Abstract
Keratinophilic fungi are mostly soil-inhabiting organisms with occasional infections in humans and animals. Even though most dermatophytes are host-adapted, cross-species infections are common by zoophilic and geophilic dermatophytes. N. nana is considered an etiological agent of ringworm in pigs but has also been isolated from other animals, including humans. However, it also possesses many characteristics of geophilic dermatophytes including the ability to grow in soil. N. nana produces characteristic pear-shaped macroconidia and usually exhibits an ectothrix pattern of hair infection. It has been isolated from dermatitis lesions as well as from soil. N. nana infections in pigs are not of much concern as far as economy or health is concerned. But it has been associated with onychomycosis and gonathritis in humans, which are significant in human medicine. The shift in the predominance of dermatophytes in humans and the ability to evolve into a potential tinea pathogen necessitates more understanding of the physiology and genetics of N. nana. In this review, we have attempted a detailed analysis of the studies about N. nana, emphasizing growth and cultural characters, physiology, isolation, infection in humans and animals, molecular characterization and antifungal susceptibility.

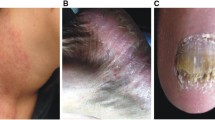
Reproduced from Gnat et al. 2020 [15] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)

Reproduced from Dukik et al. 2020 [21] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0)



Reproduced from Dukik et al. 2020 [21] under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Novak EK, Galgoczy J (1966) Notes on dermatophytes of soil origin. Mycopathologia et mycologiaapplicata 28(4):289–296
de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A (2017) Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182(1–2):5–31
Gräser Y, El Fari M, Vilgalys R, Kuijpers AFA, De Hoog GS, Presber W, Tietz HJ (1999) Phylogeny and taxonomy of the family Arthrodermataceae(dermatophytes) using sequence analysis of the ribosomal ITS region. Med Mycol 37(2):105–114
Deshmukh SK (2002) Incidence of dermatophytes and other keratinophilic fungi in the glacier bank soils of the Kashmir valley India. Mycologist 16(4):165–167
Carman MG, Rush-Munro FM, Carter ME (1979) Dermatophytes isolated from domestic and feral animals. N Z Vet J 27(7):136–144
Bontems O, Fratti M, Salamin K, Guenova E, Monod M (2020) Epidemiology of Dermatophytoses in Switzerland According to a Survey of Dermatophytes Isolated in Lausanne between 2001 and 2018. Journal of Fungi 6(2):95
Yerga AL, Rodríguez-Nevado I, Recio JF, Álvarez AJC, Durán DDAF, Eseverri EG (2007) Infeccióncutánea de Microsporum nanum. Semergen: revistaespañola de medicina de familia (3):159–160
Jain M, Shukla PK, Srivastava OP (1985) Keratinophilic fungi and dermatophytes in Lucknow soils with their global distribution:*KeratinophilePilze und DermatophytenimErdboden von Lucknow und ihrweltweitesVorkommen. Mycoses 28(3):148–153
Chmel L, Buchvald J (1970) Ecology and transmission of Microsporum gypseum from soil to man. Sabouraudia 8(2):149–156
Pontes ZBVDS, Oliveira ACD, Guerra FQS, Pontes LRDA, Santos JPD (2013) Distribution of dermatophytes from soils of urban and rural areas of cities of Paraiba State, Brazil. Rev Inst Med Trop Sao Paulo 55(6):377–383
Jain N, Sharma M (2011) Distribution of dermatophytes and other related fungi in Jaipur city, with particular reference to soil pH. Mycoses 54(1):52–58
