Abstract
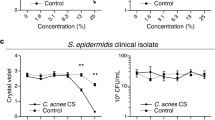
Staphylococcus spp. and Cutibacterium acnes are members of the skin microbiome but can also act as pathogens. Particularly, Staphylococcus species are known to cause medical devices-associated infections, and biofilm production is one of their main virulence factors. Biofilms allow bacteria to adhere and persist on surfaces, protecting them from antimicrobials and host defenses. Since both bacteria are found in the human skin, potentially competing for niches, we aimed to investigate if C. acnes produces molecules that affect Staphylococcus spp. biofilm formation and dispersal. Thus, we evaluated the impact of C. acnes cell-free conditioned media (CFCM) on S. aureus, S. epidermidis, S. hominis, and S. lugdunensis biofilm formation. S. lugdunensis and S. hominis biofilm formation was significantly reduced with C. acnes CFCM without impact on their planktonic growth. C. acnes CFCM also significantly disrupted S. hominis established biofilms. The active molecules against S. lugdunensis and S. hominis biofilms appeared to be distinct since initial characterization points to different sizes and sensitivity to sodium metaperiodate, although the activity is highly resistant to heat in both cases. Mass spectrometry analysis of the fractions active against S. hominis revealed several potential candidates. Investigating how species present in the same environment interact, affecting the dynamics of biofilm formation, may reveal clinically useful compounds as well as molecular aspects of interspecies interactions.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Otto, M. (2008). Staphylococcal biofilms. In: Romeo, T. (ed.) Bacterial biofilms (current topics in microbiology and immunology). Springer-Verlag, 207–228
Czekaj T, Ciszewski M, Szewczyk EM (2015) Staphylococcus haemolyticus – an emerging threat in the twilight of the antibiotics age. Microbiol (United Kingdom) 161:2061–2068. https://doi.org/10.1099/mic.0.000178
Kiedrowski MR, Horswill AR (2011) New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121. https://doi.org/10.1111/j.1749-6632.2011.06281.x
Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 5:e02192. https://doi.org/10.1016/j.heliyon.2019.e02192
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Becker K, Heilmann C, Peters G (2014) Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. https://doi.org/10.1128/CMR.00109-13
Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S et al (2012) Costs of healthcare-and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis 55:807–815. https://doi.org/10.1093/cid/cis552
Heilmann C, Ziebuhr W, Becker K (2019) Are coagulase-negative staphylococci virulent? Clin Microbiol Infect 25:1071–1080. https://doi.org/10.1016/j.cmi.2018.11.012
Boisrenoult P (2018) Cutibacterium acnes prosthetic joint infection: diagnosis and treatment. Orthop Traumatol Surg Res 104:S19–S24. https://doi.org/10.1016/j.otsr.2017.05.030
Achermann Y, Goldstein EJC, Coenye T, Shirtliffa ME (2014) Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 27:419–440. https://doi.org/10.1128/CMR.00092-13
Dessinioti C, Katsambas A (2017) Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol 35:163–167. https://doi.org/10.1016/j.clindermatol.2016.10.008
Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y et al (2013) Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One 8:e55380. https://doi.org/10.1371/journal.pone.0055380
Allhorn M, Arve S, Brüggemann H, Lood R (2016) A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci Rep 6:1–12. https://doi.org/10.1038/srep36412
Christensen GJM, Scholz CFP, Enghild J, Rohde H, Kilian M, Thürmer A et al (2016) Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics 17:1–14. https://doi.org/10.1186/s12864-016-2489-5
Grice EA, Segre JA (2013) The skin microbiome. Nat Rev Microbiol 9:244–253. https://doi.org/10.1038/nrmicro2537.The
Swetha TK, Pooranachithra M, Subramenium GA, Divya V, Balamurugan K, Pandian SK (2019) Umbelliferone impedes biofilm formation and virulence of methicillin-resistant Staphylococcus epidermidis via impairment of initial attachment and intercellular adhesion. Front Cell Infect Microbiol 9:357. https://doi.org/10.3389/fcimb.2019.00357
Malcolmson SJ, Young TS, Ruby JG, Skewes-Cox P, Walsh CT (2013) The posttranslational modification cascade to the thiopeptide berninamycin generates linear forms and altered macrocyclic scaffolds. Proc Natl Acad Sci U S A 110:8483–8488. https://doi.org/10.1073/pnas.1307111110
Claesen J, Spagnolo JB, Ramos SF, Kurita KL, Byrd AL, Aksenov AA et al (2020) A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci Transl Med 12:eaay5445. https://doi.org/10.1126/scitranslmed.aay5445
