Abstract
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection seroprevalence can be performed by detecting anti-SARS-CoV-2 antibodies. The survey is essential to understand the disease transmission’s dynamic in the studied population. This study aimed to carry out a seroepidemiological survey of SARS-CoV-2 in three hospitals located in the south of Minas Gerais state, Brazil. 859 samples were collected from August to December 2020 when SARS-CoV-2 vaccines were still not available and Enzyme-linked immunosorbent assays (ELISA) were performed on participants sera. The average age of participants was 38 years, and most were women (71.4%). Likewise, most participants were classified as health professionals with direct or indirect contact with patients with COVID-19 (74.5%). The other participants tested belonged to other sectors, such as the administrative one (11,6%). Considering clinical symptoms, 15.8% of participants reported diarrhoea, 6.4% fever, 5.8% respiratory distress, and 7.0% loss of smell and taste. Many participants reported contact with infected patients (63.35%). Regarding the ELISA tests, 21.6% of the participants had positive results and hospital 3 had the highest positivity (21.7%), followed by hospital 2 (21.6%) and hospital 1 (20.3%). The prevalence was higher in women compared to men (22,8% and 18,7%, respectively). Regarding the area of expertise, the highest positivity (20.9%) was observed among health professionals. However, professionals who worked exclusively with COVID-19 had lower positivity when compared to professionals who did not work directly with COVID-19 (22.0% and 21.5%, respectively). When analysing the correlation between the ELISA tests with the other variables, a significant association was detected with these previous serological variables, previous contact with COVID-19 and the presence of fever symptoms, loss of smell and taste. Clinical symptoms associated with serological tests are important tools for monitoring the disease among health professionals.
Similar content being viewed by others
Introduction
COVID-19 is a disease caused by the SARS-CoV-2 (Severe Acute Respiratory Syndrome Related Coronavirus 2) [1]. It was identified in December 2019 in the city of Wuhan, China. In March 2020, the World Health Organisation (WHO) declared the status of the disease as a pandemic [2]. As of April 27 2022, there were 507,184,387 cases with 6,219,657 deaths recorded globally [3]; in Brazil, there were 30,399,004 recorded cases with 663,111 deaths [4] and within the state of Minas Gerais there were 3,354,669 cases with 61,243 deaths [5].
The genome of SARS-CoV-2 is a single-stranded RNA [6]. The virus has four structural proteins: Spike (S), Envelope (E), Membrane (M) and Nucleocapsid (N). The adsorption and penetration of the virus into the host cell occurs through the binding of the viral protein S and the cellular receptor of the conservative enzyme angiotensin 2 (ACE-2) [7]. The SARS-CoV-2 is transmitted through the airways via aerosols and droplets of saliva released when talking, coughing or sneezing. As the virus has the ability to infect and replicate early in the throat, the use of protective measures such as masks, contact tracing and social distancing is necessary to prevent viral spread [8].
After infection, an individual may be asymptomatic or develop symptomatic disease. Even if they do not develop symptoms, asymptomatic people are transmitters of the virus, with epidemiological relevance. However, symptomatic people are considered more contagious [9, 10]. Symptoms begin 2 to 14 days after exposure to the virus [11] and the most common are: fever (80%), cough (between 50% and 80%) and dyspnea (30%) and may cease after the first week of infection, be persistent or progress to more severe stages of the disease [12, 13] and do not show changes in chest images. Patients with moderate symptoms have more intense clinical manifestations and changes in chest images like mild pneumonia [14]. When patients develop severe symptoms, they may experience rapid disease progression, respiratory failure, organ failure, and treatment in an intensive care unit. This lung damage occurs due to the immune response against the virus, due to the excess of secreted inflammatory cytokines [12]. The prevalence of morbidities can aggravate the condition of the disease, being considered risk factors, and associated with the death of patients [10].
The diagnosis of COVID-19 is carried out through laboratory imaging, molecular and serological tests. The RT-PCR (reverse transcription polymerase chain reaction) test is the gold standard for virus detection [15]. Serological tests such as enzyme-linked immunosorbent assays (ELISA) are suitable for seroepidemiological studies of disease transmission in a specific region or community [16]. These tests also make it possible to screen patients for convalescent plasma donation and to monitor the immune response induced by infection or vaccination [17]. ELISA tests are used to detect SARS-CoV-2 IgG, IgM and IgA antibodies, and detection using IgG antibodies is more efficient when compared to IgA and IgM [18], having a specificity of about 95.8% [16]. Seroconversion is observed in most patients between the 5th and 14th day after the onset of symptoms. Generally, ELISA tests use the virus N protein as the antigen since it induces the production of antibodies before the S protein [19].
Testing for the disease is essential to assess and understand what the immune response is, the stage of the disease, cross-reactivity between other coronaviruses, post-infection immunity and disease screening; in addition to assisting decision-making in relation to public health. With the results of seroepidemiological studies it is possible to understand the spread of the virus, the evolution of the pandemic and to propose control measures by understanding the epidemiological scenario [20].