Oyeka CA, Okoli I (2003) Isolation of dermatophytes and non-dermatophytic fungi from soil in Nigeria. Mycoses 46(8):318–320
Romano C, Massai L, Gallo A, Fimiani M (2009) Microsporum gypseum infection in the Siena area in 2005–2006. Mycoses 52(1):67–71
Roller JA, Westblom TU (1986) Microsporum nanuminfection in hog farmers. J Am Acad Dermatol 15(5):935–939
Gnat S, Łagowski D, Nowakiewicz A, Dyląg M (2020) Unusual dermatomycoses caused by Nannizzia nana: the geophilic origin of human infections. Infection 48(3):429–434. https://doi.org/10.1007/s15010-020-01416-5
Kelly R, Searls S (1977) Microsporum nanum infection in Victoria. Australas J Dermatol 18(3):137–138
Otčenašek M, Dvořak J (1975) Ecological classification of dermatophytes. Mycoses 18(10):425–434
Aly R (1994) Ecology and epidemiology of dermatophyte infections. J Am Acad Dermatol 31(3):S21–S25
Fuentes CA, Aboulafia R, Vidal RJ (1954) A dwarf form of Microsporum gypseum. J Investig Dermatol 23(1):51–61
Fuentes CA (1956) A new species of Microsporum. Mycologia 48(4)
Dukik K, de Hoog GS, Stielow JB, Freeke J, van den Ende BG, Vicente VA, Menken SBJ, Ahmed SA (2020) Molecular and phenotypic characterization of Nannizzia (Arthrodermataceae). Mycopathologia 185(1):9–35. https://doi.org/10.1007/s11046-019-00336-9
Ajello L, Varsavsky E, Ginther OJ, Bubash G (1964) The natural history of Microsporum nanum. Mycologia 56(6):873–884
Souza BDS, Sartori DS, Andrade CD, Weisheimer E, Kiszewski AE (2016) Dermatophytosis caused by Microsporum gypseum in infants: report of four cases and review of the literature. An Bras Dermatol 91:823–825
Zheng H, Blechert O, Mei H, Ge L, Liu J, Tao Y, Li D, de Hoog GS, Liu W (2020) Assembly and analysis of the whole genome of Arthroderma uncinatum strain T10, compared with Microsporum canis and Trichophyton rubrum. Mycoses 63(7):683–693
Giddey K, Favre B, Quadroni M, Monod M (2007) Closely related dermatophyte species produce different patterns of secreted proteins. FEMS Microbiol Lett 267(1):95–101
Sharma A, Chandra S, Sharma M (2012) Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses 55(5):410–415
Nardoni S, Rocchigiani G, Papini RA, Veneziano V, Brajon G, Martini M, Salari F, Mancianti F (2016) Dermatophytosis in donkeys (Equus asinus) due to Microsporum racemosum, an unusual geophilic agent. Medical mycology case reports 12:8–10
Sharifzadeh A, Shokri H, Khosravi AR (2016) In vitro evaluation of antifungal susceptibility and keratinase, elastase, lipase and DN ase activities of different dermatophyte species isolated from clinical specimens in Iran. Mycoses 59(11):710–719
Shankar SG, Ranganathan S, Ranjith MS, Vijayalakshmi GS (2002) Did earthworms contribute to the parasitic evolution of dermatophytes? Mycoses 45(9–10):399–401
Gokulshankar S, Ranjithsingh AJA, Ranjith MS, Ranganathan S, Palaniappan R (2005) Role of Chrysosporium keratinophillum in the parasitic evolution of dermatophytes. Mycoses 48(6):442–446
Morganti L, Bianchedi M, Ajello L, Padhye A (1976) First European report of swine infection by Microsporum nanum. Mycopathologia 59(3):179–182
Bubash GR, Ginther OJ, Ajello L (1964) Microsporumnanum: First recorded isolation from animals in the United States. Science 143(3604):366–367
Smith JMB and Steffert IJ (1966) Microsporum nanum in New Zealand pigs. New Zealand veterinary journal 14(7)
García-Sánchez A, Bazán J, de Mendoza JH, Martínez R, Sánchez S, de Mendoza MH (2011) Outbreak of ringworm in a traditional Iberian pig farm in Spain. Mycoses 54(2):179–181
