Abstract
Invasive candidiasis (IC) represents a growing concern worldwide, with a considerable increase in non-albicans Candida (NAC) species. The study's primary goal was to determine if species identification by semi-nested PCR (sn-PCR) with primers for the five most prevalent Candida species is sufficient to deal with the current trends of Candida infections in cancer patients. Over one year, Candida isolates were collected from samples of patients with hematological and solid organ tumors in a single center. Species of Candida were identified by chromagar and multiplex sn-PCR using specific primers for Candida albicans, Candida tropicalis, Candida glabrata, Candida krusei, and the Candida parapsilosis complex. Most Candida infection episodes are caused by NAC species (70.5% of 105 isolates). Rare species (14 isolates) accounted for 13.3% of isolates and were not identified by sn-PCR using the five most common Candida species primers. More than half of these rare species caused candidemia in cancer patients (57.1%; p = 0.011). The risk factor for candidiasis was recent surgeries (p = 0.020) in adults and chemotherapy in pediatric patients (p = 0.006). Prolonged hospitalization and genitourinary tract cancer were significantly associated with invasive infections (p = 0.005 and 0.049, respectively). Recent surgery was a significant risk factor associated with C. parapsilosis and C. glabrata infections (P = 0.038 and 0.003, respectively), while C. tropicalis was significantly more common in patients with hematological malignancies (P = 0.012). Techniques with a broader identification spectrum than the major five Candida species are crucial for the optimal management of cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive Candida infections represent a growing concern in healthcare settings, especially among high-risk immunocompromised patients such as those who have cancer [1]. Despite C. albicans being the most frequent species, NAC species have increased significantly in the last two decades [2, 3]. High mortality rates and decreased antifungal susceptibility because of this shift towards NAC necessitate rapid, accurate species identification and improving our knowledge of clinical characteristics, risk factors, and outcomes associated with these pathogens to guide the clinician for optimal therapeutic interventions [2].
Significant variations in Candida species distribution are observed within different geographical areas. It mainly depends on the patient population, age, use of central venous catheters, broad-spectrum antibiotics, and antifungal strategies [3]. Since different Candida species show variable resistance patterns, rapid identification to the species level is a substantial prerequisite for convenient antimycotic therapy management, especially if antifungal susceptibility testing is not accessible [4]. Although C. albicans is typically susceptible to commonly used antifungals, Candida guilliermondii and C. parapsilosis may gain echinocandin resistance, C. glabrata may gain resistance to azoles, Candida lusitaniae can have diminished susceptibility to amphotericin B, and C. krusei has fluconazole intrinsic resistance. Moreover, Candida auris has recently emerged as a multiresistant healthcare-associated pathogen worldwide [5].
Many studies have found that more than 90% of invasive Candida infections are caused by C. albicans, C. parapsilosis, C. tropicalis, C. krusei, and C. glabrata species [6, 7]. PCR is a milestone in Candida infection diagnosis as it detects a trace amount of the microorganism's nucleic acid. Moreover, nested PCR is considered a very accurate procedure, improving the sensitivity and specificity of detecting Candida infections due to the low chance that any improperly amplified PCR fragment will be re-amplified in the second run [8]. Building on insights from local epidemiology is an urgent prerequisite to constructing hospital antifungal protocols. Therefore, we aimed in this study to find out whether Candida species identification by sn-PCR using primers for the five major Candida species is enough to cope with current infection patterns among cancer patients. We also assess the distribution, clinical characteristics, and patient outcomes associated with invasive infections and infections caused by different species in oncological patients.
Methodology
Study design and data collection
This study was conducted at Egypt's National Cancer Institute (NCI), Cairo University. Over a year, Candida isolates cultured from various specimens from cancer patients referred to the NCI Microbiology Laboratory were collected. Inpatients and outpatients with candidiasis who showed symptoms and signs of infection were included, with only one isolate from each patient. Demographic data, underlying diseases, clinical characteristics, and outcomes of patients were obtained retrospectively. Patients' isolates with missing data were excluded. The clinical features of patients, risk factors, and microbiological characteristics of invasive candidiasis and Candida infections caused by various Candida species were analyzed. A healthcare-associated infection (HAI) was considered when a positive culture occurred more than 48 h after admission [9]. Lymphopenia was defined as the absolute lymphocyte count (ALC) being less than 0.7 × 103/μl, and neutropenia as the absolute neutrophil count (ANC) being less than 1.0 × 103/μl [10].
Candida species identification
All samples were processed under standard microbiological procedures immediately upon arrival in the laboratory. To be included, specimens must meet the following criteria: the presence of Candida in at least one positive blood culture with clinical symptoms and signs of infection; a pure growth of Candida with a significant colony count (≥ 105 colony-forming units (CFU)/mL) in urine samples from symptomatic patients; and sputum specimens where 25 or more leucocytes and fewer than ten epithelial cells per low-power field (10x) [11]. All isolates were then subcultured on Sabouraud dextrose agar (SDA), wet mount, and Gram staining as primary screening for Candida colonies. Chromagar (CHROMAgar™ Candida Becton Dickinson, Germany) and multiplex sn-PCR were used for further Candida species identification. When a strain's species could not be determined, it was classified as an "unidentified Candida species."