Antunes LCM, McDonald JAK, Schroeter K, Carlucci C, Ferreira RBR, Wang M et al (2014) Antivirulence activity of the human gut metabolome. MBio 5:1–13. https://doi.org/10.1128/mBio.01183-14
Glatthardt T, de Mello Campos JC, Chamon RC, de Sá Coimbra TF, de Almeida Rocha G, de Melo MAF et al (2020) Small molecules produced by commensal Staphylococcus epidermidis disrupt formation of biofilms by Staphylococcus aureus. Appl Environmantal Microbiol 86:02539–19
Ebeling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H (1974) Proteinase K from Tritirachium album Limber. Eur J Biochem 47:91–97. https://doi.org/10.1111/j.1432-1033.1974.tb03671.x
Buck FF, Vithayathil AJ, Bier M, Nord FF (1962) On the mechanism of enzyme action. LXXIII. Studies on trypsins from beef, sheep and pig pancreas. Arch Biochem Biophys 97:417–424. https://doi.org/10.1016/0003-9861(62)90099-1
Kumar PG, Laloraya M, Wang CY, Ruan QG, Davoodi-Semiromi A, Kao KJ et al (2001) The autoimmune regulator (AIRE) is a dna-binding protein. J Biol Chem 276:41357–41364. https://doi.org/10.1074/jbc.M104898200
Li C, Roy K, Dandridge K, Naren AP (2004) Molecular assembly of cystic fibrosis transmembrane conductance regulator in plasma membrane. J Biol Chem 279:24673–24684. https://doi.org/10.1074/jbc.M400688200
Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. https://doi.org/10.1016/S0167-7012(00)00122-6
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81:e00036–17. https://doi.org/10.1128/mmbr.00036-17
Antunes LCM, Davies JE, Finlay BB (2011) Chemical signaling in the gastrointestinal tract. F1000 Biol. Rep 3:1–8. https://doi.org/10.3410/B3-4
Burkholder KM, Fletcher DH, Gileau L, Kandolo A (2019) Lactic acid bacteria descrease Salmonella enterica Javiana virulence and modulate host inflammation during infection of an intestinal epithelial cell line. Pathog Dis 77(3):1–13. https://doi.org/10.1093/femspd/ftz025
Oladipo AO, Oladipo OG, Bezuidenhout CC (2019) Multi-drug resistance traits of methicillin-resistant Staphylococcus aureus and other Staphylococcal species from clinical and environmental sources. J Water Health 17:930–943. https://doi.org/10.2166/wh.2019.177
Dickey SW, Cheung GYC, Otto M (2017) Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16:457–471. https://doi.org/10.1038/nrd.2017.23
Babu E, Oropello J (2011) Staphylococcus lugdunensis: the coagulase-negative staphylococcus you don’t want to ignore. Expert Rev Anti Infect Ther 9:901–907. https://doi.org/10.1586/eri.11.110
Szczuka E, Telega K, Kaznowski A (2014) Biofilm formation by Staphylococcus hominis strains isolated from human clinical specimens. Folia Microbiol (Praha) 60:1–5. https://doi.org/10.1007/s12223-014-0332-4
Otto M (2018) Staphylococcal biofilms. Microbiol Spectr 176:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Frank KL, Del Pozo JL, Patel R (2008) From clinical microbiology to infection pathogenesis: How daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21:111–133. https://doi.org/10.1128/CMR.00036-07
Schilcher K, Horswill AR (2020) Staphylococcal biofilm development: structure, regulation, and treatment strategieS. Microbiol Mol Biol Rev 84:1–36. https://doi.org/10.1128/MMBR.00026-19
Acknowledgements
We thank Jefferson Bomfim Silva Cypriano and UniMicro at the Federal University of Rio de Janeiro (UFRJ) and Sara Teixeira de Macedo Silva and CENABIO at UFRJ for scanning electron microscopy and image processing. We also would like to thank the Centro de Espectrometria de Massas de Biomoléculas (CEMBIO) at UFRJ for mass spectrometry analyses.
Funding
This study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior—Brasil (CAPES—Finance Code 001), as well as the Fundação Oswaldo Cruz Inova Fiocruz/VPPCB Program. This work was supported in part by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grant # E-26/211.554/2019 (Programa Rede de Pesquisa em Saúde).
Author information
Authors and Affiliations
Contributions
Conceptualization and interpretation of data: RDL, LCMA, and RBRF. Data acquisition: RDL, GAR, JSR, TG, LSC, and COGXL. Funding acquisition: LCMA and RBRF. Supervision: RBRF. Writing: RDL, TG, LCMA, and RBRF.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Jorge Luiz Mello Sampaio
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lima, R.D., dos Reis, G.A., da Silva Reviello, J. et al. Antibiofilm activity of Cutibacterium acnes cell-free conditioned media against Staphylococcus spp.. Braz J Microbiol 52, 2373–2383 (2021). https://doi.org/10.1007/s42770-021-00617-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00617-w