Londero AT, Benevenga JP (1972) Human infection by Microsporum nanum in Brazil. Rev Inst Med Trop Sao Paulo 14(6):388–391
De Camargo RM, Silvares MR, Carvalho CR, Dillon NL, Marques SA (1992) Microsporum nanum A fourth report of human infection in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo 34(6):581
Camargo RMP, Silva NAS, Marques SA, Stolf HO, Dillon NL (1984) Report of the second human infection case by Microsporum nanum, in Brazil. Rev Inst Med Trop Sao Paulo 26(3):165–169
Camargo RM, Silvares MRC, Carvalho CR, Dillon NL, Marques SA (1992) Report of the fourth human infection case by Microsporum nanum in Brazil. Rev Inst Med Trop Sao Paulo 34(6):581–585
Carmichael JW, Reid JF (1962) Microsporum nanum infection in Alberta. Mycopathologia et mycologiaapplicata 17(4):325–326
Ponnighaus JM, Warndorff D, Port G (1995) Microsporum nanum-a report from Malawi (Africa) Microsporum nanum-einBerichtaus Malawi (Afrika). Mycoses 38(3–4):149–150
TurnerKAMINSK TWGW (1976) Microsporum nanum infection in South Australia. Med J Aust 1(20):743–744
Garg AK, Mulay DN (1972) Isolation of Microsporum nanum from man in India. Hindustan Antibiot Bull 14(3):137–139
Ranganathan S, Menon T, Balajee SAM (1997) Isolation of Microsporum nanum from a patient with tinea corporis in Madras India. Mycoses 40(5–6):229–230
Alteraş I (1970) First case of tinea infection by Microsporum nanum in Romania. Mycoses 13(9):447–450
O’Keeffe MF (1973) A report of three human infections due to Microsporum nanum. Australas J Dermatol 14(2):73–74
Sinski JT, Flouras K (1984) A survey of dermatophytes isolated from human patients in the United States from 1979 to 1981 with chronological listings of worldwide incidence of five dermatophytes often isolated in the United States. Mycopathologia 85(1–2):97–120
Sinski JT, Kelley LM (1987) A survey of dermatophytes isolated from human patients in the United States from 1982 to 1984. Mycopathologia 98(1):35–40
Weitzman I, Chin NX, Kunjukunju N, Della-Latta P (1998) A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 39(2):255–261
Mahmoud AL (2002) A study of dermatophytoses in Sana’a Yemen Republic. Mycoses 45(3–4):105–108
Chi CC, Wang SH, Chou MC (2005) The causative pathogens of onychomycosis in southern Taiwan. Mycoses 48(6):413–420
Martínez E, Ameen M, Tejada D, Arenas R (2014) Microsporum spp onychomycosis disease presentation, risk factors and treatment responses in an urban population. Brazilian J Infectious Diseas 18(2):181–186
Ratka P (1985) Microsporidgonitis caused by Microsporum nanum. Mycopathologia 92(1):45–47
Shaqra QA, Al-Jamaien H, Al Zoubi M (2012) Isolation of soil dermatophytes from three distinct geographic locations in Jordan. Fungal Ecology 5(2):274–276
Anane S (2012) Epidemiological investigation of keratinophilic fungi from soils of Djerba (Tunisia). J de mycologiemédicale 22(3):225–229
Deshmukh SK, Mandeel QA, Verekar SA (2008) Keratinophilic fungi from selected soils of Bahrain. Mycopathologia 165(3):143–147
Ranganathan S, Balajee SAM (2000) Microsporum gypseum complex in Madras India. Mycoses 43(5):177–180
Summerbell RC (2000) Form and function in the evolution of dermatophytes. Biology of dermatophytes and other keratinophilic fungi. RevistaIberoamericana de Micologia, Bilbao, Spain, pp 30–43
Kunert J (2000) Physiology of keratinophilic fungi. RevistaIberoamericana de Micologia 1:77–85
Meyers MA, Chen PY, Lin AYM, Seki Y (2008) Biological materials: structure and mechanical properties. Prog Mater Sci 53(1):1–206
