Abstract
In some governorates of Egypt, the African migratory locust L. migratoria migratorioides is in a gregarious phase. Swarm development was successfully prevented by biological control agents. In this work, the two entomopathogenic nematode species, Steinernema sp. (SII)and Heterorhabditis bacteriophora (HP88) were investigated as natural enemies against the fifth nymph and adult African locusts at various concentrations (300, 600, 900, 1200, and 1500 Infected juveniles/100 g. soil). To ascertain fatal activity at 25 °C, the nematode-inoculated sand method was used. The two nematode species were semi-field administered against fifth nymphs and adult stages at (25 ± 2 °C) and 55–60% relative humidity with concentrations (3000, 6000, 9000, 12000, and 15000 Infected juveniles/kg. soil). Protease, amylase, invertase, and trehalase levels in the digestive enzymes of both fifth nymphs and adults fed with LC50 of both nematodes significantly decreased, but lipase and chitinase levels significantly increased. Adult locusts treated with the LC50 of S. sp. SII had basophilic epithelial cells, severe lumen hemorrhage, and highly aberrant proliferating cytoplasm, whereas the LC50 of H. bacteriophora HP88 displayed necrosis in an epithelial cell with vacuoles, loss of nucleus, and loss of goblet cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because they form large swarms that travel across nations, consume crops, and disrupt agriculture, locusts have long been dreaded (Lecoq and Cease 2022). A subspecies of the migrating locust is called Locusta migratoria migratorioides. An epidemic of L.migratoria migratorioides was reported in Angola in 2020, (Dcha 2020). It was present in Egypt's western desert and played a significant role in the large land reclamation initiatives of Sharq El-Owinat and Toshka (Soliman et al. 2019). The first line of defense against wild locust swarms, which can contain up to 8 million locusts, is chemical insecticides, although the majorities of them are non-specific and can affect both beneficial and harmless living things. The food chain has also been impacted by the repeated use of pesticides that are persistent and not biodegradable, which have damaged ecosystems in the water, air, and soil (Carriger et al. 2006; Gunstone et al. 2021). Acute or chronic illnesses can develop in people who have been exposed to pesticides, either directly or indirectly (Mostafalou and Abdollahi 2012). Additionally, it has an impact on both target and non-target creatures, including birds, fish, amphibians, humans, earthworms, predators, and pollinators (Gill and Garg 2014). However, the high costs of emergency response and rising understanding of the negative environmental effects of chemical control make biological control more appealing (Lomer et al. 2001; Zhang and Hunter 2017). Nematodes, protozoa, bacteria, fungi, and viruses are examples of biological agents (Rai et al. 2013; Deka et al. 2021). Except for Antarctica, entomopathogenic nematodes (EPNs) from the families Steinernematidae and Heterorhabditidae are widespread and employed to control soil-borne insect pests (Wright et al. 2005). Both Heterorhabditis bacteriophora (Heterorhabditidae) and Steinernema sp (Steinernematidae) may survive in a wide range of climatic situations, including grasslands, forests, and coasts, from hot regions to cold mountains (Bhat et al. 2020; van der Linden et al. 2022). The EPNs, S. sp. (SII), and H. bacteriophora (HP88) have been applied against many insect pests, such as red palm weevil Rhynchophorus ferrugineus (El Sadawy et al. 2020), the sand flies Phlebotomus papatasi (El Sadawy et al. 2020). The EPNs, Steinernema sp, and H. bacteriophora, have been used against a variety of insect pests, including the tea mosquito bug Helopeltis theory and bunch caterpillar, Andrea bipunctata (Amuri and Devi 2020), locusts and grasshopper (Ibrahim et al. 2018). The current work compares the effectiveness of two EPNs, S. sp. SII and H. bacteriophora HP88, against fifth nymphs and adults of the African migratory locust, L. migratoria migratorioides, in a lab setting at 25 o C and open air in semi-field conditions.