Thus, this work aims to carry out a seroepidemiological survey of SARS-CoV-2 in hospitals in the south of Minas Gerais, Brazil. ELISA tests were performed through the quantification of IgG antibodies against the nucleocapsid protein of SARS-CoV-2 in a critical period for the virus transmission before the vaccination program started and with both state and local laws restricting the movement of people.
Material and methods
Study and sampling site
This study was conducted in three hospitals located in the south of Minas Gerais, Brazil. Three selected hospitals were chosen based on them providing COVID-19 healthcare: Santa Casa de Misericordia de Alfenas (hereinafter referred to as Hospital 1), Unimed in Poços de Caldas (hereinafter referred to as Hospital 2), and the “Covid campaign” hospital in Varginha (hereinafter referred to as Hospital 3). The chosen hospitals from Poços de Caldas, Alfenas and Varginha actually cover the healthcare needs of many smaller cities, towns and regional healthcare services, which resulted in them provided COVID-19 healthcare.
Professionals directly and indirectly involved in COVID-19 combat strategies were invited to participate. Sampling was determined by a statistical test as described by Medronho [21] for each institution based on the number of professionals working in the hospital. The following inclusion criteria were used for sample collection: a) all health professionals directly involved with suspected or confirmed individuals with COVID-19. b) professionals who worked in other sectors of the hospitals.
Individuals were invited to voluntarily participate with prior clarification of the objectives, benefits, and assurance of identity confidentiality, and each participant was asked to sign an informed consent agreement. After consent, a questionnaire was given to collect data on age, gender, previous tests to detect SARS-CoV-2 (date, result and type of test performed), previous contact with people who had a confirmed diagnosis of COVID-19, presence of comorbidities (diabetes, high blood pressure, bronchitis, asthma, etc) and regular medication intake. From each participant, 3.5 mL of venous blood was collected to obtain serum. The collection period took place between August to December 2020.
The work was carried out in a period prior to the initiation of vaccination for SARS-CoV-2, which was a critical period for the virus’ transmission and with state and municipal decrees restricting the free movement of people.
Ethical aspects
This work follows all norms and laws that regulate the human material use, according to the criteria of Resolution 466 of the National Health Council and its complementary norms and resolutions and was approved by the Research Ethics Committee of the Federal University of Alfenas under the protocol 33623320.2.0000.5142.
Enzyme immunoassay (ELISA)
Serum samples were initially tested for the detection of IgG anti-SARS-CoV-2 antibodies using an enzyme-linked immunosorbent assay (ELISA) developed by the Vaccine Technology Centre of the Federal University of Minas Gerais [22]. The test detected antibodies against the Nucleocapsid (N) protein of SARS-CoV-2. Nunc MaxiSorp® 96-well microtiter plates (eBIOSCIENCE, USA) were used to perform the ELISA. The wells were coated with 100 μL of N protein solution (0.4 mg/mL) diluted in carbonate-bicarbonate buffer (0.1 M pH 9.6) and the plate incubated for 16 hours at 4 °C. Then, the plate was washed three times with buffered saline solution (PBS) containing 0.05% Tween 20 (PBS-T) and then blocked with a 2% bovine serum albumin in PBS-T for 2 hours at 37 °C. Then, the plate was washed six times with PBS-T, 100 μL of the test sera were added at a dilution of 1:100. The plate was incubated for 1 hour at 37 °C. After this period, the plate was washed again four times with PBS-T, adding 100 μL of anti-human IgG peroxidase conjugate (Sigma-Aldrich, USA) diluted 1 to 5000. The plate was incubated for 1 hour at 37 °C. Then, the plate was washed four times with PBS-T and then 100 μL per well of a solution of O-Phenylenediamine Dihydrochloride (OPD) (Sigma-Aldrich, USA) in citrate-phosphate buffer pH 4.5 with 0.08% H2O2. After the addition of the solution, the plate was incubated for 15 minutes at room temperature, protected from light, after which the optical densities were read at 450 nm on an Anthos Zenyth 200rt microplate reader (Biochrom, UK). The cut-off value was defined as the mean of the absorbance readings of the negative control (sera collected from SARS-CoV-2 negative individuals by serological and molecular tests) plus three times the standard deviation. As positive controls for the ELISA tests, sera with a positive diagnosis for SARS-CoV-2 through molecular and serological assays were used. To determine the diagnostic value, an index was established. The index value was obtained by dividing the average optical absorption value divided by the cut-off value. Index values lower than 0.80 were considered negative. Index values between 0.81 and 1.09 were considered indeterminate. Values equal to or greater than 1.10 were considered positive. In the case of an indeterminate result, the sample was reanalysed.
Statistical analysis
Statistical analysis was performed using the SPSS v.20 software, considering the percentages and valid values of each hospital, as well as the relationship between the studied variables, using the chi-square or Fisher's exact tests, all considering a level of 5% significance.