Strnad P, Usachov V, Debes C, Gräter F, Parry DA, Omary MB (2011) Unique amino acid signatures that are evolutionarily conserved distinguish simple-type, epidermal and hair keratins. J Cell Sci 124(24):4221–4232
Rice RH (2011) Proteomic analysis of hair shaft and nail plate. J Cosmet Sci 62(2):229
Koehne GW (1962) Nutrition of three species of Microsporum. Mycopathologia et mycologiaapplicata 18(3):199–206
Wawrzkiewicz K, Wolski T, Łobarzewski J (1991) Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia 114(1):1–8
Kosanke S, Hamann L, Kupsch C, Garcia SM, Chopra A, Gräser Y (2018) Unequal distribution of the mating type (MAT) locus idiomorphs in dermatophyte species. Fungal Genet Biol 118:45–53
Metin B, Heitman J (2020) She loves me, she loves me not: on the dualistic asexual/sexual nature of dermatophyte fungi. Mycopathologia 185(1):87–101
Dawson CO, Gentles JC (1962) The perfect states of Keratinomyces ajelloi van-Breuseghem, Trichophyton terrestre Durie & Frey and Microsporum nanum Fuentes. Sabouraudia 1(1):49–57
Metin B, Heitman J (2017) Sexual reproduction in dermatophytes. Mycopathologia 182(1–2):45–55
Guarro J, Cano J, Leal JA, Gómez-Miranda B, Bernabé M (1993) Composition of the cell wall polysaccharides in some geophilic dermatophytes. Mycopathologia 122(2):69–77
Brocksins JM (1961) Microsporum nanum: A Cause of Tinea Capitis: A Case Report. Arch Dermatol 84(3):504–505
Wenyan Z, Mingyu X, Li H, Shaoxi W, Qing Z, Bian Z (1987) Electron microscopic observation on infected hairs of kerion caused by Microsporum nanum. Int J Dermatol 26(10):641–644
Mullins JF, Willis CJ, Bergeron JR, Johnson DA, Stone OJ (1966) Microsporum nanum: a review of the literature and a report of two cases. Arch Dermatol 94(3):300–303
Long JR, Brandenburg AC, Oliver PG (1972) Case report Microsporum nanum: a cause of porcine ringworm in Ontario. Canadian Veterinary J 13(7):164
Dodd DC, Newlin RW, Niksch GR (1965) Infection of Swine with Microsporum nanum. J Am Vet Med Assoc 146(5):486–489
Carter GR, Glenn MW (1966) Ringworm with complimenting acanthpsis in Swine. J Am Vet Med Assoc 149(1):42–45
Chermette R, Ferreiro L, Guillot J (2008) Dermatophytoses in animals. Mycopathologia 166(5–6):385–405
Cabo JG, Asensio MB, Rodriguez FG, Lázaro JA (1995) An outbreak of dermatophytosis in pigs caused by Microsporum canis. Mycopathologia 129(2):79–80
Cabo JG, Cequiel ML, Aisa CS, Arribas MV (1988) Dermatophytosis of pigs by Trichophyton mentagrophytes. Mycopathologia 101(3):161–164
Rippon JW (1988) Medical mycology. The pathogenic fungi and the pathogenic actinomycetes (No. Ed. 3). WB Saunders company
Mercer DK, Stewart CS (2019) Keratin hydrolysis by dermatophytes. Medical mycology 57(1):13–22
Rippon JW (1983) Host specificity in dermatophytoses. In Proceedings of the 8th Congress of the International Society for Human and Animal Mycology, 1983. Massey University Press. India
Monod M (2008) Secreted proteases from dermatophytes. Mycopathologia 166(5):285–294
Havlickova B, Czaika VA, Friedrich M (2008) Epidemiological trends in skin mycoses worldwide. Mycoses 51:2–15
Raubitschek F, Maoz R (1957) Invasion of nails in vitro by certain dermatophytes. J Invest Dermatol 28:261–267
Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y et al (2020) Trichophyton indotineae sp. nov: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 185(6):947–58
Yin H, Zhou JB, Chen YL, Ren L, Qin N, Xing YL, Zhao XJ (2022) Morphology, phylogeny, and pathogenicity of Trichothecium, Alternaria, and Fusarium species associated with panicle rot on Chenopodium quinoa in Shanxi Province China. Plant Pathology 71(2):344–360
