Abstract
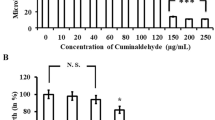
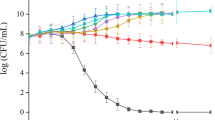
The combination of plant extract and antibiotic represents a template for developing of antibiofilm drugs. This study investigated the synergistic effects of pomegranate/rosemary/antibiotic combinations against antibiotic resistance and biofilm formation of Pseudomonas aeruginosa. The results showed that 17 (85%) of total P. aeruginosa isolates were biofilm producers; however, 5 (25%) isolates were demonstrated as a strong biofilm producer. The highest MIC level (1024 μg/ml) of tested antibiotics against strong biofilm producer isolates was observed with piperacillin, however the MIC ranges of ceftazidime, gentamycin, imipenem, and levofloxacin against these isolates were reached to (256–1024 μg/ml), (32–1024 μg/ml), (8–1024 μg/ml), and (8–512 μg/ml), respectively. PS-1 was the representative isolate for strong biofilm formation and high antibiotic resistance. 16S rRNA gene analysis suggested that PS-1 (accession No. MN619678) was identified as a strain of P. aeruginosa POA1. Pomegranate and rosemary extracts were the most effective extracts in biofilm inhibition, which significantly inhibited 91.93 and 90.83% of PS-1 biofilm, respectively. Notably, the synergism between both plant extracts and antibiotics has significantly reduced the MICs of used antibiotics at the level lower than the susceptibility breakpoints. Pomegranate/rosemary/antibiotic combinations achieved the highest biofilm eradication, which ranging from 90.0 to 99.6%, followed by the eradication ranges of pomegranate/rosemary combination, rosemary, and pomegranate extracts, which reached to (76.5–85.4%), (53.1–73.7%), and (41.2–71.5%), respectively. The findings suggest that pomegranate/rosemary/antibiotic combinations may be an effective therapeutic agent for antibiotic resistance and biofilm formation of P. aeruginosa.






Similar content being viewed by others
References
Olivares E, Badel-Berchoux S, Provot C, Prévost G, Bernardi T, Jehl F (2020) Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front Microbiol 10:2894. https://doi.org/10.3389/fmicb.2019.02894
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429. https://doi.org/10.1016/J.FOODCONT.2014.05.047
Karuppiah P, Mustaffa M (2013) Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pac J Trop Biomed 3(9):737–742. https://doi.org/10.1016/S2221-1691(13)60148-3
Lizana JA. Use of plant extracts to block bacterial biofilm formation. In: High School Students for Agricultural Science Research, Proceedings of the 3rd Congress PIIISA. (Olías, Raquel; Belver, Andrés; Sahrawy, Mariam; Serrato, Antonio Jesús; Cárdenas, Katiuska E.; Sandalio, Luisa M.; Rodríguez Serrano, María; Corpas, Francisco J.; Palma Martínez, José Manuel; Castro López AJ, ed.). CSIC - Estación Experimental del Zaidín (EEZ); 2013. http://digital.csic.es/handle/10261/99998. Accessed December 22, 2017
Roy R, Tiwari M, Donelli G, Tiwari V. (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action., 9:522, 554 https://doi.org/10.1080/21505594.2017.1313372
Cheesman MJ, Ilanko A, Blonk B, Cock IE (2017) Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn Rev 11(22):57–72. https://doi.org/10.4103/phrev.phrev_21_17
Kali A, Bhuvaneshwar D, Charles PMV, Seetha KS (2016) Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J basic Clin Pharm 7(3):93–96. https://doi.org/10.4103/0976-0105.183265
Yin H, Deng Y, Wang H, Liu W, Zhuang X, Chu W (2015) Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Sci Rep 5(1):16158. https://doi.org/10.1038/srep16158
Bassiri-Jahromi S (2018) Punica granatum (pomegranate) activity in health promotion and cancer prevention. Oncol Rev 12(1):1–7. https://doi.org/10.4081/oncol.2018.345
Mastrogiovanni F, Mukhopadhya A, Lacetera N et al (2019) Anti-inflammatory effects of pomegranate peel extracts on in vitro human intestinal caco-2 cells and ex vivo porcine colonic tissue explants. Nutrients 11(3). https://doi.org/10.3390/nu11030548
