Abstract
Background and aim
Extensively drug-resistant (XDR) Klebsiella pneumoniae represent a major threat in intensive care units. The aim of the current study was to formulate a niosomal form of azithromycin (AZM) and to evaluate its in vitro effect on XDR K. pneumoniae as a single agent or in combination with levofloxacin.
Material and methods
Forty XDR K. pneumoniae isolates (23 colistin-sensitive and 17 colistin-resistant) were included in the study. Formulation and characterization of AZM niosomes were performed. The in vitro effect of AZM solution/niosomes alone and in combination (with levofloxacin) was investigated using the checkerboard assay, confirmed with time-kill assay and post-antibiotic effect (PAE).
Results
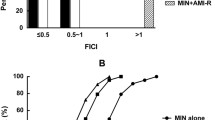
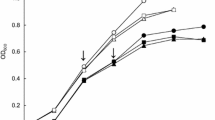
The AZM niosome mean minimal inhibitory concentration (MIC) (187.4 ± 209.1 μg/mL) was significantly lower than that of the AZM solution (342.5 ± 343.4 μg/mL). AZM niosomes/levofloxacin revealed a 40% synergistic effect compared to 20% with AZM solution/levofloxacin. No antagonistic effect was detected. The mean MIC values of both AZM niosomes and AZM solution were lower in the colistin-resistant group than in the colistin-sensitive group. The mean PAE time of AZM niosomes (2.3 ± 1.09 h) was statistically significantly longer than that of the AZM solution (1.37 ± 0.5 h) (p = 0.023).
Conclusion
AZM niosomes were proved to be more effective than AZM solution against XDR K. pneumoniae, even colistin-resistant isolates.





Similar content being viewed by others
Data availability (data transparency)
Available
References
Effah CY, Sun T, Liu S, Wu Y (2020) Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob 19(1):1. https://doi.org/10.1186/s12941-019-0343-8
Yeh YC, Huang TH, Yang SC, Chen CC, Fang JY (2020) Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front Chem 8:286. https://doi.org/10.3389/fchem.2020.00286
Patra JK, Das G, Fraceto LF, Campos E, Rodriguez-Torres M, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):71. https://doi.org/10.1186/s12951-018-0392-8
Ge X, Wei M, He S, Yuan WE (2019) Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 11(2):55. https://doi.org/10.3390/pharmaceutics11020055
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. http://www.eucast.org."
Tille P (2017) Bailey and Scott’s diagnostic microbiology. 14th. St Louis, Missouri, Mosby Elsevier
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Barakat HS, Kassem MA, El-Khordagui LK, Khalafallah NM (2014) Vancomycin-eluting niosomes: a new approach to the inhibition of staphylococcal biofilm on abiotic surfaces. AAPS PharmSciTech 15(5):1263–1274. https://doi.org/10.1208/s12249-014-0141-8
Manconi M, Sinico C, Valenti D, Lai F, Fadda AM (2006) Niosomes as carriers for tretinoin. III A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm 311(1–2):11–19. https://doi.org/10.1016/j.ijpharm.2005.11.045
Clinical and Laboratory Standards Institute (CLSI) (2012) Methods for dilution antimicrobial susceptibility tests f or bacteria that grow aerobically; Approved Standard—Ninth Edition. CLSI document M07-A9. Wayne: Clinical and Laboratory Standards Institute
Clinical and Laboratory Standards Institute (CLSI) (2020) Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100. Wayne, Pennsylvania, USA: Clinical and Laboratory Standards Institute
Schwalbe R, Steele-Moore L, Goodwin AC (2007) Antimicrobial susceptibility testing protocols. CRC Press, New York
Moody J (2007) Synergism testing: broth microdilution checkerboard and broth macrodilution methods. In: Garcia LS, Isenberg HD (eds) Clinical microbiology procedures handbook, 2nd edition update. ASM Press, Washington, DC, pp 5.12.1–5.12.23