Results
859 individuals were selected from three different hospitals in south of Minas Gerais to track the prevalence of COVID-19 through the detection of IgG antibodies against protein N of SARS-CoV-2. Demographical data showed that the mean age of the participants was 38.43 years old +/-10.025 SD, (standard deviation) (index 1.25), with 18 years being the lowest and 72 years the highest age. Table 1 shows the distribution of studied population by gender, professional category, work sector in the hospital, previously performed tests, type of test performed, test result, symptoms, previous contact with COVID-19, presence of disease, regular use of medication and ELISA results in each hospital unit. Regarding gender; 28.6% (246) of the participants were men and 71.4% (613) were women.
In the professional category, 74.5% (640) of the participants were classified in the health professional category (doctors, nurses, physiotherapists, etc.) who provided direct or indirect care to patients with COVID-19. 11.6% (100) were classified as professionals that act in the administrative area of these hospitals (secretaries, attendants, drivers, cleaning assistants, etc.). 4.2% (36) belong to other categories and 9.7% (83) did not disclose their profession.
In the work sector; 17.5% (150) of the participants were classified in hospitals providing direct care for COVID-19 patients and 58% (498) work in the non-COVID-19 hospital sector, 7.2% (62) in administration section, 3.8% (33) in the imaging and laboratory exams sector, 2.7% (23) in other sectors and 10.8% (93) did not disclose in which sector they worked.
When asked if participants had previously tested for COVID-19; 66.5% (571) of participants reported having performed COVID-19 diagnostic tests prior to this study and 32.5% (279) didn’t perform any molecular or serological test, while 1% of the individuals (9) did not respond. 9.9% (85) reported having performed RT-PCR, 47.1% (405) performed serological tests, 4.7% (40) both molecular and serological tests, and 38.3% (329) did not respond or they did not know. 5.4% (46) of the participants reported a positive test result, 59.5% (511) a negative result, 0.2% (2) reported an indeterminate result, and 38.3% (329) did not know or did not respond.
When investigated for the presence of clinical symptoms of COVID-19; 6.4% (55) reported fever, 5.8% (50) breathing trouble, 7% (60) loss of smell and taste, and 15.8% (136) diarrhoea. Therefore, diarrhoea was the most common symptom reported by the participants.
When evaluating previous contact with symptomatic people infected with COVID-19; 63.3% (544) reported contact with positive testing people, 30.3% (260) said they had not had contact with COVID-19 patients and 6.4% (55) did not respond or did not know if this contact took place.
Another important aspect is to know if the participants had comorbidities. This factor can aggravate the disease. 9.3% (80) reported having hypertension, 4% (34) bronchitis, 2.2% (19) asthma, 1.7% (15) diabetes, 1% (9) bronchitis and hypertension and 0.9 % (8) diabetes and hypertension and 80.7% (693) of the participants did not respond or did not know if they had any health problems.
When investigated about the regular use of medication; 62.9% (540) of the participants reported not using any kind of medication. 9.5% (82) reported using anti-hypertensive drugs, 2.3% (20) reported using medication for diabetes, 0.8% (7) medication for heart problems and 24.4% (210) reported using other kinds of medication.
Regarding the result of the ELISA test; 21.6% (186/859) of the individuals had a positive result, 62.7% (539/859) had a negative result and 15.6% (134/859) had an indeterminate result (p<0.001) (Table 1). Hospital 3 (campaign hospital in Varginha) had the highest percentage of positivity (22.9%, 75/327), followed by Hospital 2 (Unimed hospital in Poços de Caldas) (21.6%, 50/232) and Hospital 1 (Santa Casa hospital in Alfenas) (20.3%, 61/300) (p=0.079).
Then, we performed an association between positivity in ELISA test and previously studied variables. Table 2 shows the distribution of ELISA test positivity regarding the parameters previously analysed in table 1. Once the sample was characterised, the variables studied were associated with positivity in the ELISA test, in order to assess which public was more vulnerable to the virus infection, as well as which reported symptoms were related to the infection. Table 2 shows the distribution of positivity in the ELISA test regarding the parameters previously analysed in Table 1, analysing the value and percentage of the result and individually by variable group.
Regarding the distribution by gender in relation to the ELISA test, a higher prevalence was observed in women (75.3%, 140/186) with a positive test compared to men (24.7%, 46/186) (p<0.001). And when evaluating positivity within the group of variables, the percentage of women continued to be higher (22.8%, 140/613) compared to men (18.7%, 46/246) (p=0.537).
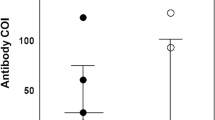
Referring to the distribution by profession, 82.2% (134/163) of the participants from the health area were positive in the test, 13.5% (22/163) of the participants were from the administrative area and 4.3% (7/163) of the participants were from other professions (p<0.001). When evaluating the percentage of the variable groups, the highest percentage was among professionals in the administrative area (22.9%, 22/100), followed by the health area (20.9%, 134/640) and other areas (19.4%, 7/36) (p=0.893) (Fig 1a, b).