Multiplex semi-nested PCR
DNA extraction was done according to the manufacturer's instructions using QIAGEN (QIAamp DNA Mini Kit). The PCR amplification was performed using the fungus-specific oligonucleotides with internal transcribed spacers 1 and 4 (ITS1 and ITS4) as outer primers (5'-TCCGTAGGTGAACCTGCGG-3' and 5'-TCCTCCGCTTATTGATATGC-3', respectively). Then the inner primers for C. krusei, C. glabrata, C. albicans, C. parapsilosis complex, and C. tropicalis were used. The primer sequences were published in [12] and were as follows: CKR (F- 5' ACTACACTGCGTGAGCGGAA 3') (R- 5' AAAAAGTCTAGTTCGCTCGG 3'); CGL (F- 5' TTATCACACGACTCGACACT 3'), (R- 5' CCCACATACTGATATGGCCTACAA 3'); CALB (F- 5' TTTATCAACTTGTCACACCAGA 3'), (R- 5' ATCCCGCCTTACCACTACCG 3'); CPAR (F- 5' GCCAGAGATTAAACTCAACCAA 3'), (R- 5' CCTATCCATTAGTTTATACTCCGC 3'); and CTR (F- 5' CAATCCTACCGCCAGAGGTTAT 3') (R- 5' TGGCCACTAGCAAAATAAGCGT 3'), respectively. The sizes of the amplicons were 362 bp, 423 bp, 272 bp, 297 bp, and 357 bp, respectively. The protocol described in [12] was used for DNA extraction, quantification, and amplification. The amplified PCR products were run on 2% agarose gel electrophoresis and then visualized by a UV transilluminator (Biometra).
Statistical analysis
The qualitative data were shown as frequency and percentage, while the numerical data were shown as mean and standard deviation, or median and range, as convenient. Comparisons between qualitative variables were examined by the Chi-square or Fisher’s exact test; however, the Mann–Whitney test was used to compare continuous variables. The correlation between diagnostic techniques was examined by the Kappa test, where range values of 1% to 20% indicate minor agreement, 21% to 40% fair agreement, 41% to 60% median agreement, 61% to 80% significant agreement, and 81% to 100% nearly perfect agreement. Risk estimates were measured and expressed as an odds ratio and a 95% confidence interval (CI) to identify risk factors for different groups. The Kaplan–Meier test was used in survival analysis, and the log-rank test was used to compare survival between groups. To detect the independent risk factors for thirty-day post-infection mortality, binary logistic regression was used to analyze factors with a p-value less than 0.1 in univariate analysis. A p-value less than 0.05 was regarded as significant. The data were statistically analyzed using version 25 of IBM SPSS (SPSS Inc., Armonk, NY, USA).
Results
Clinical characteristics of Candida-infected cancer patients
The demographic characteristics of 105 cancer patients diagnosed with Candida infections during the study period are summarized in Table 1. Candida infections were more prevalent in the over-50 age group, followed by the 18–50 age group and children (< 18 years) (n = 46, 43.8%, n = 38, 36.2%, and n = 21, 20.0%, respectively). Infected children had a median age of 6 years, ranging from 1 to 17 years, whereas adults had a median age of 47 years, ranging from 19 to 80 years. Hematological malignancies were observed in 37.1% and solid organ tumors in 62.9% of all patients. Gastrointestinal tract (GIT) tumors were significantly more common in Candida-infected adult patients, while central nervous system (CNS) cancers were more common in children (p = 0.011 and p < 0.001, respectively). Neutropenia and lymphopenia were present in 33.3% and 42.9% of patients, respectively.
Most Candida infection episodes were HAIs (97.1%), with the median time spent in the hospital before the onset of infection being ten days. Also, the presence of Candida infections was linked to a longer hospital stay, admission to the intensive care unit (ICU), previous exposure to broad-spectrum antibiotics, receiving chemotherapy, and recent surgical procedures (65.7%, 53.3%, 96.2%, 49.5%, and 36.2%, respectively). Recent surgeries were a significant risk factor for candidiasis in adult patients, whereas chemotherapy was in pediatric patients (p = 0.020 and p = 0.006, respectively).
Identification of Candida species
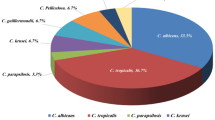
Regarding clinical sites of infection, 30 patients had candidemia, 25 had surgical site infections (SSIs), 17 had urinary tract infections (UTIs), and 33 had respiratory tract infections (RTIs) (28.6%, 23.8%, 16.2%, and 31.4%, respectively). The frequency of different Candida species identified by sn-PCR is shown in Fig. 1a. NAC species represented 70.5% of isolates (n = 74). C. tropicalis was the most frequently isolated NAC species, followed by C. parapsilosis, C. glabrata, and C. krusei (24.8%, 14.3%, 13.3%, and 4.8%, respectively). Fourteen isolates (13.3%) were not identified to the species level. Furthermore, 33 patients (31.4%) had concomitant Candida infections in other body sites.