Aaron SD, Ferris W, Henry DA, Speert DP, Macdonald NE (2000) Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am J Respir Crit Care Med 161(4Pt1):1206–1212. https://doi.org/10.1164/ajrccm.161.4.9907147
Lavigne JP, Bonnet R, Michaux-Charachon S, Jourdan J, Caillon J, Sotto A (2004) Post-antibiotic and post-beta-lactamase inhibitor effects of ceftazidime plus sulbactam on extended-spectrum beta-lactamase-producing Gram-negative bacteria. J Antimicrob Chemother 53(4):616–619. https://doi.org/10.1093/jac/dkh140
Chan Y (2003) Biostatistics 102: quantitative data–parametric & non-parametric tests. Blood Press 140(24.08):79
Ma YX, Wang CY, Li YY, Li J, Wan QQ, Chen JH, Tay FR, Niu LN (2019) Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv Sci (Weinh) 7(1):1901872. https://doi.org/10.1002/advs.201901872
Santimaleeworagun W, Thunyaharn S, Juntanawiwat P, Thongnoy N, Harindhanavudhi S, Nakeesathit S, Teschumroon S (2020) The prevalence of colistin-resistant Gram-negative bacteria isolated from hospitalized patients with bacteremia. J Appl Pharm Sci 10(02):056–059. https://doi.org/10.7324/JAPS.2020.102009
Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) (2015) Risk factors for bloodstream infections due to colistin-resistant KPC-producing klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21(12):1106.e1–1106.e8. https://doi.org/10.1016/j.cmi.2015.08.001
Ullah S, Shah MR, Shoaib M, Imran M, Shah SW, Ali I, Ahmed F (2017) Creatinine-based non-phospholipid vesicular carrier for improved oral bioavailability of azithromycin. Drug Dev Ind Pharm 43(6):1011–1022. https://doi.org/10.1080/03639045.2017.1291667
Gomes C, Martínez-Puchol S, Palma N, Horna G, Ruiz-Roldán L, Pons MJ, Ruiz J (2017) Macrolide resistance mechanisms in Enterobacteriaceae: focus on azithromycin. Crit Rev Microbiol 43(1):1–30. https://doi.org/10.3109/1040841X.2015.1136261
Eleraky NE, Allam A, Hassan SB, Omar MM (2020) Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics 12(2):142. https://doi.org/10.3390/pharmaceutics12020142
Khan S, Akhtar MU, Khan S, Javed F, Khan AA (2020) Nanoniosome-encapsulated levoflaxicin as an antibacterial agent against Brucella. J Basic Microbiol 60(3):281–290. https://doi.org/10.1002/jobm.201900454
Ghafelehbashi R, Akbarzadeh I, Tavakkoli Yaraki M, Lajevardi A, Fatemizadeh M, Heidarpoor Saremi L (2019) Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int J Pharm 569:118580. https://doi.org/10.1016/j.ijpharm.2019.118580
Saini H, Chhibber S, Harjai K (2015) Azithromycin and ciprofloxacin: a possible synergistic combination against Pseudomonas aeruginosa biofilm-associated urinary tract infections. Int J Antimicrob Agents 45(4):359–367. https://doi.org/10.1016/j.ijantimicag.2014.11.008
Saiman L, Chen Y, Gabriel PS, Knirsch C (2002) Synergistic activities of macrolide antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 46(4):1105–1107. https://doi.org/10.1128/aac.46.4.1105-1107.2002
Kolumbić Lakos A, Skerk V, Maleković G, Dujnić Spoljarević T, Kovacic D, Pasini M, Markotić A, Magri V, Perletti G (2011) A switch therapy protocol with intravenous azithromycin and ciprofloxacin combination for severe, relapsing chronic bacterial prostatitis: a prospective non-comparative pilot study. J Chemother 23(6):350–353. https://doi.org/10.1179/joc.2011.23.6.350
Magri V, Montanari E, Škerk V, Markotić A, Marras E, Restelli A, Naber KG, Perletti G (2011) Fluoroquinolone-macrolide combination therapy for chronic bacterial prostatitis: retrospective analysis of pathogen eradication rates, inflammatory findings and sexual dysfunction. Asian J Androl 13(6):819–827. https://doi.org/10.1038/aja.2011.36
Kumaraswamy M, Lin L, Olson J, Sun CF, Nonejuie P, Corriden R, Döhrmann S, Ali SR, Amaro D, Rohde M, Pogliano J, Sakoulas G, Nizet V (2016) Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother 71(5):1264–1269. https://doi.org/10.1093/jac/dkv487
