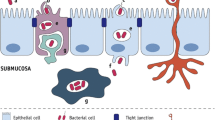
Abstract
Uropathogenic Escherichia coli (UPEC) have the potential to receive the virulence markers of intestinal pathotypes and transform into various important hybrid pathotypes. This study aimed to investigate the frequency and characteristics of hybrid enteroaggregative E. coli (EAEC)/UPEC strains. Out of 202 UPEC strains, nine (4.5%) were detected as hybrid EAEC/UPEC. These strains carried one to four iron uptake systems. Among nine investigated pathogenicity islands (PAIs), PAI IV536, PAI II536, and PAI ICFT073 were found in 9 (100%), 3 (33.3%), and 1 (11.1%) strains, respectively. The chuA and sitA genes were detected in 5 (55.5%) and 3 (33.3%) hybrid strains, respectively. Six hybrid strains were found to be typical extraintestinal pathogenic E. coli (ExPEC) according to their virulence traits. Most of the hybrid strains belonged to the phylogenetic group E (6/9). Among the hybrid strains, seven (7/9) were able to form biofilm and adhere to cells; however, only two strains penetrated into the HeLa cells. Our findings reveal some of the virulence characteristics of hybrid strains that lead to fitness and infection in the urinary tract. These strains, with virulence factors of intestinal and non-intestinal pathotypes, may become emerging pathogens in clinical settings; therefore, further studies are needed to reveal their pathogenicity mechanisms and so that preventive measures can be taken.


Similar content being viewed by others
References
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201
Gati NS, Temme IJ, Middendorf-Bauchart B, Kehl A, Dobrindt U, Mellmann A (2021) Comparative phenotypic characterization of hybrid Shiga toxin-producing / uropathogenic Escherichia coli, canonical uropathogenic and Shiga toxin-producing Escherichia coli. Int J Med Microbiol 311:151533
França FL, Wells TJ, Browning DF, Nogueira RT, Sarges FS, Pereira AC et al (2013) Genotypic and phenotypic characterisation of enteroaggregative Escherichia coli from children in Rio de Janeiro, Brazil. PLoS One 8:e69971
Huang DB, Mohanty A, DuPont HL, Okhuysen PC, Chiang T (2006) A review of an emerging enteric pathogen: enteroaggregative Escherichia coli. J Med Microbiol 55:1303–1311
Nataro JP, Deng Y, Cookson S, Cravioto A, Savarino SJ, Guers LD et al (1995) Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis 171:465–468
Smith HR, Scotland SM, Willshaw GA, Rowe B, Cravioto A, Cv E (1994) Isolates of Escherichia coli O44:H18 of diverse origin are enteroaggregative. J Infect Dis 170:1610–1613
Tanabe RHS, Dias RCB, Orsi H, de Lira DRP, Vieira MA, Dos Santos LF et al (2022) Characterization of Uropathogenic Escherichia coli reveals hybrid isolates of Uropathogenic and Diarrheagenic (UPEC/DEC) E. coli. Microorganisms 10(3):645. https://doi.org/10.3390/microorganisms10030645
Olesen B, Scheutz F, Andersen RL, Menard M, Boisen N, Johnston B et al (2012) Enteroaggregative Escherichia coli O78:H10, the cause of an outbreak of urinary tract infection. J Clin Microbiol 50:3703–3711
Nascimento JAS, Santos FF, Santos-Neto JF, Trovão LO, Valiatti TB, Pinaffi IC et al (2022) Molecular epidemiology and presence of hybrid pathogenic Escherichia coli among isolates from community-acquired urinary tract infection. Microorganisms 10(2):302. https://doi.org/10.3390/microorganisms10020302
Abe CM, Salvador FA, Falsetti IN, Vieira MA, Blanco J, Blanco JE et al (2008) Uropathogenic Escherichia coli (UPEC) strains may carry virulence properties of diarrhoeagenic E. coli. FEMS Immunol Med Microbiol 52:397–406
Lara FB, Nery DR, de Oliveira PM, Araujo ML, Carvalho FR, Messias-Silva LC et al (2017) Virulence markers and phylogenetic analysis of Escherichia coli strains with hybrid EAEC/UPEC genotypes recovered from sporadic cases of extraintestinal infections. Front Microbiol 8:146
Modgil V, Kaur H, Mohan B, Taneja N (2020) Molecular, phylogenetic and antibiotic resistance analysis of enteroaggregative Escherichia coli/uropathogenic Escherichia coli hybrid genotypes causing urinary tract infections. Indian J Med Microbiol 38:421–429
Desvaux M, Dalmasso G, Beyrouthy R, Barnich N, Delmas J, Bonnet R (2020) Pathogenicity factors of genomic islands in intestinal and extraintestinal Escherichia coli. Front Microbiol 11:2065
Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H et al (1994) Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun 62:606–614
Dobrindt U, Hochhut B, Hentschel U, Hacker J (2004) Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol 2:414–424
Schmidt H, Hensel M (2004) Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev 17:14–56
Okeke IN, Nataro JP (2001) Enteroaggregative Escherichia coli. Lancet Infect Dis 1:304–313
Schubert S, Rakin A, Karch H, Carniel E, Heesemann J (1998) Prevalence of the "high-pathogenicity island" of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun 66:480–485