Frequency of different Candida species identified by multiplex semi-nested PCR (a) and by chromagar (b). non-albicans Candida (NAC) species represented more than 2/3 of species (n=74; 70.5% and n=69; 65.7% as identified by sn-PCR (semi-nested PCR) and chromagar, respectively). sn-PCR and chromagar were unable to identify 13.3% and 34.3% of Candida isolates. C. tropicalis and C. krusei were detected in 24.8% and 4.8% of all Candida isolates by PCR and 26.7% and 4.8% by chromagar, respectively. C. parapsilosis and C. glabrata were identified in 14.3% and 13.3% by PCR but could not be identified by chromagar
Figure 1b illustrates the frequency of Candida species identified by chromagar. The results of chromagar showed a high degree of correspondence with those of sn-PCR, as presented in Table 2. Thus, the overall concordance of the chromagar compared to sn-PCR tests was found to be 80.7% (p < 0.001). There was a discrepancy between both results in 14 isolates (13.3%) (two C. albicans, two C. tropicalis, one C. glabrata, six C. parapsilosis, and three other Candida species as identified by sn-PCR). Besides, the exact species of 36 Candida isolates (34.3%) could not be determined by chromagar.
Risk factors associated with invasive candidiasis
We divided Candida infections into two groups based on clinical sites: invasive candidiasis (infections in sterile body sites such as the bloodstream and surgical sites) and non-invasive candidiasis (urine and respiratory tract infections). Table 3 compares the two groups' risk factors, clinical features, microbiological characteristics, and patient outcomes. Invasive Candida infections were found to be significantly associated with prolonged hospital stay (≥ seven days) (p = 0.005). Furthermore, the type of underlying malignancy was a significant variable in invasive infections. Invasive infections were significantly higher in patients with genitourinary tract cancer and lower in patients with solid cancers other than the GIT, genitourinary tract (GUT), and CNS (p = 0.049 and p = 0.038, respectively). On the other hand, age, gender, hospitalization, ICU admission, chemotherapy, recent surgery, antibiotic intake, neutropenia, lymphopenia, Candida species, and multifocal Candida infections in other body sites did not differ statistically between the two groups.
Risk factors associated with infection by different Candida species
In the present study, Candida isolates were grouped into five categories according to the identified species: C. albicans (n = 31), C. tropicalis (n = 26), C. parapsilosis (n = 15), C. glabrata (n = 14), and other Candida species (n = 19), which included C. krusei (n = 5) and all unidentified species (n = 14). Table 4 compares groups in terms of risk factors, clinical characteristics, outcomes of patients, and associated microbiological features. C. albicans infections were significantly lower in lymphopenic patients (OR: 0.348, 95% CI: 0.138 to 0.877, P = 0.022). C. tropicalis was significantly more frequently detected in patients with hematological malignancies but significantly less frequently in patients who had recently undergone surgery (OR: 3.125, 95% CI: 1.253 to 7.794, P = 0.012; OR: 0.332, 95% CI: 0.114 to 0.970, P = 0.038, respectively). This species was significantly more frequently isolated from UTIs and less from RTIs (OR: 6.429, 95% CI: 2.124 to 19.456, P < 0.001; OR: 0.313, 95% CI: 0.098 to 0.999, P = 0.042, respectively).
While C. parapsilosis infections were significantly more common in patients who had recently undergone surgery, they were significantly less common in male patients, those with hematological malignancies, and neutropenic patients (OR: 3.155, 95% CI: 1.026 to 9.705, P = 0.038; OR: 0.231, 95% CI: 0.068 to 0.784, P = 0.013; OR: 0.220, 95% CI: 0.047 to 1.035, P = 0.039; OR: 0.118, 95% CI: 0.015 to 0.935, P = 0.018, respectively). The preceding species produced significantly more SSIs (OR: 4.908, 95% CI: 1.564 to 15.398, P = 0.004). However, infections by C. glabrata were significantly revealed in patients with solid organ tumors in locations other than GIT, GUT, and CNS and who had recently had surgeries but were significantly less frequently isolated from patients who had hematological malignancies and who were exposed to chemotherapy (OR: 3.657, 95% CI: 1.047 to 12.773, P = 0.033; OR: 5.625, 95% CI: 1.624 to 19.478, P = 0.003; OR: 0.107, 95% CI: 0.013 to 0.856, P = 0.013; OR: 0.234, 95% CI: 0.061 to 0.894, P = 0.024, respectively). The former species were significantly more commonly isolated from SSIs (OR: 4.056, 95% CI: 1.262 to 13.037, P = 0.013) and less from bloodstream infections (BSIs), with a p-value of 0.011.