Methods
Locust rearing
Adult locusts, L. migratoria migratorioides (♂ and ♀), were cultured in a laboratory using samples taken from field-grown maize in the Abu Rawash town of Giza, Egypt. Locusts were raised for more than two generations in the gregarious phase in wooden cages (40 × 40 × 30 cm) at a density of (30–60) adults/cage. For the locusts to lay the eggs, each cage had a third of the sand. Instead of using artificial light, locusts were exposed to sunlight for more accurate findings (Hill and Taylor 1933). Every day, cages were cleaned. Depending on the season, fresh alfalfa (Medicago sativa) or corn (Zea mays) leaves were fed to the locusts.
Entomopathogenic nematode species
S. sp. SII and H. bacteriophora HP88, two EPNs, were acquired from the Department of Parasitology and Animal Diseases at the National Research Centre in Dokki, Egypt, and were raised for numerous generations in the Plant Protection Institute at the Agricultural Research Center. Infectious juveniles (IJs) of the two EPNs were housed in the last instar larvae of the larger wax moth Galleria mellonella.
Bioassay test
At the Department of Pest Physiology, Plant Protection Institute, ARC, Egypt, studies were conducted using doses of 300, 600, 900, 1200, and 1500 IJs/100 g. soil for two tested EPN species at 25 o C 60% for 7 days against fifth nymphs and adults of L. migratoria migratorioides. Nematode-inoculated Sand as a method: 100 g of sterilized, air-dried sandy soil (60 sand, 20 clay, and 20% silt) was combined with the aforementioned amounts of two applied EPNs in aerated plastic jars (8 × 10 × 10 cm), (Van Sambeek and Wiesner 1999). The soil's moisture content was preserved at 10% (v/w) by adding distilled water. Freshly cleaned maize leaves were fed to the locusts. Each nematode species' IJs were exposed to three adults or five nymphs /aerated plastic jars. A seven-day check was carried out to determine the fatality rate. Two nematode species, five concentrations, one species of locust, two instars, and three vessels/concentrations were employed in total for the testing. Nymphs and adults were kept under control with only water. The test was repeated in three trials over time using the two nematode species.
Semi-field experiment
The experiment's goal was to investigate the effects of EPNs SII and HP88 infection on fifth- and adult-stage African locusts in settings that were more like nature. To prevent external infestations and prevent the experimental locusts from escaping, all containers in this study were covered with netting material. In this study, 50 fifth instar nymphs or 30 adults / plastic containers (20 × 15 × 15 cm) containing 1 kg of moistened, sterile sand. 3000, 6000, 9000, 12000, and 15000 (IJs/ kg. soil) were the two EPN concentrations. Three replicates, fifty 5th nymphs or thirty adults, and either a concentration or control were employed. The experiment was conducted three times using the two nematode species. At a temperature of 25° C 2, a soil moisture content of 20%, and a relative humidity of 55–60%, all containers were placed near the field-grown corn in the Plant Protection Institute, Giza, Egypt, in September. After 7 days of treatment, the mortality rates of both the 5th nymphs and the adults of the L. migratoria migratorioides were recorded (Nouh 2022).
Reproduction of EPNs in L. migratoria migratorioides
The bioassay experiment produced dead locust cadavers, which were collected and stored in white traps (White 1927; Sobhy et al. 2020). A single adult or nymph locust was placed into each trap, which was subsequently incubated at 25 °C. The nearby water was used to introduce the newly emerged worms. These were taken out of the water and placed in storage bottles. It took up to 10 to 14 days for all of the EPNs to be harvested. Statistics were run on the nematode emergence data that were collected.
Digestive enzyme activities assayed for 5th nymphs and adult locusts
Fifth nymphs and adults of locusts were used as controls, and after being exposed for 72 h in semi-field plastic containers, they were given the LC50 doses of SII and HP88. After the locusts' wings and legs were removed, they were homogenized to create a sample solution and test the digestive enzymes' [protease, lipase, amylase, invertase, trehalase, and chitinase] activities by the procedures outlined by (Kreema et al. 2021), with a slight modification to the lipase activity outlined by (Singab et al. 2022). Each replication was taken from 30 locusts, both fifth nymphs and adults, and three replicates were analyzed.