Concerning the distribution by sector of work for the participants that were positive in the test; 63.7% (107/168) of the participants did not work directly with COVID-19 (non-Covid hospital) reported more positivity in the test, while 19.6% (33/168) of participants working in areas directly related to treating patients with COVID-19 had a positive ELISA test. 8.3% (14/168) of the participants reported working in administrative areas, as well as 6.0% (10/168) were professionals in the sector of diagnosis by imaging or laboratory tests and 2.4% (4/168) professionals who worked in other areas (p<0.001).
However, when evaluating the work sector as a variable, the highest percentage was of professionals who performed imaging and laboratory tests (30.3%, 10/33), followed by the administrative sector (22.6%, 14/62), workers with direct contact with patients with COVID-19 (22.0%, 33/150), workers who did not work directly with patients with COVID-19 (21.5%, 107/498) and other sectors (17.4%, 4/23) (p=0.433) (Fig 2a, b).
The comparison between the positive result of the ELISA tests with the diagnostic tests previously performed by the participants was 75.4% (89/118) of the participants regarding the serological tests, 17.8% (21/118) regarding the RT-PCR and 6.8% (8/118) for both tests (p<0.001). Analysing the test performed by variable, the highest percentage was in relation to RT-PCR (24.7%, 21/85), followed by serological tests (22.0%, 89/405) and both tests (20.0%, 8/40) (p=0.753).
When the data from positive ELISA tests and the presence of signs and symptoms for COVID-19 were crossed, 10.3% (19/185) had fever, 9.2% (17/185) respiratory difficulty, 18.4% (34/185) loss of smell and taste and 21.1% (39/185) diarrhoea (p=0.004). The last one was the most common symptom amongst the positive participants.
However, when evaluating the crossing between positive ELISA test and the presence of signs and symptoms for COVID-19 as a variable, the highest percentage was in the loss of smell and taste (56.7%, 34/60), followed by fever (34. 5%, 19/55), breathing distress (34.0%, 17/50) and diarrhoea (28.7%, 39/136) (p=0.007).
When comparing positivity in the ELISA test and previous contact with symptomatic people, 67.1% (116/173) reported contact and had a positive test, 32.9% participants (57/173) did not know or did not have contact with infected people (p<0.001), but these participants had a positive result in the ELISA tests, which shows that they may have been contaminated by asymptomatic people. However, when evaluating the percentage as a variable, the highest percentage of positive cases was from participants who did not know or had no contact with positive people (21.9%, 57/260) followed by people who had contact with positive people (21.3%, 116/544) (p<0.001).
When comparing positivity in the ELISA test and morbidity prior to COVID-19, 51.3% (20/39) reported having hypertension, 23.1% (9/39) bronchitis, 10.3% (4/39) asthma, 7.7% (3/39) diabetes and the association between bronchitis and high blood pressure, and the association between diabetes and high blood pressure and other types of diseases, no positive cases were recorded (p<0.001). However, when analysing this association as a variable, the highest percentage was in relation to the association between bronchitis and hypertension (33.3%, 3/9), bronchitis (26.5%, 9/34), hypertension (25.0%, 20/80), asthma (21.1%, 4/19) and diabetes (20.0%, 3/15), and the association between diabetes and high blood pressure and other types of diseases, no positive cases were recorded (p=0.365).
When comparing positivity in the ELISA test and medication intake, 29.4% (20/68) took medication for hypertension, 5.9% (4/68) took medication to control diabetes, 1.5% (1/68) took medication for the heart and 63.2% (43/68) of other types of medication unrelated to comorbidities that increase the risk of severe disease (p<0.001). In the same way, when evaluating this association by variables, the highest percentage was related to the use of drugs for hypertension (24.4%, 20/82), other drugs (20.5%, 43/210), drugs to control diabetes (20.0%, 4/20) and heart medications (14.3%, 1/7) (p=0.445).
The association between the positivity of the ELISA tests and the studied variables showed a statistically significant value for the positive result in the tests previously performed in the three hospitals (p=0.011; 0.016 and <0.001), for the loss of smell and taste in two hospitals (p<0.001 and <0.001) and for the presence of fever reported in only one hospital (p=0.03) (Table 3).
Discussion
Healthcare professionals and those that work in hospital settings are at high risk of exposure to SARS-CoV-2 [23]. Assessment of the serological response during the COVID-19 pandemic was crucial to understanding the risk of infection in a population exposed to this virus. This study aimed to carry out a seroepidemiological survey of SARS-CoV-2 in 2020 in workers from hospitals located in south of Minas Gerais, Brazil. The work was carried out before the start of vaccination programme, critical for the transmission of the virus and during the quarantine, when people were advised to stay home and wear basic protection like masks and sanitising alcohol.
The age and gender of the participants are important factors to understand the dynamics of the disease in population, because they are considered a risk factor for the disease, with a higher rate of deaths in patients over 50 years old in the first and second wave in 2020 in Italy [24]. The average age of the participants was 38 years, which is similar to that found in the population of Minas Gerais state infected with the virus (42 years old) [25]. In a study carried out with hospital workers in Denmark, the average age was 44.4 years, with positivity in antibody tests of 13.5% under this average [26].