Other Candida species infections were significantly less common in patients who had a prolonged hospital stay (≥ seven days) (OR: 0.168, 95% CI: 0.057 to 0.496, P = 0.001). That C. krusei and unidentified Candida species were significantly detected at a higher rate in BSIs and a lower rate in SSIs (OR: 3.667, 95% CI: 1.309 to 10.272, P = 0.010; OR: 0.144, 95% CI: 0.018 to 1.135, P = 0.036, respectively). Moreover, Fig. 2 illustrates the distribution of clinical sites of infection caused by different Candida species among cancer patients. The urinary tract was the most common infection site caused by C. tropicalis species (38.5%). However, nearly half of the C. parapsilosis and C. glabrata species infections were SSIs (53.3% and 50%, respectively). Interestingly, BSI was the most frequent infection produced by unidentified Candida species (57.1%, p = 0.011).
Clinical sites of Candida infections in 105 cancer patients. Nearly half of C. parapsilosis and C. glabrata species infections were SSIs, while 57.1% of patients infected by unidentified Candida species had candidemia. BSIs: bloodstream infections, SSIs: surgical site infections, UTIs: urinary tract infections, RTIs: respiratory tract infections
Antifungal therapy
Forty-seven patients (44.8%) received antifungal therapy after documented proof of infection. Triazoles (fluconazole or voriconazole) were the most frequently used antifungals (21/47, 44.7%), followed by echinocandins (caspofungin or micafungin) and amphotericin B (n = 7, 14.9%, and n = 6, 12.8%, respectively). Some patients got more than one antifungal treatment (n = 13, 27.7%). All 13 combination antifungal treatments include at least fluconazole or voriconazole. Most of the 58 patients who didn’t receive antifungal treatment (79.3%) had solid organ malignancies (OR: 5.175, 95% CI: 2.192 to 12.218, P < 0.001).
Outcome
The overall thirty-day post-infection cumulative mortality rate was 32.4% (n = 34). Figure 3 clarifies the 30-day cumulative mortality of patients infected with different Candida species. No significant differences in crude mortality rates were observed among the species groups. Furthermore, univariate regression analysis for associated risk factors for 30-day mortality in Candida-infected cancer patients is shown in Table 5. ICU admission and multifocal Candida infections were associated risk factors for increased 30-day mortality (OR: 7.167, 95% CI: 2.631 to 19.520, P < 0.001; OR: 2.824, 95% CI: 1.187 to 6.715, P = 0.017, respectively). However, logistic regression analysis revealed that ICU stay was an independent risk factor for 30-day cumulative mortality in infected patients (adjusted OR: 6.172, 95% CI: 2.210 to 17.231, P = 0.001) (Table 6). Thirty-day mortality rates did not differ statistically according to the clinical site of infection; however, the highest rate was seen in patients with candidemia (32.4%, p = 0.553).
The thirty-day cumulative mortality curves of 105 cancer patients infected with different Candida species. The overall 30-day post-infection cumulative mortality curve is shown in (a). The cumulative mortality curves for C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, and other Candida species are also presented in (b, c, d, e, and f, respectively). Other species include C. krusei (n = 5) and unidentified Candida species (n = 14)
Discussion
Invasive Candida infections have become a public health concern, highly connected to immunocompromised states such as cancer patients. Furthermore, the COVID-19 pandemic has been implicated in a rise in fungal infections [13]. Additionally, there has been a remarkable increase in morbidity and mortality associated with Candida infections and decreased susceptibility to commonly used antifungal agents in recent years. This situation necessitates understanding local trends and species distribution among cancer patients with hematological and solid organ malignancies to properly improve the management of those high-risk patients [6].
The changing pattern of Candida infections in high-risk cancer patients, manifested by increasing NAC species, is due to previous exposure to antifungals, broad-spectrum antibiotics, chemotherapy, invasive devices, surgeries, prolonged hospital stay, ICU admissions, and the presence of neutropenia [14], in addition to immunocompromised conditions, comorbidities, and the severity of the underlying malignancy in such patients [15]. Furthermore, multidrug-resistant species like C. auris are becoming more prevalent [13]. This current epidemiology necessitates improving the diagnostic skills of microbiology laboratories. Rapid species identification is necessary because of the increased mortality rates associated with these infections, particularly in high-risk cancer patients [16].
Local trends of Candida species are crucial since the variable distribution is observed between geographical regions and is influenced by underlying conditions and risk factors [7]. Candida pneumonia diagnosis is challenging because the definitive diagnosis is based on histologic evidence of yeast and inflammatory cells in lung tissue and the failure of less invasive methods to confirm infection. Thus, antimycotic therapy should be reserved for immunocompromised patients with evidence of pneumonia, alongside physicians' clinical judgment [17]. Furthermore, some studies stated that Candida detection in the respiratory tract of critically ill patients increased morbidity and mortality, and even if it is thought to be colonization, it may contribute to poor outcomes [18]. Therefore, we included Candida isolated from respiratory specimens, particularly considering the immunocompromised state of our patients. In the current study, NAC accounted for 60.6% of the Candida identified from thirty-three respiratory samples of cancer patients.