Uchida T, Makimura K, Ishihara K, Goto H, Tajiri Y, Okuma M, Fujisaki R, Uchida K, Abe S, Iijima M (2009) Comparative study of direct polymerase chain reaction, microscopic examination and culture-based morphological methods for detection and identification of dermatophytes in nail and skin samples. J Dermatol 36(4):202–208
Okeke CN, Tsuboi R, Kawai M, Hiruma M, Ogawa H (2001) Isolation of an intron-containing partial sequence of the gene encoding dermatophyte actin (ACT) and detection of a fragment of the transcript by reverse transcription-nested PCR as a means of assessing the viability of dermatophytes in skin scales. J Clin Microbiol 39(1):101–106
Pounder JI, Williams S, Hansen D, Healy M, Reece K, Woods GL (2005) Repetitive-sequence-PCR-based DNA fingerprinting using the Diversilab system for identification of commonly encountered dermatophytes. J Clin Microbiol 43(5):2141–2147
Kanbe T, Suzuki Y, Kamiya A, Mochizuki T, Kawasaki M, Fujihiro M, Kikuchi A (2003) Species-identification of dermatophytes Trichophyton, Microsporum and Epidermophyton by PCR and PCR-RFLP targeting of the DNA topoisomerase II genes. J Dermatol Sci 33(1):41–54
Liu D, Coloe S, Baird R, Pedersen J (1997) Rapid differentiation of Microsporumdermatophytes based on arbitrarily primed PCR amplification. Opportunistic pathogens 9(1):3–6
Leon-Mateos A, Paredes-Suárez C, Pereiro M Jr, Toribio J (2006) Study of the ITS region in an atypical isolate and comparison with six species of Microsporum. Mycoses 49(6):452–456
Rezaei-Matehkolaei A, Makimura K, Shidfar MR, Zaini F, Eshraghian MR, Jalalizand N, Nouripour-Sisakht S, Hosseinpour L, Mirhendi H (2012) Use of single-enzyme PCR-restriction digestion barcode targeting the internal transcribed spacers (ITS rDNA) to identify dermatophyte species. Iran J Public Health 41(3):82
Liu D, Coloe S, Baird R, Pedersen J (1997) Molecular determination of dermatophyte fungi using the arbitrarily primed polymerase chain reaction. Br J Dermatol 137(3):351–355
L’Ollivier C, Cassagne C, Normand AC, Bouchara JP, Contet-Audonneau N, Hendrickx M, Fourquet P, Coulibaly O, Piarroux R, Ranque S (2013) A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med Mycol 51(7):713–720
Nenoff P, Erhard M, Simon JC, Muylowa GK, Herrmann J, Rataj W, Gräser Y (2013) MALDI-TOF mass spectrometry–a rapid method for the identification of dermatophyte species. Med Mycol 51(1):17–24
Theel ES, Hall L, Mandrekar J, Wengenack NL (2011) Dermatophyte identification using matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 49(12):4067–4071
Panda A, Ghosh AK, Mirdha BR, Xess I, Paul S, Samantaray JC, Srinivasan A, Khalil S, Rastogi N, Dabas Y (2015) MALDI-TOF mass spectrometry for rapid identification of clinical fungal isolates based on ribosomal protein biomarkers. J Microbiol Methods 109:93–105
Rychert J (2019) Benefits and limitations of MALDI-TOF mass spectrometry for the identification of microorganisms. J Infectiol Epidemiol 2(4)
Erhard M, Hipler UC, Burmester A, Brakhage AA, Wöstemeyer J (2008) Identification of dermatophyte species causing onychomycosis and tinea pedis by MALDI-TOF mass spectrometry. Exp Dermatol 17(4):356–361
Frías-De-León MG, Martínez-Herrera E, Atoche-Diéguez CE, González-Cespón JL, Uribe B, Arenas R, Rodríguez-Cerdeira C (2020) Molecular identification of isolates of the Trichophyton mentagrophytes complex. Int J Med Sci 17(1):45