Histopathological effects of the midgut of the adult locust
As controls, ten adult locusts were employed, and the same number of them received the LC50 concentrations of both species' EPNs. According to (Nasiruddin and Mordue 1993), insects were dissected in Ringer solution (pH 6.8), and the midgut was isolated from the rest of the gut, fixed in Bonn's fluid, and embedded in paraffin.
Statistics
The lethal activity of EPNs species was analyzed using (SPSS) version 27 software (SPSS Inc 2020), to determine the LC50, LC90, lower bound, and upper bound (95% confidence limits). P < 0.05 denoted a significant difference between groups when analyzing the reproduction rate of the tested EPNs species and the impact of EPNs on biochemical analyses.
Results
Lethal activity
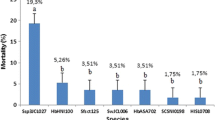
In Abu Rawash, Giza, Egypt, the destruction of greenery by locusts is represented in (Fig. 1a). The LC50 values for the fifth nymphs and adults, when SII was delivered via the nematode-inoculated sand approach, were (1201.953, 1279.473 IJs/100 g. soil), respectively, according to (Table 1). The LC50 values for the treatment with HP88 utilizing the nematode-inoculated sand method were (1960.94, 2071.085 IJs/100 g. soil) for the fifth nymphs and adults, respectively (Table 1), (Fig. 1b, c). The semi-field application of the EPN, SII indicated LC50 values for fifth nymphs and adults of (6695.01, 8123.9 IJs/kg soil), whereas the treatment with HP88 revealed LC50 values for fifth nymphs and adults of (10119.7, 12285.4 IJs/kg soil), respectively (Table 2). The treated deformed adults were shown in (Fig. 2a–c) as the fifth nymphs with the highest concentrations of SII and HP88 (15000 IJs/ kg).
a Severe damage to the green leaves of some plant leaves was affected by the African migratory locust, Locusta migratoria migratorioides, in Abu Rawash, Giza, Egypt. b Died fifth nymph of L. migratoria migratorioides treated with SII and HP88. c Died adult's ♀ and ♂ of L. migratoria migratorioides treated with SII and HP88
Reproduction of EPNs in L. migratoria migratorioides
The results of the reproductive experiment showed that every examined EPN species was able to enter and reproduce inside the hemocoel of adult locusts that were in their fifth instar (Figs. 3 and 4). For fifth-stage nymphs, there were significant variations in the production of IJs between the two strains (F = 13.96; d f = 2; P = 0.001), and for adults (F = 9.24; d f = 2; P = 0.004). The highest progeny output among the examined EPNs was noted for SII (78333 881.61, 200333 1201.47) for 5th nymph, adults, respectively. HP88 (32666 333.1, 95000 1154.7) was the next best progeny producer for 5th nymph, and adults, respectively. Additionally, for the two EPN isolates studied, the study found a good connection between mean reproduction rates and nematode concentrations (r = 0.998 in SII, r = 0.999 in HP88) for the 5th instar, (r = 0.989 in SII, r = 0.955 in HP88) for the adult stage.
Digestive enzyme activities assayed for 5th nymphs and adult locusts
The results of the digestive enzyme activities in 5th instar nymphs and adults of L. migratoria migratorioides treated with LC50 of the EPNs, S. sp. (SII), and H. bacteriophora (HP88) are shown in (Table 3). These results revealed a statistically significant decrease in protease activity in 5th instar nymphs (d f = 2, f = 165.82, P > 0.001), adults (d f = 2, f = 160.92, P > 0.001) in comparison to control 5th nymphs and adults. While 5th instar nymphs exposed to LC50 of the EPNs, (SII), (HP88) demonstrated a statistically significant increase in lipase activity in contrast to untreated adults (d f = 2, f = 102.15, P > 0.001). Amylase, invertase, and trehalase activities that hydrolyze carbohydrates exhibited a statistically significant decrease in 5th nymphs and adults as compared to the controls. The results of amylase activity in 5th instar nymphs were (d f = 2, f = 4200.41, P > 0.001), in invertase (d f = 2, f = 757, P > 0.001), and in trehalase (d f = 2, f = 4939.6, P > 0.001). Also, in adults, the amylase activity was (d f = 2, f = 35048.99, P > 0.001), in invertase (d f = 2, f = 85837.23, P > 0.001), and trehalase (d f = 2, f = 16869.29, P > 0.001). Finally, the chitinase activity showed a statistically significant increase in treated 5th nymphs (d f = 2, f = 67.65, P > 0.001), and adults (d f = 2, f = 297.37, P > 0.001).
