, S.S., Chaturvedi, V.K. et al. Recent progress and major gaps in the vaccine development for African swine fever. Braz J Microbiol 55, 997–1010 (2024). https://doi.org/10.1007/s42770-024-01264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01264-7