Makimura K, Tamura Y, Murakami A, Kano R, Nakamura Y, Hasegawa A, Uchida K, Yamaguchi H (2001) Cluster analysis of human and animal pathogenic Microsporum species and their teleomorphic states, Arthroderma species, based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1. Microbiol Immunol 45(3):209–216
Rezaei-Matehkolaei A, Mirhendi H, Makimura K, de Hoog GS, Satoh K, Najafzadeh MJ, Shidfar MR (2014) Nucleotide sequence analysis of beta tubulin gene in a wide range of dermatophytes. Med Mycol 52(7):674–688
Mirhendi H, Makimura K, de Hoog GS, Rezaei-Matehkolaei A, Najafzadeh MJ, Umeda Y, Ahmadi B (2015) Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol 53(3):215–224
Houšť J, Spížek J, Havlíček V (2020) Antifungal drugs. Metabolites 10(3):106
Carrillo-Muñoz AJ, Giusiano G, Guarro J, Quindós G, Guardia C, Del Valle O, Rodríguez V, Estivill D, Cárdenes CD (2007) In vitro activity of voriconazole against dermatophytes, Scopulariopsis brevicaulis and other opportunistic fungi as agents of onychomycosis. Int J Antimicrob Agents 30(2):157–161
Fernández-Torres B, Carrillo AJ, Martın E, Del Palacio A, Moore MK, Valverde A, Serrano M, Guarro J (2001) In vitro activities of 10 antifungal drugs against 508 dermatophyte strains. Antimicrob Agents Chemother 45(9):2524–2528
Perea S, Fothergill AW, Sutton DA, Rinaldi MG (2001) Comparison of in vitro activities of voriconazole and five established antifungal agents against different species of dermatophytes using a broth macrodilution method. J Clin Microbiol 39(1):385–388
Ali-Shtayeh MS, Arda HM, Hassouna M, Shaheen SF (1988) Keratinophilic fungi on the hair of cows, donkeys, rabbits, cats, and dogs from the West Bank of Jordan. Mycopathologia 104(2):109–121
Ali-Shtayeh MS, Arda HM, Hassouna M, Shaheen SF (1989) Keratinophilic fungi on sheep hairs from the West Bank of Jordan. Mycopathologia 106(2):95–101
Nweze EI (2011) Dermatophytoses in domesticated animals. Rev Inst Med Trop Sao Paulo 53(2):94–99
Seker E, Dogan N (2011) Isolation of dermatophytes from dogs and cats with suspected dermatophytosis in Western Turkey. Prev Vet Med 98(1):46–51
Ilhan Z, Karaca M, Ekin IH, Solmaz H, Akkan HA, Tutuncu M (2016) Detection of seasonal asymptomatic dermatophytes in Van cats. Brazilian J Microbiol 47(1):225–230
Mahmoud ALE (1993) Dermatophytes and other associated fungi isolated from ringworm lesions of camels. Folia Microbiol 38(6):505–508
Ramesh VM, Hilda A (1998) Incidence of keratinophilic fungi in the soil of primary schools and public parks of Madras city India. Mycopathologia 143(3):139
Dolenc-Voljč M, Gasparič J (2017) Human infections with Microsporum gypseum complex (Nannizzia gypsea) in Slovenia. Mycopathologia 182(11):1069–1075
Acknowledgements
We thank Indian Council of Medical Research for granting a project on dermatophytes titled 'Unraveling the Pheno-Genotyping Linking and Distrubution Dynamics of Trichophyton mentagrophytes among Human and Animals'. Figure 3 is created using Biorender.com.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SSN and A both conceptualized the idea and wrote the review article along with the valuable inputs from all other authors. All authors read, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors confirm that no ethical approval was required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Luis Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, S.S., Abhishek, Saini, S. et al. Dermatophytosis caused by Nannizzia nana (Microsporum nanum): a comprehensive review on a novel pathogen. Braz J Microbiol 54, 509–521 (2023). https://doi.org/10.1007/s42770-022-00880-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00880-5