Histopathological observations of the adult L. migratoria migratorioides treated with SII and HP88
In the present study, the histological characteristics of the midgut were explored. The basic cell type of the midgut in adult locusts is the goblet cell, which is located between the columnar epithelial cells and degenerative cells at the basement membrane (Fig. 5a). Numerous villi were observed, which were well supplied with blood vessels. Villi had apical microvilli that projected into the lumen. Goblet cells, interstitial glands, and columnar cells constitute the main bulk of the epithelium of the midgut (Fig. 5b). Large numbers of goblet cells were found, while the interstitial cells occupied the spaces between the goblet cells. In addition, longitudinal and circular muscles were present. After injection of SII, the epicuticle showed severe corrugation, and the epidermal layer was completely split. The epithelial cells showed basophilic, the lumen showed severe hemorrhage, and the cytoplasm showed severely abnormally proliferated (Fig. 6a, b). Injection with HP88 induced a thinning and corrugated cuticular surface with distortion of subcuticular layers. The musculature region showed a disorganized appearance. These changes included necrotic epithelial cells with vacuoles, loss of nuclei, and loss of goblet cells (Fig. 7a–c).
a L.S of the anterior part of control mid-gut adult L. migratoria migratorioides showed normally arranged epithelial cells (StainH&EX400). b L.S. of the posterior part of control mid-gut adult L. migratoria migratorioides showed normally arranged epithelial cells, normal regenerative cells (long arrows), and goblet cells (short arrow) (StainH&EX200)
a L.S of treated adult L. migratoria migratorioides midgut with LC50 of EPN S. sp. (SII) appears basophilic epithelial cells (long arrow) hemorrhage (star) with bacteria in the middle (short arrow) (StainH&EX400 b L.S of treated adult L. migratoria migratorioides midgut with LC50 of S. sp. (SII) showing hemorrhage in the lumen (long arrow) and severe abnormally proliferated and basophilic cytoplasm (short arrow) (StainH&EX400)
a L.S of treated adult L. migratoria migratorioides midgut with LC50 of H. bacteriophora (HP88) showed necrosis of epithelial cells with loss of nucleus (long arrow), loss of goblet cells (short arrow). (StainH&EX200) b L.S. of treated adult L. migratoria migratorioides midgut with LC50 of H. bacteriophora (HP88) showed severe necrosis of the epithelial cells (arrow). (StainH&EX400) c L.S of treated adult L. migratoria migratorioides midgut with LC50 of H. bacteriophora (HP88) showed degenerated and destructed epithelial cells with vacuoles (arrow). (StainH&EX200)
Discussion
The results of the current study showed that the EPNs S. sp. (SII) and H. bacteriophora (HP88) were effective against the fifth nymph and adult L. migratoria migratorioides. The H. bacteriophora (HP88) was less effective than the S. sp. (SII), which was also shown to be effective against the sand fly (El Sadawy et al. 2020). According to (Ahmed et al. 2014), H. bacteriophora appeared to have a higher deadly effect on Spodoptera littoralis, a cotton leaf worm, than S. riobraveand, S. feltiae (Noctuoidea: Lepidoptera). The effectiveness of EPN species against insect hosts varies depending on EPN traits (EPN species/stain, application methods, behavior, and type of bacteria hosted), and host traits (species, development, immune system, and molecules emitted by the host), additionally, the abiotic environment (temperature, humidity, UV radiation, soil characteristics, and chemicals) and the biotic environment (rhizosphere characteristics, molecules emitted by damaged roots, and natural enemies) are divided into two categories (Mráček et al. 2005; Labaude and Griffin 2018). The symbiotic bacterium Xenorhabdus nematophila, which is connected to the intravascular structure, was found in significant numbers of Steinernema sp. IJs. The insecticidal toxins it produced after being released into the host insect's hemolymph traveled to the connective tissues around the midgut, muscle fibers, and tracheae, where they caused harm and served as a source of nutrients for bacterial and nematode development. In addition, a significant portion of the H. bacteriophora IJs included the symbiotic bacterium Photorhabdus luminescens linked to the intestinal lumen. They moved to the region between the extracellular midgut epithelium after being discharged into the hemolymph of the host insect. There, they produced insecticidal toxins that damaged the insect tissues and turned them into a nutritional soup for bacteria and nematodes (Hinchliffe et al. 2010; Da Silva et al. 2020; Santhoshkumar et al. 2021). According to (Chapman and Chapman 1998), digestive enzymes are primarily generated and secreted by the midgut epithelium of insect alimentary ducts from the brush edge of microvilli on the apical membrane. Amylase and protease, two digesting enzymes, were also found in the salivary gland complex (Li et al. 2017). It depends on feeding habits, the quality, and quantity of the food consumed, as well as the specific midgut habitats, how the digestive enzymes in the gut are made is complex (and frequently species-specific) (Holtof et al. 2019). The digestive enzymes lipase, amylase, and protease, which metabolize sugars, lipids, cellulose, and proteins in the insect midgut, are crucial for the production of energy and the metabolism of insect nutrition (Gökkuş et al. 2016; Bonelli et al. 2020). The digestive enzymes in this study's locusts were protease, lipase, amylase, invertase, trehalase, and chitinase since they consumed maize leaves. Different levels of the evaluated digestive enzymes were found in treated locusts compared to control ones after treatment with the LC50 of both EPNs. Nymphal and adult-treated locusts showed a considerable reduction in protease activity. This is supported by numerous kinds of research on pests in general (Wang et al. 2012; Ibrahim, et al. 2015, 2018). The hydrolysis of triacylglycerol into its free fatty acids and glycerol backbone is one of the many reactions that lipase is in charge of (Yao et al. 2021). Because X. nematophila or P. luminescens bacteria secrete various secreted enzymes, such as hemolysis, lipases, and proteases that contribute to pathogenicity or nutrient acquisition for the bacterium and its nematode host, lipase activity was dramatically boosted (Richards and Goodrich-Blair 2010). Due to the toxic effects of the EPNs, S. sp. (SII), the H. bacteriophora (HP88), and their associated bacteria, the treatment of 5th nymph and adult locusts considerably reduced the levels of amylase, invertase, and trehalase when compared to the control (Muhammad et al. 2022). This was accepted (Ibrahim, et al. 2015; Tang et al. 2018). Chitin, a polymer of N-acetyl-D-glucosamine, is mostly produced by fungi, arthropods, and nematodes and makes up a significant portion of the insect cuticle. It supports the cuticles of the skin and trachea, the peritrophic matrices lining the gut epithelium, and insect development and morphogenesis in insects (Kramer and Muthukrishnan 1997; Merzendorfer and Zimoch 2003; Subbanna et al. 2018; Da Silva et al. 2020 and Henriques et al. 2020). Due to the presence of X. nematophila or P. luminescens bacteria, which create chitinase for their growth rate, the amount of chitinase was greatly enhanced in this study (Chen et al. 1996).
Data availability
Data is available.