Most of the participants are health professionals (74.5%). 17.5% of the professionals were in direct care of patients with COVID-19, while 58% of them did not work directly with these patients (Table 1). However, when analysing only the sample of health professionals (640), the participants who had direct contact with patients with COVID-19 was 23.1% and without direct contact with patients with COVID-19 was 76.8%.
The present study showed a positivity of 21.6% in the seroprevalence of SARS-CoV-2 in the three researched hospitals (Table 1). The campaign hospital (Hospital 3) and Santa Casa de Misericórdia of Alfenas (Hospital 1) worked specifically with the treatment of patients with COVID-19, with positivity rates of 22.9% and 20.3% respectively. These two hospitals serve several cities in their region because they are regional health centres. Hospital 2 (a private hospital) had a positivity rate of 21.6%. In a study carried out in two hospitals in Germany with their professionals, between July and September 2020, it revealed a positivity percentage of 38.5% in the first hospital and 61.5% in the second hospital, using IgG antibody tests. However, in relation to the total sample, the positivity in the tests was 1.4% [27]. In another study carried out in Denmark, health professionals in direct contact with COVID-19 patients from different hospitals were invited to participate voluntarily. In April 2020 they presented a positive result for antibody tests, 2.67% presented a result for IgG tests, 2.81% developed IgM antibodies and 4.04% had IgG, IgM or both [26]. A study carried out in a university hospital in Ireland, between May and June 2020, showed a positivity of 15.5% in IgG antibody tests [28]. In the study carried out in Milan, between February and May 2020, in a university hospital, with samples collected at three different times, the initial phase of the pandemic, 1 and 2 months, with a progressive increase in positive tests at each time of the test performed, 0.4%, 4.2% and 4.6% respectively [29]. In turn, in a hospital in Belgium between May and June 2020, the positivity rate in health professionals was 7.4% [30]. It is observed that the positivity rates in the tests in each hospital, in this study, were higher than in some studies carried during previous periods of time and lower in similar periods.
When analysing the percentage of positive cases notified by the municipal health departments (in relation to the total population of municipality) where the ELISA tests were carried out; by December 30th 2020 in Alfenas they were 3.3% (2,429/73,774), in Poços de Caldas 1.7% (2,625/152,435) and in Varginha 1.7% (2,174/123,081) [31]. Prakash et al. [32], evaluating seropositivity for IgG antibodies against SARS-CoV-2 in the city of Ahmedabad, India, found 17.61% seropositivity and no statistically significant difference for both sexes. These data may suggest an underreporting of positive cases in the cities where the tests were performed.
The total number of participants in terms of gender was 71.4% women and 28.6% men (Table 1), however seropositivity in women was 22.8% and in men 18.7% (table 2). This data is similar to the data found in the study by Hildebrandt et al. [27], with a greater number of positives in women: 76.9% women and 23.1% men. Data from the epidemiological bulletin of the State of Minas Gerais with cumulative frequency up to December 30th 2020, shows a higher rate of female positivity with 51% [31]. This data is based on the general population of the state, and according to this result in the ELISA tests the percentage of positivity was 75.3% of women and 24.7% of men.
Health professionals had 20.9% of positive results (Table 2). This demonstrates how these professionals were exposed to the virus in their workdays. Venugopal et al. [33] assessed seroprevalence among healthcare workers in a New York City hospital and found 27% positivity for the SARS-CoV-2 antibody. Bryan et al. [34] reported 29% positive cases for the presence of SARS-CoV-2 antibodies also in New York City. Gomez-Ochoa et al. [23] showed that seropositivity in health professionals was 7%.
When classifying professionals by work sector, it was observed that professionals in direct contact with patients had 22.0% positivity, while professionals in indirect contact had 21.5% positivity (Table 2). Gomez-Ochoa et al. [23] showed that 43% of professionals with a positive result worked in hospital wards and non-emergency sectors during patient triage. In turn, Prakash et al. [32], showed a significantly lower seropositivity (13.64%) for health professionals compared to non-health professionals (18.71%). The work by Purswani et al. [35] showed that one-third of hospital healthcare workers were seropositive for SARS-CoV-2 by the end of the first wave in New York. Seroprevalence differs by job function and workplace, with the highest estimated risk for nurses and the emergency department, respectively. In turn, professionals in the administrative, laboratory and imaging areas had a high positivity rate (22.6% and 30.3% respectively) (Table 2). Brousseau et al. [36] show 11.7% positive serology for SARS-CoV-2 among healthcare workers in Quebec, Canada. Of these, 71.0% had been previously diagnosed with COVID-19. Seroprevalence varied between hospitals, from 2.4% to 3.7% in low-incidence regions and from 17.9% to 32.0% in hospitals with outbreaks involving 5 or more health workers. The highest seroprevalence was associated with working in a hospital where they occurred, being a nurse or nursing assistant, or an orderly and black or Hispanic ethnicity. Lower seroprevalence was associated with work in the intensive care unit or emergency department.
