Abstract
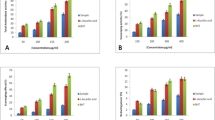
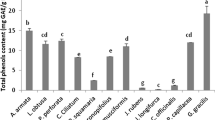
Macroalgae comprise a vast group of aquatic organisms known for their richness in phytochemicals. In this sense, the lipophilic profile of five Antarctic seaweed species was characterized by chromatographic and spectroscopic analysis and their antioxidant and antimicrobial potential was evaluated. Results showed there were 31 lipophilic substances, mainly fatty acids (48.73 ± 0.77 to 331.91 ± 10.79 mg.Kg−1), sterols (14.74 ± 0.74 to 321.25 ± 30.13 mg.Kg−1), and alcohols (13.07 ± 0.04 to 91.87 ± 30.07 mg.Kg−1). Moreover, Desmarestia confervoides had strong antioxidant activity, inhibiting 86.03 ± 1.47% of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical at 1 mg.mL−1. Antimicrobial evaluation showed that extracts from Ulva intestinalis, Curdiea racovitzae, and Adenocystis utricularis inhibited the growth of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhimurium (ATCC 14028) from concentrations of 1.5 to 6 mg.mL−1. Therefore, the evaluated brown, red, and green macroalgae contained several phytochemicals with promising biological activities that could be applied in the pharmaceutical, biotechnological, and food industries.


Similar content being viewed by others
References
Shobier AH, Abdel Ghani SA, Barakat KM (2016) GC/MS spectroscopic approach and antifungal potential of bioactive extracts produced by marine macroalgae. Egypt J Aquat Res 42:289–299. https://doi.org/10.1016/j.ejar.2016.07.003
Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P (2013) Valuable compounds in macroalgae extracts. Food Chem 138:1819–1828. https://doi.org/10.1016/j.foodchem.2012.11.081
Santos SAO, Oliveira CSD, Trindade SS, Abreu MH, Rocha SSM, Silvestre AJD (2016) Bioprospecting for lipophilic-like components of five Phaeophyta macroalgae from the Portuguese coast. J Appl Phycol 28:3151–3158. https://doi.org/10.1007/s10811-016-0855-y
Santos MAZ, Colepicolo P, Pupo D, Fujii MT, de Pereira CMP, Mesko MF (2017) Antarctic red macroalgae: a source of polyunsaturated fatty acids. J Appl Phycol 29:759–767. https://doi.org/10.1007/s10811-016-1034-x
Passos LF, Berneira LM, Poletti T, Mariotti KC, Carreño NLV, Hartwig CA, Pereira CMP (2020) Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust J Forensic Sci:1–10. https://doi.org/10.1080/00450618.2020.1715478
Martins RM, dos Santos MAZ, Pacheco BS et al (2016) Fatty acid profile of the Chlorophyta species from Chile’s sub-Antarctic region. Acad J Sci Res 4:93–98. https://doi.org/10.15413/ajsr.2015.0154
Schmid M, Kraft LGK, van der Loos LM, Kraft GT, Virtue P, Nichols PD, Hurd CL (2018) Southern Australian seaweeds: a promising resource for omega-3 fatty acids. Food Chem 265:70–77. https://doi.org/10.1016/j.foodchem.2018.05.060
Berneira L, da Silva C, Poletti T, Ritter M, dos Santos M, Colepicolo P, de Pereira CMP (2020) Evaluation of the volatile composition and fatty acid profile of seven Antarctic macroalgae. J Appl Phycol 32:3319–3329. https://doi.org/10.1007/s10811-020-02170-9
Santos SAO, Vilela C, Freire CSR, Abreu MH, Rocha SM, Silvestre AJD (2015) Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem 183:122–128. https://doi.org/10.1016/j.foodchem.2015.03.006
Pereira CMP, Nunes CFP, Zambotti-Villela L, Streit NM, Dias D, Pinto E, Gomes CB, Colepicolo P (2017) Extraction of sterols in brown macroalgae from Antarctica and their identification by liquid chromatography coupled with tandem mass spectrometry. J Appl Phycol 29:751–757. https://doi.org/10.1007/s10811-016-0905-5
Martins RM, Nedel F, Guimarães VBS, da Silva AF, Colepicolo P, de Pereira CMP, Lund RG (2018) Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front Microbiol 9:1–10. https://doi.org/10.3389/fmicb.2018.00412
Pellati F, Benvenuti S, Magro L, Melegari M, Soragni F (2004) Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. Pharm and Biomedical Anal 35:289–301. https://doi.org/10.1016/S0731-7085(03)00645-9
CLSI, M07-A10: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI (Clinical and Laboratory Standards Institute). 2015.