References
Ahmed NF, Maklad AM, Yassin SA, Abolmaaty SM (2014) Biochemical effects of Steinernema feltiae, Steinernema riobrave, and Heterorhabditis bacteriophora on Spodoptera littoralis larvae. Egypt Acad J Biol Sci C Physiol Mol Biol 6(1):23–34. https://doi.org/10.21608/EAJBSC.2014.16044
Amuri B, Devi G (2020) Bio efficacy of Heterorhabditis bacteriophora and Oscheius chongmingensis against Helopeltis theivora and Andraca bipunctata. Int J Curr Microbiol Appl Sci 9(4):2460–2473. https://doi.org/10.20546/ijcmas.2020.904
Bhat AH, Chaubey AK, Askary TH (2020) Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt J Biol Pest Control 30(1):1–15. https://doi.org/10.1186/s41938-020-0212-y
Bonelli M, Bruno D, Brilli M, Gianfranceschi N, Tian L, Tettamanti G, Caccia S, Casartelli M (2020) Black soldier fly larvae adapt to different food substrates through morphological and functional responses of the midgut. Int J Mol Sci 21(14):4955. https://doi.org/10.3390/ijms21144955
Carriger JF, Rand GM, Gardinali PR, Perry WB, Tompkins MS, Fernandez AM (2006) Pesticides of potential ecological concern in sediment from South Florida canals: an ecological risk prioritization for aquatic arthropods. Soil Sediment Contam 15(1):21–45. https://doi.org/10.1080/15320380500363095
Chapman RF, Chapman RF (1998) The insects: structure and function. Cambridge University Press
Chen G, Zhang Y, Li J, Dunphy GB, Punja ZK, Webster JM (1996) Chitinase activity of xenorhabdus and photorhabdus species, bacterial associates of entomopathogenic nematodes. J Invertebr Pathol 68(2):101–108. https://doi.org/10.1006/JIPA.1996.0066
da Silva WJ, Pilz-Júnior HL, Heermann R, da Silva OS (2020) The great potential of entomopathogenic bacteria Xenorhabdus and Photorhabdus for mosquito control: A review. Parasit Vectors 13(1):1–14. https://doi.org/10.1186/S13071-020-04236-6/FIGURES/3328
Deka B, Baruah C, Babu A (2021) Entomopathogenic microorganisms: their role in insect pest management. Egypt J Biol Pest Control 31(1):1–8. https://doi.org/10.1186/S41938-021-00466-7
Dcha Y (2020) ETOP Bulletin for May 2020TYB. http://www.fao.org/ag/locusts/en/info/info/index.html
El Sadawy HA, Namaky AHE, Al Omari F, Bahareth OM (2020) Susceptibility of Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae) to entomopathogenic nematodes with regard to its immune response. Biol Control 148:104308. https://doi.org/10.1016/J.BIOCONTROL.2020.104308_338
El-Sadawy HA, Ramadan MY, Abdel Megeed KN, Ali HH, El Sattar SA, Elakabawy LM (2020) Biological control of Phlebotomus papatasi larvae by using entomopathogenic nematodes and its symbiotic bacterial toxins. Trop Biomed 37(2):288–302. PMID: 33612799
Gill HK, Garg H (2014) Pesticides: Environmental impacts and management strategies. Pesticides - Toxic Aspects. https://doi.org/10.5772/57399
Gökkuş A, Kahriman F, Alatürk F, Ali B (2016) Variation of nutritional values in leaves and stalks of different maize genotypes having high protein and high oil during vegetation. Agric Agric Sci Procedia 10:18–25. https://doi.org/10.1016/J.AASPRO.2016.09.004
Gunstone T, Cornelisse T, Klein K, Dubey A, Donley N (2021) Pesticides and soil invertebrates: A hazard assessment. Front Environ Sci 122. https://doi.org/10.3389/FENVS.2021.643847/BIBTEX
Henriques BS, Garcia ES, Azambuja P, Genta FA (2020) Determination of chitin content in insects: an alternate method based on calcofluor staining. Front Physiol 117. https://doi.org/10.3389/FPHYS.2020.00117/BIBTEX
Hill L, Taylor HJ (1933) Locusts in sunlight. Nature 132(3329):276–276. https://doi.org/10.1038/132276a0