The results of contamination in hospital environments may be related to several factors, such as the incorrect use of PPE, the non-use of such equipment or PPE, or the use of less efficient PPE. In relation to administrative professionals, since they have contact with the public that arrives at hospitals, both asymptomatic and symptomatic. In this sense, the work by Gómez-Ochoa et al. [23] presented 4.7% positivity in professionals without proper use of PPE. In this way, the proper use of PPE and social distancing measures are of fundamental importance to reduce the risk of contamination by SARS-CoV-2 [37].
66.5% of the participants reported having performed tests for COVID-19 and of these, 5.4% with positive results in comparison to the total number of participants (Table 1). However, when performing the percentage considering the total number of reports of previous tests, the percentage is 8.0% (46/571). The comparison between the positivity of the ELISA tests with the results of the tests previously performed by the participants showed that 22.3% (118/530) had a positive result in the ELISA tests (table 2), suggesting a concordance between our tests and the tests previously performed. Factors such as the time of sample collection can directly impact the test result [15]. In this sense, the ELISA test was adequate to assess the prevalence of antibodies against the virus [15, 38].
Another important variable was to assess whether the participants had contacts with symptomatic people for the disease [37]. 63.3% of the participants reported direct contact with patients with COVID-19 (Table 1). Our results showed that 21.3% of the participants who reported having contact with sick people had a positive result in the ELISA tests (Table 2), which may be associated with the observed seropositivity rate. Prakash et al. [39] found a seroprevalence of 31.92% considering people who had contact with COVID-19 cases in the city of Ahmedabad, India.
Understanding how the disease behaves in the participants is important to analyse whether the symptoms presented are common, which are the most recurrent and, therefore, be able to seek medical assistance when these symptoms develop. Among the symptoms most reported by the participants, diarrhoea is the most common with 15.8% and 28.7% of positivity in the ELISA tests, the percentage of positivity on loss of smell and taste with 7% of reports and 56.7% of positivity, fever with 6.4% of reports and 34.5% of positivity and respiratory difficulty with 5.8% of reports and 34.0% of positivity (Tables 1 and 2 respectively). The symptom of diarrhoea, in addition to COVID-19, can have different etiologies, however it is one of the most frequent symptoms reported by patients, with about 10.4% of reports [40]. The work by Mair et al. [41] carried out with data from hospitalised patients showed that 69% had fever, 38% loss of smell, 29% loss of taste, 9% diarrhoea and 10 to 20% respiratory difficulty. The other evaluated the presence of 11 symptoms common to the disease, 63.5% had three or more symptoms, 56.5% had changes in smell and taste, 52.1% fever, 25.6% diarrhoea, 23.1 difficulty breathing [42].
Another important discussion is whether the participants have a prevalence of diseases and the use of drugs to control them, which are directly related to the development of a serious COVID-19 outcome [10, 43]. 19.2% (166/859) of the participants (Table 1) had some type of morbidity and their positivity in the ELISA tests was 25.0% hypertension, 26.5% bronchitis, 21.1% asthma, 20 .0% diabetes, 33.3% bronchitis and hypertension (Table 2).
12.6% (109/859) of the participants were taking medication related to the comorbidities mentioned above (Table 1), and among these, in relation to the ELISA tests, the use of hypertensive drugs (29.4%) for diabetes was positive (5.9%) and for the heart (1.5%) (Table 2). In addition, it is observed that most participants who reported using some medication not related to the disease as a risk factor for COVID-19 (24.4%) had a positive result in the ELISA tests of 63.2% (table 1 and 2) that is, there were more people who took medication (312 participants, 19.2%) than those who reported having some type of disease (166 participants, 37.0%).
The association between ELISA test positivity and the studied variables indicated a statistically significant association for previous ELISA test results for COVID-19 in the three hospitals, loss of smell and taste in hospitals 1 and 2, and fever in hospital 3 (table 3). This result highlights the relevance of the data found.
Thus, in the population studied, the highest exposure of health professionals with the lowest exposure to professionals from other areas with patients with COVID-19 did not show a significant difference in prevalence between the groups. However, there was a significant difference in relation to reported symptoms associated with positive ELISA test results and with contact with a COVID-19 patient.
Conclusion
It can be concluded that the ELISA test was effective in detecting antibodies to SARS-CoV-2 in hospital workers, with data on symptoms such as fever, loss of taste and smell, it was an important tool for monitoring exposure, especially among health professionals.