Pérez-Sánchez A, Barrajón-Catalán E, Ruiz-Torres V et al (2019) Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci Rep. 9(1). https://doi.org/10.1038/s41598-018-37173-7
O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. [6] Genetic approaches to study of biofilms Methods Enzymol 1999;310:91–109. doi:https://doi.org/10.1016/S0076-6879(99)10008-9
Stiefel P, Rosenberg U, Schneider J, Mauerhofer S, Maniura-Weber K, Ren Q (2016) Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl Microbiol Biotechnol 100(9):4135–4145. https://doi.org/10.1007/s00253-016-7396-9
Alcaráz LE, Satorres SE, Lucero RM, Puig De Centorbi ON (2003) Species identification, slime production and oxacillin susceptibility in coagulase-negative staphylococci isolated from nosocomial specimens. Brazilian J Microbiol. 34:45–51 https://pdfs.semanticscholar.org/0891/b942d8ff7367be36ec92dc602797ea36c318.pdf.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement Clinical and Laboratory Standards Institute. 2011;31(15). http://vchmedical.ajums.ac.ir/_vchmedical/documents/CLSI 2011.pdf. Accessed December 22, 2017
CLSI. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. CLSI. 1999;19(18):7. https://clsi.org/media/1462/m26a_sample.pdf. Accessed December 22, 2017
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard-Ninth Edition. CLSI document M07-A9. 2012;32(2):92
Kawsud P, Puripattanavong J, Teanpaisan R (2014) Screening for anticandidal and antibiofilm activity of some herbs in Thailand. Trop J Pharm Res 13(9):1495. https://doi.org/10.4314/tjpr.v13i9.16
Ivanova V, Stefova M, Chinnici F (2010) Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J Serb Chem Soc 7585285366324(6345). https://doi.org/10.2298/JSC1001045I
Goupy P, Hugues M, Boivin P, Amiot MJ (1999) Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric 79(12):1625–1634. https://doi.org/10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8
Abd El-Salam AE, Abd-El-Haleem D, Youssef AS, Zaki S, Abu-Elreesh G, El-Assar SA (2017) Isolation, characterization, optimization, immobilization and batch fermentation of bioflocculant produced by Bacillus aryabhattai strain PSK1. J Genet Eng Biotechnol 15:335–344. https://doi.org/10.1016/j.jgeb.2017.07.002
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. https://doi.org/10.1093/molbev/msr121
Manandhar S, Luitel S, Dahal RK (2019) In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria 2019. doi:https://doi.org/10.1155/2019/1895340, 1, 5
Sabaeifard P, Abdi-Ali A, Soudi MR, Dinarvand R (2014) Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J Microbiol Methods 105:134–140. https://doi.org/10.1016/j.mimet.2014.07.024
O’May C, Tufenkji N (2011) The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol 77(9):3061–3067. https://doi.org/10.1128/AEM.02677-10
White RL, Burgess DS, Manduru M, Bosso JA (1996) Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40(8):1914–1918 http://www.ncbi.nlm.nih.gov/pubmed/8843303. Accessed December 22, 2017
Ceylan O, Uğur A, Saraç N, Ozcan F, Baygar T (2014) The in vitro antibiofilm activity of Rosmarinus officinalis L essential oil against multiple antibiotic resistant Pseudomonas sp and Staphylococcus sp 12(3&4):82–86
Pang Z, Raudonis R, Glick BR, Lin T-J, Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37(1):177–192. https://doi.org/10.1016/j.biotechadv.2018.11.013
Li M-Y, Zhang J, Lu P, Xu J-L, Li S-P (2009) Evaluation of biological characteristics of bacteria contributing to biofilm formation. Pedosphere. 19(5):554–561. https://doi.org/10.1016/S1002-0160(09)60149-1
Neopane P, Nepal HP, Shrestha R, Uehara O, Abiko Y (2018) In vitro biofilm formation by <em>Staphylococcus aureus</em> isolated from wounds of hospital-admitted patients and their association with antimicrobial resistance. Int J Gen Med 11:25–32. https://doi.org/10.2147/IJGM.S153268
Perez LRR, Costa MCN, Freitas ALP, Barth AL (2011) Evaluation of biofilm production by Pseudomonas aeruginosa isolates recovered from cystic fibrosis and non-cystic fibrosis patients. Brazilian J Microbiol 42:476–479 http://www.scielo.br/pdf/bjm/v42n2/11.pdf.
