Abstract
Background
Traditional culture-based microbiological methods remain the most used for defining the etiology of urinary tract infections and antibiotic susceptibility testing (AST) of isolated uropathogens. They are time-consuming and lead to delays of several days when obtaining the final results of microbiological tests.
Objectives
In this study, we validate the possibility of using a microbiological CFN analyzer combined with MALDI-TOF mass spectrometry (MS) for fast conclusive urine testing (1 day) without obtaining pure cultures.
Materials and methods
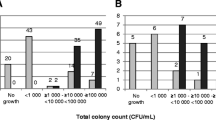
The study included three stages: detection of urine microflora growth using the CFN analyzer to separate positive and negative samples within 2–4 h; fast MS identification of positive samples without isolating uropathogens; fast AST using CFN analyzer within 3–6 h. In parallel, all urine samples were tested by traditional culture-based microbiological methods.
Result
In total, 194 urine samples were tested, and 22 urine cultures were identified by MS, among them, 20 monocultures with bacterial counts ≥ 105 and 2 mixed cultures. The AST of these 22 urine cultures and additional 88 pure clinical cultures was performed using eight antibiotics. Overall, 276 tests were performed. The results of AST obtained using the CFN analyzer and traditional methods were in good agreement (98.2%). Although two mixed cultures were falsely identified as monocultures, their susceptibility determined by the CFN analyzer was correct.
Conclusions
The CFN analyzer is promising and effective for fast AST. Combined with MS identification, it allows to perform full urine analysis in 1 day without the lengthy isolation of pure cultures.





Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Najar MS, Saldanha CL, Banday KA (2009) Approach to urinary tract infections. Indian J Nephrol 19:129–139. https://doi.org/10.4103/0971-4065.59333
Nicolle LE (2014) Catheter associated urinary tract infections. Antimicrob Resist Infect Control 3:23. https://doi.org/10.1186/2047-2994-3-23
Sadar M (1998) Turbidity Science, Technical Information Series, Booklet No. 11. Hach Company. https://www.hach.com/asset-get.download.jsa?id=7639984474. Accessed May 2003
McFarland J (1907) The nephelometer: and instrument for estimating the number of bacteria in suspensions used for calculation of opsonic index and for vaccines. JAMA 14:1176–1178
Davenport M, Mach KE, Dairiki Shortliffe LM, Banaei N, Wang TH, Liao JC (2017) New and developing diagnostic technologies for urinary tract infections. Nat Rev Uro 14:296–310. https://doi.org/10.1038/nrurol.2017.20
Athamna A, Zbriger A, Avadov S, Shapira M, Tal Y, Freimann S (2020) Rapid identification of uropathogens by combining Alfred 60 system with matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry technology. Eur J Clin Microbiol Infect Dis 39(10):1855–1863. https://doi.org/10.1007/s10096-020-03919-3
Rastopov SF (2011) Coherent fluctuation nephelometry: a high-sensitivity method for detecting particles in liquids. Instrum Exp Tech 54:837–840. https://doi.org/10.1134/S0020441211060194
EUCAST (2019) Breakpoint tables for interpretation of MICs and zone diameters Version 9.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf. Accessed 1 January 2019
Gurèv AS, Yudina IE, Lazareva AV, Ayu Volkov (2018) Coherent fluctuation nephelometry as a promising method for diagnosis of bacteriuria. Pract Lab Med 12:e00106. https://doi.org/10.1016/j.plabm.2018.e00106
Roberts AL, Joneja U, Villatoro T, Andris E, Boyle JA, Bondi J (2017) Evaluation of the BacterioScan 216Dx for standalone preculture screen of preserved urine specimens in a clinical setting. Lab Med 49:35–40. https://doi.org/10.1093/labmed/lmx052
Lahanas S, Stathopoulos G, Chan RC, van Hal SJ (2013) Evaluation of the Alfred 60/AST device as a screening test for urinary tract infections. J Clin Microbiol 51:3406–3408. https://doi.org/10.1128/JCM.01126-13
Gur’ev AS, Kuznetsova OYu, Kraeva LA, Rastopov SF, Verbov VN, Vasilenko IA, Rusanova EV, Volkov AYu (2018) Development of microbiological analyzer based on coherent fluctuation nephelometry. In: Hu Z, Petoukhov S, He M (eds) Advances in artificial systems for medicine and education. AIMEE 2017. Advances in intelligent systems and computing, vol 658. Springer, Cham, pp 198–206. https://doi.org/10.1007/978-3-319-67349-3_18
EUCAST (2016) Expert Rules Version 3.1, Intrinsic resistance and exceptional phenotypes tables. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/Expert_rules_intrinsic_exceptional_V3.1.pdf. Accessed 9 September 2016
Prasetyoputri A, Jarrad AM, Cooper MA, Blaskovich MAT (2019) The eagle effect and antibiotic-induced persistence: two sides of the same coin? Trends Microbiol 27:339–354. https://doi.org/10.1016/j.tim.2018.10.007
Eagle H, Musselman AD (1948) The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J Exp Med 88:99–131. https://doi.org/10.1084/jem.88.1.99
Author information
Authors and Affiliations
Contributions
Conceptualization: AG, AB, MI, and AV; methodology: AG, MT, AB, MI, and AV; research performing: MT, AB, and MI; data analysis and interpretation: AG and MT; analyzer and reagents: OS and SR; writing — original draft preparation: AG; writing — review and editing: AG, MT, OS, SR, AB, MI, and AV; supervision: MI and AV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of East-Tallinn Central Hospital.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests..
Additional information
Responsible Editor: Nilton Lincopan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gur’ev, A.S., Tigasson, M., Shalatova, O.Y. et al. Fast antibiotic susceptibility testing of urine microflora using a microbiological analyzer based on coherent fluctuation nephelometry. Braz J Microbiol 53, 195–204 (2022). https://doi.org/10.1007/s42770-021-00671-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00671-4