Previous studies from various parts of the world revealed a predominance of NAC species, with varying trends and Candida species rankings. In the present study, NAC species accounted for 70.5% of isolates cultured from different body sites. C. tropicalis was the predominant cause of NAC infections, followed by C. parapsilosis, C. glabrata, and C. krusei (24.8%, 14.3%, 13.3%, and 4.8%). Our results were comparable to those of the Assiut University Hospitals in Egypt study. In that study, 75% of isolated species were NAC species, with C. tropicalis being the most prevalent species (46.5%) cultured from different specimens of ICU patients [19].
Another surveillance study was done in 21 hospitals in seven Latin American countries, where NAC species accounted for 62.4% of candidemia infections. C. parapsilosis was the most frequent NAC species isolated (26.5%) [20]. Similarly, a surveillance study in the United States from 2009 to 2017 demonstrated that NAC species caused 52% of IC infections, and C. glabrata was the most common one responsible for these infections [21]. On the other hand, a study by Lindberg et al. [22] showed a lower rate of NAC species (35% of all isolates) causing candidemia in the Swedish University Hospital. C. glabrata was the most identified NAC species, accounting for 19% of all isolates.
Many studies have reported that the main five Candida species we tested (C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, and C. krusei) are responsible for over 90% of Candida infections [6]. In the current study, 13.3% of Candida isolates were not identified by sn-PCR, indicating an increase in infections caused by uncommon Candida species among cancer patients. This percentage was higher than in previous surveillance studies conducted in other centers. In a multi-center study involving five years of surveillance of IC in China, 8829 Candida isolates from 65 tertiary hospitals were collected. Although 32 Candida species were identified using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) combined with Vitek MS and DNA sequencing, uncommon Candida species contributed 6% (5.7%) of the total isolates [23]. Similarly, Ying et al. [24] revealed rare species accounting for 1.7% (n = 53) of the total isolates recovered from various clinical specimens. These isolates were identified by Vitek 2 system.
However, in another study, these rare Candida species were detected at a higher rate (10.2%) and identified using PCR-restriction fragment length polymorphism (PCR–RFLP), duplex-PCR, and multiplex-PCR techniques [14]. Interestingly, uncommon species accounted for 21.1% of candidemia in patients with acute leukemia in a cancer center in the USA [25]. Moreover, 31.9% of isolates causing candidemia in patients admitted to the ICU at a tertiary care center in India were uncommon Candida species. PCR–RFLP could not identify 32 isolates (26.9% of a total of 119 isolates) but was then identified by MALDI-TOF MS [26]. These findings emphasize the increased rates of NAC species, notably rare species, among high-risk patients. Thus, accurate Candida species identification is crucial, particularly for those causing invasive fungal infections (IFIs).
Early antifungal treatment has a significant impact on the patient’s outcome. Thus, a more rapid and accurate diagnosis is crucial [13, 27]. Molecular techniques are more precise, faster, and less liable to growth condition variations and phenotypic changes. Multiplex PCR, PCR–RFLP, and sequencing of specific genome regions are some of the best-known molecular approaches [28]. A broader primer library should be used as Candida distribution expands beyond the five major Candida species previously known to cause invasive Candida infections in high-risk patients [29]. Based on our findings, 13.3% of isolates were not identified using multiplex sn-PCR with the primers of the five common Candida species, despite the significant detection of these unidentified species in invasive BSIs (57.1%, p = 0.011). In addition, our patients had a high 30-day crude mortality rate of 32.4%. So, probably Vitek, MALDI-TOF, or combined methodologies with a broader identification spectrum are extensively recommended.
Chromagar is an inexpensive and easy method for primarily identifying common Candida species, particularly in areas with limited resources. In addition, this medium allows for rapid IC diagnosis and epidemiological surveillance in high-risk units; however, it is still insensitive and unable to identify all species [16]. Moreover, chromagar does not recognize the emergent yeast C. auris [13]. Despite the high degree of concordance between chromagar and sn-PCR (80.7%, p < 0.001), a discrepancy between the two methods was observed in 13.3% of our isolates. Furthermore, 34.3% of isolates could not be identified to the species level by chromagar since it fails to correctly identify all but three species: C. albicans, C. tropicalis, and C. krusei. In a study conducted in Japan, chromagar could not determine the species of more than half of the Candida isolates (66.7%) cultured from various specimens [28]. Thus, the chromogenic medium is unreliable compared to molecular methods because of the inaccurate identification of many Candida species [29].