Tanniou A, Vandanjon L, Gonçalves O, Kervarec N, Stiger-Pouvreau V (2015) Rapid geographical differentiation of the European spread brown macroalga Sargassum muticum using HRMAS NMR and Fourier-Transform Infrared spectroscopy. Talanta 132:451–456. https://doi.org/10.1016/j.talanta.2014.09.002
Li-Beisson Y, Thelen JJ, Fedosejevs E, Harwood JL (2019) The lipid biochemistry of eukaryotic algae. Prog Lipid Res 74:31–68. https://doi.org/10.1016/j.plipres.2019.01.003
Loftsson T, Ilievska B, Asgrimsdottir GM, Ormarsson OT, Stefansson (2016) Fatty acids from marine lipids: Biological activity, formulation and stability. J Drug Deliv Sci Technol 34:71–75. https://doi.org/10.1016/j.jddst.2016.03.007
Kumari P, Kumar M, Reddy CRK, Jha B. Algal lipids, fatty acids and sterols (2013) Functional Ingredients from Algae for Foods and Nutraceuticals. Woodhead Publishing 7-134. https://doi.org/10.1533/9780857098689.1.87
Graeve M, Kattner G, Wiencke C, Karsten U (2002) Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser 231:67–74. https://doi.org/10.3354/meps231067
Becker S, Graeve M, Bischof K (2010) Photosynthesis and lipid composition of the Antarctic endemic rhodophyte Palmaria decipiens: effects of changing light and temperature levels. Polar Biol 33:945–955. https://doi.org/10.1007/s00300-010-0772-5
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Fitton A, Goa KL (1991) Azelaic Acid. Drugs. 41(5):780–798. https://doi.org/10.2165/00003495-199141050-00007
Widhalm JR, Dudareva N (2015) A familiar ring to it: Biosynthesis of plant benzoic acids. Mol Plant 8(1):83–97. https://doi.org/10.1016/j.molp.2014.12.001
Torres P, Santos JP, Chow F, dos Santos DYAC (2019) A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res 37:288–306. https://doi.org/10.1016/j.algal.2018.12.009
Torres P, Novaes P, Ferreira LG et al (2018) Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res 32:142–149. https://doi.org/10.1016/j.algal.2018.03.016
Santos MAZ, Miguel R, Pereira CMP, Freitag RA, Bairros AV (2014) Analysis of Phytosterols in Plants and Derived Products by Gas Chromatography – A Short Critical Review. Austin Publ Gr 1(5):1–4
Boulom S, Robertson J, Hamid N, Ma Q, Lu J (2014) Seasonal changes in lipid, fatty acid, α-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds. New Zealand Food Chem 161:261–269. https://doi.org/10.1016/j.foodchem.2014.04.007
Honya M, Kinoshita T, Ishikawa M, Mori H, Nisizawa K (1994) Seasonal variation in the lipid content of cultured Laminaria japonica: fatty acids, sterols, β-carotene and tocopherol. J Appl Phycol 6:25–29. https://doi.org/10.1007/BF02185900
de Alencar DB, Diniz JC, Rocha SAS, dos Santos Pires-Cavalcante KM, Freitas JO, Nagano CS, Sampaio AH, Saker-Sampaio S (2017) Chemical composition of volatile compounds in two red seaweeds, Pterocladiella capillacea and Osmundaria obtusiloba, using static headspace gas chromatography mass spectrometry. J Appl Phycol 29(3):1571–1576. https://doi.org/10.1007/s10811-016-1020-3
Bruckner CG, Heesch S, Liland N et al (2017) In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res 26:240–249. https://doi.org/10.1016/j.algal.2017.08.001
Sun SM, Chung GH, Shin TS (2012) Volatile compounds of the green alga, Capsosiphon fulvescens. J Appl Phycol 24:1003–1013. https://doi.org/10.1007/s10811-011-9724-x
Le Pape M-A, Grua-Priol J, Prost C, Demaimay M (2004) Optimization of Dynamic Headspace Extraction of the Edible Red Algae Palmaria palmata and Identification of the Volatile Components. J Agric Food Chem 52:550–556. https://doi.org/10.1021/jf030478x
Tellez MR, Schrader KK, Kobaisy M (2001) Volatile components of the cyanobacterium Oscillatoria perornata (Skuja). J Agric Food Chem 49:5989–5992. https://doi.org/10.1021/jf010722p
Yamamoto M, Baldermann S, Yoshikawa K, Fujita A, Mase N, Watanabe N (2014) Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci World J 2014:1–8. https://doi.org/10.1155/2014/289780
Kakinuma M, Park CS, Amano H (2001) Distribution of free L-cysteine and glutathione in seaweeds. Fish Sci 67:194–196. https://doi.org/10.1046/j.1444-2906.2001.00223.x