Carniel E (2001) The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect 3:561–569
Schubert S, Cuenca S, Fischer D, Heesemann J (2000) High-pathogenicity island of Yersinia pestis in enterobacteriaceae isolated from blood cultures and urine samples: prevalence and functional expression. J Infect Dis 182:1268–1271
Schubert S, Rakin A, Heesemann J (2004) The Yersinia high-pathogenicity island (HPI): evolutionary and functional aspects. Int J Med Microbiol 294:83–94
Harrington SM, Dudley EG, Nataro JP (2006) Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett 254:12–18
Garénaux A, Caza M, Dozois CM (2011) The Ins and Outs of siderophore mediated iron uptake by extra-intestinal pathogenic Escherichia coli. Vet Microbiol 153:89–98
Erjavec MS, Arbiter T, Bertok DŽ (2009) Pathogenicity islands, plasmids and iron uptake systems in extraintestinal pathogenic Escherichia coli strains. Acta Biol Slov 52:73–83
Toma C, Lu Y, Higa N, Nakasone N, Chinen I, Baschkier A et al (2003) Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J Clin Microbiol 41:2669–2671
Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH et al (1997) Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun 65:4135–4145
Okhuysen PC, DuPont HL (2010) Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. J Infect Dis 202:503–505
Bafandeh S, Haghi F, Zeighami H (2015) Prevalence and virulence characteristics of enteroaggregative Escherichia coli in a case–control study among patients from Iran. J Medical Microbiol 64:519–524
Rodriguez-Siek KE, Giddings CW, Doetkott C, Johnson TJ, Nolan LK (2005) Characterizing the APEC pathotype. Veterinary research 36:241–256
Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK (2008) Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol 46:3987–3996
Maurer JJ, Brown TP, Steffens W, Thayer SG (1998) The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin Tsh among avian Escherichia coli. Avian Dis 42(1):106–18
Spurbeck RR, Dinh PC Jr, Walk ST, Stapleton AE, Hooton TM, Nolan LK et al (2012) Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect Immun 80:4115–4122
Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA et al (2003) Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob Agents Chemother 47:2161–2168
Rahmani HK, Tabar GH, Askari Badouei M, Khoramian B (2020) Development of three multiplex-PCR assays for virulence profiling of different iron acquisition systems in Escherichia coli. Iran J Microbiol 12:281
Johnson JR, Russo TA, Tarr PI, Carlino U, Bilge SS, Vary JC et al (2000) Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect Immun 68:3040–3047
Clermont O, Christenson JK, Denamur E, Gordon DM (2013) The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65
Wang S, Meng Q, Dai J, Han X, Han Y, Ding C et al Development of an allele-specific PCR assay for simultaneous serotyping of avian pathogenic Escherichia coli predominant O1, O2, O18 and O78 Strains. PLoS One 9(5):e96904. https://doi.org/10.1371/journal.pone.0096904
Sabaté M, Moreno E, Pérez T, Andreu A, Prats G (2006) Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin Microbiol Infect 12:880–886
Weigel RM, Qiao B, Teferedegne B, Suh DK, Barber DA, Isaacson RE et al (2004) Comparison of pulsed field gel electrophoresis and repetitive sequence polymerase chain reaction as genotyping methods for detection of genetic diversity and inferring transmission of Salmonella. Vet Microbiol 100:205–217
Toval F, Köhler C-D, Vogel U, Wagenlehner F, Mellmann A, Fruth A et al (2014) Characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. J Clin Microbiol 52:407–418
Alipour T, Poursina F (2021) The frequency of hybrid Enteroaggregative/Uropathogenic Escherichia coli isolated from clinical samples of Isfahan hospitals, Iran. Gene Reports 23:101042
Yousefipour M, Rezatofighi SE, Roayaei Ardakani M (2023) Detection and characterization of hybrid uropathogenic Escherichia coli strains among E. coli isolates causing community-acquired urinary tract infection. J Med Microbiol 72:001660
Monteiro BT, Campos LC, Sircili MP, Franzolin MR, Bevilacqua LF, Nataro JP et al (2009) The dispersin-encoding gene (aap) is not restricted to enteroaggregative Escherichia coli. Diagn Microbiol Infect Dis 65:81–84
Jenkins C, Van Ijperen C, Dudley EG, Chart H, Willshaw GA, Cheasty T et al (2005) Use of a microarray to assess the distribution of plasmid and chromosomal virulence genes in strains of enteroaggregative Escherichia coli. FEMS Microbiol Lett 253:119–124
Cordeiro F, da Silva Gomes Pereira D, Rocha M, Asensi MD, Elias WP, Campos LC (2008) Evaluation of a multiplex PCR for identification of enteroaggregative Escherichia coli. J Clin Microbiol 46:828–829
Yamamoto T, Echeverria P (1996) Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun 64:1441–1445