References
Dhama K, Khan S, Tiwari R et al (2020) Coronavirus disease 2019–COVID-19. Clin Microbiol Rev 33(4):1–48. https://doi.org/10.1128/CMR.00028-20
WHO. Coronavirus Disease 2019 (COVID-19) Situation Report – 51; 2020. Accessed June 13, 2022. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10
WHO. COVID-19 Weekly Epidemiological Update - 27 April 2022; 2022. https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update
Brasil. Covid-19: situação epidemiológica do Brasil nesta quarta-feira (27) — Português (Brasil). Ministério da Saúde. Published April 27, 2022. Accessed May 4, 2022. https://www.gov.br/saude/pt-br/coronavirus/informes-diarios-covid-19/covid-19-situacao-epidemiologica-do-brasil-nesta-quarta-feira-27
Minas Gerais. Boletim Epidemiológico de 27 de Abril de 2022; 2022. Accessed May 4, 2022. https://coronavirus.saude.mg.gov.br/images/2022/04/Boletim_Completo_27.04.2022.pdf
Chan JF-W, Kok K-H, Zhu Z et al (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9(1):221. https://doi.org/10.1080/22221751.2020.1719902
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181(2):271. https://doi.org/10.1016/J.CELL.2020.02.052
Jin Y, Yang H, Ji W et al (2020) Virology, epidemiology, pathogenesis, and control of covid-19. Viruses 12(4):1–17. https://doi.org/10.3390/v12040372
Gao Z, Xu Y, Sun C et al (2021) A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 54(1):12. https://doi.org/10.1016/J.JMII.2020.05.001
Chilamakuri R, Agarwal S (2021) COVID-19: Characteristics and Therapeutics. Cells 10(2):1–29. https://doi.org/10.3390/cells10020206
CDC. Symptoms of COVID-19. Centers for Disease Control and Prevention. Published February 22, 2021. Accessed September 15, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
Machhi J, Herskovitz J, Senan AM et al (2020) The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol 15(3):359–386. https://doi.org/10.1007/s11481-020-09944-5
Ortiz-Prado E, Simbaña-Rivera K, Gómez- Barreno L et al (2020) Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis 98(1). https://doi.org/10.1016/j.diagmicrobio.2020.115094
Hozhabri H, Sparascio FP, Sohrabi H et al (2020) The global emergency of novel coronavirus (SARS-CoV-2): An update of the current status and forecasting. Int J Environ Res Public Health 17(16):1–35. https://doi.org/10.3390/ijerph17165648
La Marca A, Capuzzo M, Paglia T, Roli L, Trenti T, Nelson SM (2020) Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online 41(3):483–499. https://doi.org/10.1016/j.rbmo.2020.06.001
Cota G, Freire ML, de Souza CS et al (2020) Diagnostic performance of commercially available COVID-19 serology tests in Brazil. Int J Infect Dis 101:382–390. https://doi.org/10.1016/j.ijid.2020.10.008
Tozetto-Mendoza TR, Kanunfre KA, Vilas-Boas LS et al (2021) Nucleoprotein-based ELISA for detection of SARS-COV-2 IgG antibodies: Could an old assay be suitable for serodiagnosis of the new coronavirus? J Virol Methods:290. https://doi.org/10.1016/j.jviromet.2021.114064
Tré-Hardy M, Wilmet A, Beukinga I et al (2021) Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol 93(2):803–811. https://doi.org/10.1002/jmv.26303
Guo L, Ren L, Yang S et al (2020) Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 71(15):778–785. https://doi.org/10.1093/cid/ciaa310
Cheng MP, Yansouni CP, Basta NE et al (2020) Serodiagnostics for Severe Acute Respiratory Syndrome-Related Coronavirus 2 : A Narrative Review. Ann Intern Med 173(6):450–460. https://doi.org/10.7326/M20-2854
Medronho RA, Bloch KV, Luiz RR, Werneck GL. Epidemiologia. Vol 1. 2nd ed. Atheneu; 2009. Accessed December 29, 2021. https://plataforma.bvirtual.com.br/Leitor/Publicacao/185965/pdf/0?code=BEcNwqFq/Ydl1o0pSmq7fJtGdBZR7BwBR3AGKKDxdFcaq/8cu6goHTpFeH03tJvGslWhTxynhFne599qeQvfFQ==
Bagno FF, Sérgio SAR, Figueiredo MM et al (2022) Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19. Journal of Clinical Virology Plus 2(3):100101. https://doi.org/10.1016/J.JCVP.2022.100101
Gómez-Ochoa SA, Franco OH, Rojas LZ et al (2021) COVID-19 in Healthcare Workers: A Living Systematic Review and Meta-analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am J Epidemiol 190(1):161–175. https://doi.org/10.1093/AJE/KWAA191
Dorrucci M, Minelli G, Boros S et al (2021) Excess Mortality in Italy During the COVID-19 Pandemic: Assessing the Differences Between the First and the Second Wave, Year 2020. Front Public Health:927. https://doi.org/10.3389/FPUBH.2021.669209/FULL
Minas Gerais. Boletim Epidemiológico de 21 de janeiro de 2022. Secretaria de Estado de Saúde de Minas Gerais. Published January 21, 2022. Accessed January 22, 2022. https://coronavirus.saude.mg.gov.br/images/2022/01/COVID-19_-_BOLETIM20220121.pdf