Yayan J, Ghebremedhin B, Rasche K (2015) Antibiotic Resistance of Pseudomonas aeruginosa in pneumonia at a Single University Hospital Center in Germany over a 10-year period. Webber MA, ed. PLoS One 10(10):e0139836. https://doi.org/10.1371/journal.pone.0139836
Zhang Y, Xu D, Shi L, Cai R, Li C, Yan H (2018) Association between agr type, virulence factors, Biofilm formation and antibiotic resistance of Staphylococcus aureus isolates From Pork Production. Front Microbiol 9:1876. https://doi.org/10.3389/fmicb.2018.01876
Qi L, Li H, Zhang C, Liang B, Li J, Wang L, du X, Liu X, Qiu S, Song H (2016) Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol 7:483. https://doi.org/10.3389/fmicb.2016.00483
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet (London, England) 358(9276):135–138. https://doi.org/10.1016/s0140-6736(01)05321-1
Akinbobola AB, Sherry L, Mckay WG, Ramage G, Williams C (2017) Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J Hosp Infect 97(2):162–168. https://doi.org/10.1016/j.jhin.2017.06.024
Husain FM, Ahmad I, Khan MS, Ahmad E, Tahseen Q, Khan MS, Alshabib NA (2015) Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of gram-negative bacteria. Front Microbiol 6:420. https://doi.org/10.3389/fmicb.2015.00420
Romero CM, Vivacqua CG, Abdulhamid MB, Baigori MD, Slanis AC, Allori MCG, Tereschuk ML (2016) Biofilm inhibition activity of traditional medicinal plants from northwestern Argentina against native pathogen and environmental microorganisms. Rev Soc Bras Med Trop 49(6):703–712. https://doi.org/10.1590/0037-8682-0452-2016
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25(2):361–366. https://doi.org/10.1016/j.sjbs.2017.02.004
López D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2(7):a000398. https://doi.org/10.1101/cshperspect.a000398
Breidenstein EBM, de la Fuente-Núñez C, Hancock REW (2011) Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 19(8):419–426. https://doi.org/10.1016/j.tim.2011.04.005
Gloag ES, Turnbull L, Huang A, Vallotton P, Wang H, Nolan LM, Mililli L, Hunt C, Lu J, Osvath SR, Monahan LG, Cavaliere R, Charles IG, Wand MP, Gee ML, Prabhakar R, Whitchurch CB (2013) Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci U S A 110(28):11541–11546. https://doi.org/10.1073/pnas.1218898110
Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by Pseudomonas aeruginosa : a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int 2015:1–17. https://doi.org/10.1155/2015/759348
Gopu V, Kothandapani S, Shetty PH (2015) Quorum quenching activity of Syzygium cumini (L.) Skeels and its anthocyanin malvidin against Klebsiella pneumoniae. Microb Pathog 79:61–69. https://doi.org/10.1016/j.micpath.2015.01.010
Liu Q, Meng X, Li Y, Zhao C-N, Tang G-Y, Li H-B (2017) Antibacterial and antifungal activities of spices. Int J Mol Sci 18(6):1283. https://doi.org/10.3390/ijms18061283
Silva LN, Zimmer KR, Macedo AJ, Trentin DS (2016) Plant natural products targeting bacterial virulence factors. Chem Rev 116(16):9162–9236. https://doi.org/10.1021/acs.chemrev.6b00184
Defoirdt T, Pande GSJ, Baruah K, Bossier P (2013) The apparent quorum-sensing inhibitory activity of pyrogallol is a side effect of peroxide production. Antimicrob Agents Chemother 57(6):2870–2873. https://doi.org/10.1128/AAC.00401-13
Rudrappa T, Quinn WJ, Stanley-Wall NR, Bais HP (2007) A degradation product of the salicylic acid pathway triggers oxidative stress resulting in down-regulation of Bacillus subtilis biofilm formation on Arabidopsis thaliana roots. Planta. 226(2):283–297. https://doi.org/10.1007/s00425-007-0480-8
Plyuta V, Zaitseva J, Lobakova E, Zagoskina N, Kuznetsov A, Khmel I (2013) Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS. 121(11):1073–1081. https://doi.org/10.1111/apm.12083
Ta C, Freundorfer M, Mah T-F, Otárola-Rojas M, Garcia M, Sanchez-Vindas P, Poveda L, Maschek J, Baker B, Adonizio A, Downum K, Durst T, Arnason J (2014) Inhibition of bacterial quorum sensing and biofilm formation by extracts of neotropical rainforest plants. Planta Med 80(04):343–350. https://doi.org/10.1055/s-0033-1360337