Jønsson R, Liu B, Struve C, Yang Y, Jørgensen R, Xu Y et al (2017) Structural and functional studies of Escherichia coli aggregative adherence fimbriae (AAF/V) reveal a deficiency in extracellular matrix binding. Biochim Biophys Acta 1865:304–311
Sheikh J, Hicks S, Dall'Agnol M, Phillips AD, Nataro JP (2001) Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli. Mol Microbiol 41:983–997
Yamazaki M, Inuzuka K, Matsui H, Sakae K, Suzuki Y, Miyazaki Y et al (2000) Plasmid encoded enterotoxin (Pet) gene in enteroaggregative Escherichia coli isolated from sporadic diarrhea cases. Jap J Infect Dis 53:248–249
Czeczulin JR, Whittam TS, Henderson IR, Navarro-Garcia F, Nataro JP (1999) Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun 67:2692–2699
Dudley EG, Thomson NR, Parkhill J, Morin NP, Nataro JP (2006) Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol Microbiol 61:1267–1282
Galardini M, Clermont O, Baron A, Busby B, Dion S, Schubert S et al (2020) Major role of iron uptake systems in the intrinsic extra-intestinal virulence of the genus Escherichia revealed by a genome-wide association study. PLoS Genet 16:e1009065
Schmidt H, Michael Hensel M (2007) Pathogenicity islands in bacterial pathogenesis. Clinic Microbiol Rew 17:14–56
Paauw A, Leverstein-van Hall MA, van Kessel KP, Verhoef J, Fluit AC (2009) Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS one 4:e8240
Garcia EC, Brumbaugh AR, Mobley HL (2011) Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun 79:1225–1235
Nègre VL, Bonacorsi S, Schubert S, Bidet P, Nassif X, Bingen E (2004) The siderophore receptor IroN, but not the high-pathogenicity island or the hemin receptor ChuA, contributes to the bacteremic step of Escherichia coli neonatal meningitis. Infect Immun 72:1216–1220
Torres AG, Redford P, Welch RA, Payne SM (2001) TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun 69:6179–6185
Clermont O, Condamine B, Dion S, Gordon DM, Denamur E (2021) The E phylogroup of Escherichia coli is highly diverse and mimics the whole E. coli species population structure. Environ Microbiol 23:7139–7151
Arimizu Y, Kirino Y, Sato MP, Uno K, Sato T, Gotoh Y et al (2019) Large-scale genome analysis of bovine commensal Escherichia coli reveals that bovine-adapted E. coli lineages are serving as evolutionary sources of the emergence of human intestinal pathogenic strains. Genome Res 29:1495–1505
Touchon M, Perrin A, De Sousa JAM, Vangchhia B, Burn S, O’Brien CL et al (2020) Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. PLoS Genet 16:e1008866
Kallonen T, Brodrick HJ, Harris SR, Corander J, Brown NM, Martin V et al (2017) Systematic longitudinal survey of invasive Escherichia coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res 27:1437–1449
de Lastours V, Laouénan C, Royer G, Carbonnelle E, Lepeule R, Esposito-Farèse M et al (2020) Mortality in Escherichia coli bloodstream infections: antibiotic resistance still does not make it. J Antimicrob Chemother 75:2334–2343
Denamur E, Clermont O, Bonacorsi S, Gordon D (2021) The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol 19:37–54
Martínez-Santos VI, Ruíz-Rosas M, Ramirez-Peralta A, García OZ, Resendiz-Reyes LA, Romero-Pineda OJ et al (2021) Enteroaggregative Escherichia coli is associated with antibiotic resistance and urinary tract infection symptomatology. PeerJ 9:e11726
Valiatti TB, Santos FF, Santos AC, Nascimento JA, Silva RM, Carvalho E et al (2020) Genetic and virulence characteristics of a hybrid atypical enteropathogenic and uropathogenic Escherichia coli (aEPEC/UPEC) Strain. Front Cell Infect Microbiol 10:492
Funding
The present study was partially funded by Ferdowsi University of Mashhad (Grant No. FUM. 48976).
Author information
Authors and Affiliations
Contributions
MAB designed and supervised the study. SM conducted the main laboratory experiments and some complementary tests were carried out by SER and FM. SM, MAB, GH, SER and FM contributed in data analysis. SM, MAB and SER contributed in writing the initial draft and all authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Ferdowsi University of Mashhad. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The research was performed on bacterial isolates collected from clinical samples that were already cultured as part of the routine work in the Microbiology laboratories of hospitals. Before collecting information, participants or parents (for children cases) were asked to read, accept and sign an informed consent form.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Beatriz Ernestina Cabilio Guth
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moazeni, S., Askari Badouei, M., Hashemitabar, G. et al. Detection and characterization of potentially hybrid enteroaggregative Escherichia coli (EAEC) strains isolated from urinary tract infection. Braz J Microbiol 55, 1–9 (2024). https://doi.org/10.1007/s42770-023-01195-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01195-9