In this study, Candida infections were more prevalent in the over-50 age group, with a median age of 50 years among all infected cancer patients. This was in agreement with previous studies [6, 22, 27]. Most Candida isolates in our study were from hospitalized patients (97.1%). Other risk factors for Candida infection observed in our patients included a prolonged duration of hospitalization of ≥ 7 days (65.7%), an ICU stay (53.3%), previous antibiotic exposure (96.2%), chemotherapy (49.5%), and recent surgeries (36.2%). Similarly, it was reported that the age of less than one or more than sixty-five years, a central venous catheter (CVC), surgical procedures, exposure to chemotherapy, and prior antibiotic uptake are all main risk factors for invasive Candida infections [1, 30]. Furthermore, invasive Candida infections were significantly higher in patients with prolonged hospital stays and genitourinary tract cancer (p = 0.005 and p = 0.049, respectively). Similarly, Xia et al. [31] found that increased hospitalization duration was significantly associated with invasive Candida infections (p = 0.037). Additionally, invasive infections were significantly more often detected in patients with cancer as an underlying comorbidity (p = 0.006) [31].
In our study, the incidence of C. glabrata and C. parapsilosis infections was significantly higher in patients who had recent surgeries, and they were significantly revealed much more frequently from SSIs. Infections with C. tropicalis species were significantly associated with hematological malignancies and were more commonly detected in UTIs. Negri et al. [32] also stated the link of C. tropicalis with UTIs and hematological malignancies. Furthermore, Lortholary et al. [33] and Wu et al. [2] also reported a significant association between C. tropicalis and hematological malignancies. Interestingly, unidentified Candida species were significantly more frequently isolated from BSIs (p = 0.011), emphasizing the importance of accurate Candida species identification.
In our study, the thirty-day crude mortality rate of infected cancer patients was 32.4%, with candidemia patients having the highest mortality rate of 32.4%. Reports from other studies showed nearly the same mortality rates of 30% to 40%. At 28 days, mortality was 26.3% among candidemia-infected pediatric cancer patients [1]. Similarly, a prospective multicenter study of invasive candidiasis found a 30-day mortality rate of 38.8% among ICU surgical patients [34]. Another study in China on cancer patients with candidemia showed a 30.0% mortality rate at 30 days. This rate was significantly higher than and roughly twice as high as bacterial BSIs (p = 0.006) [35]. In agreement with previous studies [30, 33], we revealed that ICU admission was an independent risk factor for increased 30-day mortality (p = 0.001).
Conclusion
NAC species infections predominate, with increasing rates of rare Candida species other than the five common species (C. tropicalis, C. albicans, C. glabrata, C. krusei, and C. parapsilosis), particularly in critically ill patients. This epidemiology necessitates rapid, accurate species identification and an understanding of local trends, distribution, and risk factors associated with these pathogens for optimal management of cancer patients. Multiplex sn-PCR is one of the best-known molecular techniques that could be used for species identification, while chromagar is an unreliable method compared to PCR. Other techniques with an extended identification spectrum, such as MALDI-TOF or combined methods, are still highly recommended to deal with the increasing rates of uncommon species and their significant involvement in invasive infections. Patients with hematological and solid organ tumors were at risk of developing IC, with the main risk factors being previous exposure to antibiotics, prolonged hospitalization, ICU, recent surgeries, and chemotherapy. Underlying cancers, chemotherapy, and recent surgeries were all risk factors significantly linked to infection with a given species. Moreover, ICU and multifocal Candida infections were significant risk factors for higher mortality rates, with ICU being independently associated with 30-day post-infection mortality. We provide a thorough overview of Candida infections at various body sites. Our institute treats cancer patients from all over the country. So, we suggest our results represent this high-risk group of patients in Egypt.
Data availability
The corresponding author will provide the required data upon reasonable request.
Code availability
Not applicable.