Annapoorani A, Umamageswaran V, Parameswari R, Pandian SK, Ravi AV (2012) Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J Comput Aided Mol Des 26(9):1067–1077. https://doi.org/10.1007/s10822-012-9599-1
Nasri H, Bahmani M, Shahinfard N, Moradi Nafchi A, Saberianpour S, Rafieian KM (2015) Medicinal plants for the treatment of acne vulgaris: a review of recent evidences. Jundishapur J Microbiol 8(11):e25580. https://doi.org/10.5812/jjm.25580
Pramila DM, Xavier R, Marimuthu K et al (2012) Phytochemical analysis and antimicrobial potential of methanolic leaf extract of peppermint (Mentha piperita: Lamiaceae). J Med Plants Res 6(3):331–335. https://doi.org/10.5897/JMPR11.1232
Cheesman M, Ilanko A, Blonk B, Cock I (2017) Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn Rev 11(22):57–72. https://doi.org/10.4103/phrev.phrev_21_17
Luana Kamila A. Braga. Potentiation of in vitro antibiotic activity by Ocimum gratissimum L. African J Pharm Pharmacol. 2011;5(19). doi:https://doi.org/10.5897/AJPP11.414
Stefanović OD, Stanković MS, Čomić LR. In vitro antibacterial efficacy of Clinopodium vulgare L. extracts and their synergistic interaction with antibiotics. 2011. https://www.semanticscholar.org/paper/In-vitro-antibacterial-efficacy-of-Clinopodium-L.-Stefanović-Stanković/604bae6a4a7f305d3cd647fdd3fbc743c426641a.
Miklasińska-Majdanik M, Kępa M, Wojtyczka R, Idzik D, Wąsik T (2018) Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int J Environ Res Public Health 15(10):2321. https://doi.org/10.3390/ijerph15102321
Xie Y, Yang W, Tang F, Chen X, Ren L (2015) Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem 22(1):132–149 http://www.ncbi.nlm.nih.gov/pubmed/25245513.
Sanhueza L, Melo R, Montero R, Maisey K, Mendoza L, Wilkens M (2017) Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. Agbor G, ed. PLoS One 12(2):e0172273. https://doi.org/10.1371/journal.pone.0172273
Lima VN, Oliveira-Tintino CDM, Santos ES, Morais LP, Tintino SR, Freitas TS, Geraldo YS, Pereira RLS, Cruz RP, Menezes IRA, Coutinho HDM (2016) Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: gallic acid, caffeic acid and pyrogallol. Microb Pathog 99:56–61. https://doi.org/10.1016/j.micpath.2016.08.004
Amin MU, Khurram M, Khattak B, Khan J (2015) Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement Altern Med 15(1):59. https://doi.org/10.1186/s12906-015-0580-0
Fazly Bazzaz BS, Sarabandi S, Khameneh B, Hosseinzadeh H (2016) Effect of catechins, green tea extract and methylxanthines in combination with gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa: - combination therapy against resistant bacteria. Aust J Pharm 19(4):312–318. https://doi.org/10.3831/KPI.2016.19.032
Yi Z-B, Yu Y, Liang Y-Z, Bao Z (2007) Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J Pharm Biomed Anal 44(1):301–304. https://doi.org/10.1016/j.jpba.2007.02.018
Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR (2010) Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int J Biol Sci 6(6):556–568. https://doi.org/10.7150/ijbs.6.556
Chusri S, Villanueva I, Voravuthikunchai SP, Davies J (2009) Enhancing antibiotic activity: a strategy to control Acinetobacter infections. J Antimicrob Chemother 64(6):1203–1211. https://doi.org/10.1093/jac/dkp381
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editorial Responsibility: Luis Henrique Souza Guimaraes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abu El-Wafa, W.M., Ahmed, R.H. & Ramadan, M.AH. Synergistic effects of pomegranate and rosemary extracts in combination with antibiotics against antibiotic resistance and biofilm formation of Pseudomonas aeruginosa. Braz J Microbiol 51, 1079–1092 (2020). https://doi.org/10.1007/s42770-020-00284-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00284-3