Iversen K, Bundgaard H, Hasselbalch RB et al (2020) Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 20(12):1401. https://doi.org/10.1016/S1473-3099(20)30589-2
Hildebrandt A, Hökelekli O, Uflacker L, Rudolf H, Gatermann SG (2021) COVID-19: Hotspot hospital?- seroprevalence of SARS-CoV-2 antibodies in hospital employees in a secondary care hospital network in Germany: Intermediate results of a prospective surveillance study. Int J Hyg Environ Health 235:113771. https://doi.org/10.1016/J.IJHEH.2021.113771
Faller E, Wyse A, Barry R et al (2021) Original research: Seroprevalence study of SARS-CoV-2 antibodies in healthcare workers following the first wave of the COVID-19 pandemic in a tertiary-level hospital in the south of Ireland. BMJ Open 11(6). https://doi.org/10.1136/BMJOPEN-2021-051415
Milazzo L, Lai A, Pezzati L et al (2021) Original research: Dynamics of the seroprevalence of SARS-CoV-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup Environ Med 78(8):541–547. https://doi.org/10.1136/OEMED-2020-107060
De Geyter D, Vancutsem E, Van Laere S et al (2021) SARS-CoV-2 seroprevalence among employees of a university hospital in Belgium during the 2020 COVID-19 outbreak (COVEMUZ-study). Epidemiol Infect 149. https://doi.org/10.1017/S0950268821001540
Minas Gerais (2020) Boletim Epidemiológico: Cenário em Minas Gerais. Coronavírus. https://coronavirus.saude.mg.gov.br/boletim2020. Accessed 29 Oct 2022.
Prakash O, Solanki B, Sheth JK et al (2021) Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: A cross-sectional study. BMJ Open 11(1). https://doi.org/10.1136/bmjopen-2020-044101
Venugopal U, Jilani N, Rabah S et al (2021) SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis 102:63–69. https://doi.org/10.1016/J.IJID.2020.10.036
Bryan A, Tatem K, Diuguid-Gerber J et al (2021) Original research: Cross-sectional study evaluating the seroprevalence of SARS-CoV-2 antibodies among healthcare workers and factors associated with exposure during the first wave of the COVID-19 pandemic in New York. BMJ Open 11(11). https://doi.org/10.1136/BMJOPEN-2021-053158
Purswani MU, Bucciarelli J, Tiburcio J et al (2021) SARS-CoV-2 Seroprevalence Among Healthcare Workers by Job Function and Work Location in a New York Inner-City Hospital. J Hosp Med 16(5):282–289. https://doi.org/10.12788/JHM.3627
Brousseau N, Morin L, Ouakki M et al (2021) SARS-CoV-2 seroprevalence in health care workers from 10 hospitals in Quebec, Canada: a cross-sectional study. CMAJ. 193(49):E1868–E1877. https://doi.org/10.1503/CMAJ.202783/TAB-RELATED-CONTENT
Chen Y, Tong X, Wang J et al (2020) High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect 81(3):420–426. https://doi.org/10.1016/J.JINF.2020.05.067
Gillot C, Douxfils J, Cadrobbi J et al (2020) An Original ELISA-Based Multiplex Method for the Simultaneous Detection of 5 SARS-CoV-2 IgG Antibodies Directed against Different Antigens. J Clin Med 9(11):3752. https://doi.org/10.3390/jcm9113752
Prakash O, Solanki B, Sheth JK, Kadam M, Vyas S (2021) Severe acute respiratory syndrome coronavirus 2 immunoglobulin G antibody: Seroprevalence among contacts of COVID-19 cases. Indian J Public Health 65(1):5–10. https://doi.org/10.4103/IJPH.IJPH_1199_20
D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L (2020) Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol 18(8):1663. https://doi.org/10.1016/J.CGH.2020.04.001
Mair M, Singhavi H, Pai A et al (2021) A Meta-Analysis of 67 Studies with Presenting Symptoms and Laboratory Tests of COVID-19 Patients. Laryngoscope 131(6):1254–1265. https://doi.org/10.1002/LARY.29207
Menezes AMB, Victora CG, Hartwig FP et al (2021) High prevalence of symptoms among Brazilian subjects with antibodies against SARS-CoV-2. Sci Rep 11(1). https://doi.org/10.1038/S41598-021-92775-Y
Fang X, Li S, Yu H et al (2020) Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 12(13):12493. https://doi.org/10.18632/AGING.103579
Acknowledgment
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior -Brasil (CAPES) - Finance Code 001 (Duillio Alves Caixeta - DAC). We are deeply grateful to Prof. Jamie A. Hawkes FRSC. who made a complete revision of the English language in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that the present study had no conflict of interest.
Additional information
Responsible Editor: Fernando R. Spilki
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caixeta, D.A., do Carmo, M.A.V., da Fonseca, F.G. et al. Seroprevalence of SARS-CoV-2 in hospital workers in the southern region of Minas Gerais state in Brazil: An analysis of the pre-vaccine period. Braz J Microbiol 54, 859–871 (2023). https://doi.org/10.1007/s42770-023-00966-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00966-8