References
Vasileiou E, Paisiou A, Tsipou C, Pourtsidis A, Galani V, Katzilakis N, Antoniadi K, Papakonstantinou E, Ioannidou E, Stiakaki E, Baka M, Kattamis A, Kitra V, Tragiannidis A (2020) Candidemia in Children with Malignancies: Report from the Infection Working Group of the Hellenic Society of Pediatric Hematology-Oncology. J Fungi (Basel) 6(4). https://doi.org/10.3390/jof6040276
Wu PF, Liu WL, Hsieh MH, Hii IM, Lee YL, Lin YT, Ho MW, Liu CE, Chen YH, Wang FD (2017) Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients. Emerg Microbes Infect 6(10):e87. https://doi.org/10.1038/emi.2017.74
Khan Z, Ahmad S, Al-Sweih N, Mokaddas E, Al-Banwan K, Alfouzan W, Al-Obaid I, Al-Obaid K, Asadzadeh M, Jeragh A, Joseph L, Varghese S, Vayalil S, Al-Musallam O (2019) Changing trends in epidemiology and antifungal susceptibility patterns of six bloodstream Candida species isolates over a 12-year period in Kuwait. PLoS One 14(5):e0216250. https://doi.org/10.1371/journal.pone.0216250
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36(2):288–305. https://doi.org/10.1111/j.1574-6976.2011.00278.x
Camp I, Spettel K, Willinger B (2020) Molecular Methods for the Diagnosis of Invasive Candidiasis. J Fungi (Basel) 6(3). https://doi.org/10.3390/jof6030101
Xiao Z, Wang Q, Zhu F, An Y (2019) Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: a retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob Resist Infect Control 8:89. https://doi.org/10.1186/s13756-019-0534-2
Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):5–10. https://doi.org/10.1111/1469-0691.12539
Arafa SH, Elbanna K, Osman GEH, Abulreesh HH (2023) Candida diagnostic techniques: a review. J Umm Al-Qura Univ Appl Sci. https://doi.org/10.1007/s43994-023-00049-2
Zhong L, Zhang S, Tang K, Zhou F, Zheng C, Zhang K, Cai J, Zhou H, Wang Y, Tian B, Zhang Z, Cui W, Dong Z, Zhang G (2020) Clinical characteristics, risk factors and outcomes of mixed Candida albicans/bacterial bloodstream infections. BMC Infect Dis 20(1):810. https://doi.org/10.1186/s12879-020-05536-z
Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA, Nastoupil LJ, Rajotte M, Rolston KV, Strasfeld L, Flowers CR (2018) Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol 36(30):3043–3054. https://doi.org/10.1200/JCO.18.00374
Saukkoriipi A, Palmu AA, Jokinen J (2019) Culture of all sputum samples irrespective of quality adds value to the diagnosis of pneumococcal community-acquired pneumonia in the elderly. Eur J Clin Microbiol Infect Dis 38(7):1249–1254. https://doi.org/10.1007/s10096-019-03536-9
Taira CL, Okay TS, Delgado AF, Ceccon ME, de Almeida MT, Del Negro GM (2014) A multiplex nested PCR for the detection and identification of Candida species in blood samples of critically ill paediatric patients. BMC Infect Dis 14:406. https://doi.org/10.1186/1471-2334-14-406
Bayona JVM, Garcia CS, Palop NT, Martin AV, Padron CG, Rodriguez JC, Peman J, Cardona CG (2022) Novel Chromogenic Medium CHROMagar(TM) Candida Plus for Detection of Candida auris and Other Candida Species from Surveillance and Environmental Samples: A Multicenter Study. J Fungi (Basel) 8(3). https://doi.org/10.3390/jof8030281
Taei M, Chadeganipour M, Mohammadi R (2019) An alarming rise of non-albicans Candida species and uncommon yeasts in the clinical samples; a combination of various molecular techniques for identification of etiologic agents. BMC Res Notes 12(1):779. https://doi.org/10.1186/s13104-019-4811-1
Ho J, Camilli G, Griffiths JS, Richardson JP, Kichik N, Naglik JR (2021) Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 162(1):11–16. https://doi.org/10.1111/imm.13255
Nejad EE, Almani PGN, Mohammadi MA, Salari S (2020) Molecular identification of Candida isolates by Real-time PCR-high-resolution melting analysis and investigation of the genetic diversity of Candida species. J Clin Lab Anal 34(10):e23444. https://doi.org/10.1002/jcla.23444
Fisher BT, Smith PB, Zaoutis TE (2019) Chapter 200: candidiasis. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ (eds) Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 8th edn. Elsevier, pp 2030–2047. https://www.clinicalkey.com.au/#!/content/book/3-s2.0-B9780323376921002008
Pendleton KM, Dickson RP, Newton DW, Hoffman TC, Yanik GA, Huffnagle GB (2018) Respiratory Tract Colonization by Candida species Portends Worse Outcomes in Immunocompromised Patients. Clin Pulm Med 25(6):197–201. https://doi.org/10.1097/CPM.0000000000000279
Daef E, Moharram A, Eldin SS, Elsherbiny N, Mohammed M (2014) Evaluation of chromogenic media and seminested PCR in the identification of Candida species. Braz J Microbiol 45(1):255–262. https://doi.org/10.1590/S1517-83822014005000040
Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI, Colombo AL, Latin American Invasive Mycosis N (2013) Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One 8(3):e59373. https://doi.org/10.1371/journal.pone.0059373