Tenorio-Rodriguez PA, Murillo-Álvarez JI, Campa-Cordova ÁI, Angulo C (2017) Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. J Food Sci Technol 54:422–429. https://doi.org/10.1007/s13197-016-2478-3
Paiva L, Lima E, Isabel A, Marcone M, Baptista J (2016) Health-promoting ingredients from four selected Azorean macroalgae. Food Res Int 89:432–438. https://doi.org/10.1016/j.foodres.2016.08.007
Alves C, Pinteus S, Simões T, Horta A, Silva J, Tecelão C, Pedrosa R (2016) Bifurcaria bifurcata: a key macro-alga as a source of bioactive compounds and functional ingredients. Int J Food Sci Technol 51:1638–1646. https://doi.org/10.1111/ijfs.13135
Zubia M, Fabre MS, Kerjean V, Lann KL, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701. https://doi.org/10.1016/j.foodchem.2009.03.025
Fernandes F, Andrade PB, Ferreres F, Gil-Izquierdo A, Sousa-Pinto I, Valentão P (2017) The chemical composition on fingerprint of Glandora diffusa and its biological properties. Arab J Chem 10:583–595. https://doi.org/10.1016/j.arabjc.2015.01.012
Feller R, Matos ÂP, Mazzutti S, Moecke EHS, Tres MV, Derner RB, Oliveira JV, Junior AF (2017) Polyunsaturated Ω-3 and Ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO2 and subcritical n-butane. J Supercrit Fluids 133:437–443. https://doi.org/10.1016/j.supflu.2017.11.015
Guedes EAC, dos Santos Araújo MA, Souza AKP, de Souza LIO, de Barros LD, de Albuquerque Maranhão FC, Sant’Ana AEG (2012) Antifungal Activities of Different Extracts of Marine Macroalgae Against Dermatophytes and Candida Species. Mycopathologia. 174:223–232. https://doi.org/10.1007/s11046-012-9541-z
Pacheco BS, Dos Santos MAZ, Schultze E et al (2018) Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front Bioeng Biotechnol 6:185. https://doi.org/10.3389/fbioe.2018.00185
Cortés Y, Hormazábal E, Leal H, Urzúa A, Mutis A, Parra L, Quiroz A (2014) Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta:Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica agents that cause disease. Electron J Biotechnol 17(3):126–131. https://doi.org/10.1016/j.ejbt.2014.04.005
Shanmughapriya S, Manilal A, Sujith S, Selvin J, Kiran GS (2008) Antimicrobial activity of seaweeds extracts against multiresistant pathogens. Ann Microbiol 58:535–541
Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, Reddy CRK, Jha B (2011) Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol 23:797–810. https://doi.org/10.1007/s10811-010-9578-7
Berneira LM, da Silva CC, Passos LF, Mansilla A, dos Santos MAZ, de Pereira CMP (2021) Evaluation of volatile organic compounds in brown and red sub-Antarctic macroalgae. Braz J Bot 44:79–84. https://doi.org/10.1007/s40415-020-00684-7
Funding
We are grateful for the logistical support provided by the Brazilian Antarctic Program and for the financial support by the Office to Coordinate Improvement of University Personnel (CAPES—grant 99999.002378/2015-9), Rio Grande do Sul State Research Foundation (FAPERGS—grant 2010/50193-1), São Paulo State Research Foundation (FAPESP) and National Council for Scientific and Technological Development (CNPq—grant 407588/2013-2).
Author information
Authors and Affiliations
Contributions
Conceptualization: Dalila Venzke, Pio Colepicolo; Methodology: Ivandra S. de Santi; Formal analysis and investigation: Marco A. Z. ds Santos, Rodrigo de A. Vaucher; Writing (original draft preparation): Lucas M. Berneira; Writing (review and editing): Caroline C. da Silva; Funding acquisition: Claudio M. P. Pereira.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ernani Pinto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1343 kb)
Rights and permissions
About this article
Cite this article
Berneira, L.M., de Santi, I.I., da Silva, C.C. et al. Bioactivity and composition of lipophilic metabolites extracted from Antarctic macroalgae. Braz J Microbiol 52, 1275–1285 (2021). https://doi.org/10.1007/s42770-021-00475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00475-6