Meerwein M, Tarnutzer A, Böni M, Van Bambeke F, Hombach M, Zinkernagel AS (2020) Increased azithromycin susceptibility of multidrug-resistant gram-negative bacteria on RPMI-1640 agar assessed by disk diffusion testing. Antibiotics (Basel) 9(5):E218. https://doi.org/10.3390/antibiotics9050218
Gordon NC, Png K, Wareham DW (2010) Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 54(12):5316–5322. https://doi.org/10.1128/AAC.00922-10
Vidaillac C, Benichou L, Duval RE (2012) In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 56(9):4856–4861. https://doi.org/10.1128/AAC.05996-11
Ulloa ER, Kousha A, Tsunemoto H, Pogliano J, Licitra C, LiPuma J, Sakoulas G, Nizet V, Kumaraswamy M (2020) Azithromycin exerts bactericidal activity and enhances innate immune mediated killing of MDR Achromobacter xylosoxidans. Infect Microb Dis 2(1):10–17. https://doi.org/10.1097/IM9.0000000000000014
Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr, Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V (2015) Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. EBioMedicine 2(7):690–698. https://doi.org/10.1016/j.ebiom.2015.05.021
Bremmer DN, Bauer KA, Pouch SM, Thomas K, Smith D, Goff DA, Pancholi P, Balada-Llasat JM (2016) Correlation of checkerboard synergy testing with time-kill analysis and clinical outcomes of extensively drug-resistant Acinetobacter baumannii respiratory infections. Antimicrob Agents Chemother 60(11):6892–6895. https://doi.org/10.1128/AAC.00981-16
Flamm RK, Rhomberg PR, Lindley JM, Sweeney K, Ellis-Grosse EJ, Shortridge D (2019) Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against gram-negative bacterial strains by using time-kill curves. Antimicrob Agents Chemother 63(5):e02549–e02518. https://doi.org/10.1128/AAC.02549-18
Mezzatesta ML, Caio C, Gona F, Zingali T, Salerno I, Stefani S (2016) Colistin increases the cidal activity of antibiotic combinations against multidrug-resistant Klebsiella pneumoniae: an in vitro model comparing multiple combination bactericidal testing at one peak serum concentration and time-kill method. Microb Drug Resist 22(5):360–363. https://doi.org/10.1089/mdr.2015.0160
Debbia EA, Molinari G, Paglia P, Schito GC (1990) Post-antibiotic effect of azithromycin on respiratory tract pathogens. Drugs Exp Clin Res 16(12):615–619
Author information
Authors and Affiliations
Contributions
MMB and HAE were responsible for the idea, study design, and revision of the manuscript. HSB was responsible for the formulation and characterization of niosomes. NSA was responsible for the data collection. HAO was responsible for the laboratory work, data analysis, and drafting of the manuscript. MAM was responsible for the idea, study design, laboratory work supervision, data analysis and interpretation, and writing of the manuscript. All authors contributed to the interpretation of data and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not required
Consent for publication
The author accepts the responsibility for releasing this material on behalf of any and all co-authors. This transfer of publication rights covers the nonexclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online), or any other reproductions of similar nature.
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability (software application or custom code)
Not applicable
Additional information
Responsible Editor: Luis Henrique Souza Guimaraes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Owais, H.M., Baddour, M.M., El-Metwally, H.A.ER. et al. Assessment of the in vitro activity of azithromycin niosomes alone and in combination with levofloxacin on extensively drug-resistant Klebsiella pneumoniae clinical isolates. Braz J Microbiol 52, 597–606 (2021). https://doi.org/10.1007/s42770-021-00433-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00433-2