Ricotta EE, Lai YL, Babiker A, Strich JR, Kadri SS, Lionakis MS, Prevots DR, Adjemian J (2021) Invasive Candidiasis Species Distribution and Trends, United States, 2009–2017. J Infect Dis 223(7):1295–1302. https://doi.org/10.1093/infdis/jiaa502
Lindberg E, Hammarstrom H, Ataollahy N, Kondori N (2019) Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci Rep 9(1):3838. https://doi.org/10.1038/s41598-019-40280-8
Xiao M, Sun ZY, Kang M, Guo DW, Liao K, Chen SC, Kong F, Fan X, Cheng JW, Hou X, Zhou ML, Li Y, Yu SY, Huang JJ, Wang H, Xu YC, China Hospital Invasive Fungal Surveillance Net Study G (2018) Five-Year National Surveillance of Invasive Candidiasis: Species Distribution and Azole Susceptibility from the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) Study. J Clin Microbiol 56(7). https://doi.org/10.1128/JCM.00577-18
Ying Y, Zhang J, Huang SB, Liu FD, Liu JH, Zhang J, Hu XF, Zhang ZQ, Liu X, Huang XT (2015) Fluconazole susceptibility of 3,056 clinical isolates of Candida species from 2005 to 2009 in a tertiary-care hospital. Indian J Med Microbiol 33(3):413–415. https://doi.org/10.4103/0255-0857.158569
Wang E, Farmakiotis D, Yang D, McCue DA, Kantarjian HM, Kontoyiannis DP, Mathisen MS (2015) The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother 70(8):2362–2368. https://doi.org/10.1093/jac/dkv087
Pandey N, Gupta MK, Paul P, Tilak R (2020) Necessity to identify candida species accurately with minimum inhibitory concentration determination in each case of bloodstream infections. J Infect Public Health 13(5):753–758. https://doi.org/10.1016/j.jiph.2019.12.002
Ngamchokwathana C, Chongtrakool P, Waesamaae A, Chayakulkeeree M (2021) Risk Factors and Outcomes of Non-albicans Candida Bloodstream Infection in Patients with Candidemia at Siriraj Hospital-Thailand's Largest National Tertiary Referral Hospital. J Fungi (Basel) 7(4). https://doi.org/10.3390/jof7040269
Jafari Z, Motamedi M, Jalalizand N, Shokoohi GR, Charsizadeh A, Mirhendi H (2017) Comparison of CHROMagar, polymerase chain reaction-restriction fragment length polymorphism, and polymerase chain reaction-fragment size for the identification of Candida species. Curr Med Mycol 3(3):10–15. https://doi.org/10.29252/cmm.3.3.10
Sankari SL, Mahalakshmi K, Kumar VN (2019) Chromogenic medium versus PCR-RFLP in the speciation of Candida: a comparative study. BMC Res Notes 12(1):681. https://doi.org/10.1186/s13104-019-4710-5
Zhang W, Song X, Wu H, Zheng R (2020) Epidemiology, species distribution, and predictive factors for mortality of candidemia in adult surgical patients. BMC Infect Dis 20(1):506. https://doi.org/10.1186/s12879-020-05238-6
Xia J, Huang W, Lu F, Li M, Wang B (2022) Comparative Analysis of Epidemiological and Clinical Characteristics Between Invasive Candida Infection versus Colonization in Critically Ill Patients in a Tertiary Hospital in Anhui, China. Infect Drug Resist 15:3905–3918. https://doi.org/10.2147/IDR.S368792
Negri M, Silva S, Breda D, Henriques M, Azeredo J, Oliveira R (2012) Candida tropicalis biofilms: effect on urinary epithelial cells. Microb Pathog 53(2):95–99. https://doi.org/10.1016/j.micpath.2012.05.006
Lortholary O, Renaudat C, Sitbon K, Desnos-Ollivier M, Bretagne S, Dromer F, French Mycoses Study G (2017) The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med 43(5):652-662.https://doi.org/10.1007/s00134-017-4743-y
Klingspor L, Tortorano AM, Peman J, Willinger B, Hamal P, Sendid B, Velegraki A, Kibbler C, Meis JF, Sabino R, Ruhnke M, Arikan-Akdagli S, Salonen J, Doczi I (2015) Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin Microbiol Infect 21(1):87 e81–87 e10. https://doi.org/10.1016/j.cmi.2014.08.011
Li D, Xia R, Zhang Q, Bai C, Li Z, Zhang P (2017) Evaluation of candidemia in epidemiology and risk factors among cancer patients in a cancer center of China: an 8-year case-control study. BMC Infect Dis 17(1):536. https://doi.org/10.1186/s12879-017-2636-x
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support were obtained for this study.
Author information
Authors and Affiliations
Contributions
Hadir El-Mahallawy and Mona Wassef conceptualized and designed the study. Nesma Abdelfattah and Rasha Abdel Hamid performed practical parts and acquired data. Rasha Abdel Hamid conducted statistical analysis, analyzed data, prepared figures and tables, and wrote the manuscript. Hadir El-Mahallawy did data analysis and manuscript writing. Mona Wassef revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study proposal, CPCP22301-503–026, was approved by the NCI ethical committee. Participants or their guardians provided informed consent.
Conflict of interest
The authors disclose no relevant financial or non-financial interests.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Hamid, R.M., El-Mahallawy, H.A., Abdelfattah, N.E. et al. The impact of increasing non-albicans Candida trends on diagnostics in immunocompromised patients. Braz J Microbiol 54, 2879–2892 (2023). https://doi.org/10.1007/s42770-023-01163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01163-3