Hinchliffe SJ, Hares MC, Dowling AJ (2010) Insecticidal toxins from the Photorhabdus and Xenorhabdus bacteria. Open Toxinol J 3(1). https://doi.org/10.1007/S00441-019-03031-9
Holtof M, Lenaerts C, Cullen D, Vanden Broeck J (2019) Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res 377(3):397–414. https://doi.org/10.1007/S00441-019-03031-9
Ibrahim E, Dobeš P, Kunc M, Hyršl P, Kodrík D (2018) Adipokinetic hormone and adenosine interfere with nematobacterial infection and locomotion in Drosophila melanogaster. J Insect Physiol 107:167–174. https://doi.org/10.1016/j.jinsphys.2018.04.002
Ibrahim SAM, Taha MA, Salem HHA, Farghaly DS (2015) Changes in enzyme activities in Agrotisipsilon (Lepidoptera, Noctuidae) as a response to entomopathogenic nematode infection. Int J Adv Res 3(5):111–118. http://www.journalijar.com
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27(11):887–900. https://doi.org/10.1016/S0965-1748(97)00078-7
Kreema A, Sorour HA, El-Metwally F, El-Hefny AA (2021) Egypt J Plant Protect Res Inst. Retrieved May 22, from www.ejppri.eg.net
Labaude S, Griffin CT (2018) Transmission success of entomopathogenic nematodes used in pest control. Insects 9(2):72. https://doi.org/10.3390/INSECTS9020072
Lecoq M, Cease A (2022) What have we learned after millennia of locust invasions? Agronomy 12(2):472. https://doi.org/10.3390/agronomy12020472
Li W, Zhao X, Yuan W, Wu K (2017) Activities of digestive enzymes in the omnivorous pest Apolygus lucorum (Hemiptera: Miridae). J Econ Entomol 110(1):101–110. https://doi.org/10.1093/JEE/TOW263
Lomer CJ, Bateman RP, Johnson DL, Langewald J, Thomas M (2001) Biological control of locusts and grasshoppers. Ann Rev Entomol 46(1):667–702. www.annualreviews.org
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206(24):4393–4412. https://doi.org/10.1242/JEB.00709
Muhammad J, Zeinab F, Saad M (2022) Entomopathogenic bacteria as natural enemy against the African migratory locust, (Reiche&Fairmaire, 1849) (Orthoptera: Acrididae). Egypt J Biol Pest Control 32:92. https://doi.org/10.1186/s41938-022-00592-w
Mostafalou S, Abdollahi M (2012) Concerns of environmental persistence of pesticides and human chronic diseases. Clin Exp Pharmacol 01(S5). https://doi.org/10.4172/2161-1459.s5-e002
Mráček Z, Bečvář S, Kindlmann P, Jersáková J (2005) Habitat preference for entomopathogenic nematodes, their insect hosts and new faunistic records for the Czech Republic. Biol Control 34(1):27–37. https://doi.org/10.1016/J.BIOCONTROL.2005.03.023
Nasiruddin M, Mordue AJ (1993) The effect of azadirachtin on the midgut histology of the locusts, Schistocerca gregaria and Locusta migratoria. Tissue Cell 25(6):875–884. https://doi.org/10.1016/0040-8166(93)90036-K
Nouh GM (2022) Effect of temperature and soil moisture on the efficacy of indigenous and imported strains of the entomopathogenic nematode, Heterorhabditis sp. against the black cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera/Noctuidae). Egypt J Biol Pest Control 32(1):1–7. https://doi.org/10.1186/s41938-022-00507-9
Rai MM, Gore DG, Rathod MK, Khurad AM (2013) Evidence of transovarial transmission of Bacillus subtilis in the silkworm, Bombyx mori L. J Pharm Res 7(4):318–323. https://doi.org/10.1016/J.JOPR.2013.03.022
Richards GR, Goodrich-Blair H (2010) Examination of Xenorhabdus nematophila lipases in pathogenic and mutualistic host interactions reveals a role for xlpA in nematode progeny production. Appl Environ Microbiol 76(1):221–229. https://doi.org/10.1128/AEM.01715-09
Santhoshkumar K, Mathur C, Mandal A, Dutta TK (2021) A toxin complex protein from Photorhabdus akhurstii conferred oral insecticidal activity against Galleria mellonella by targeting the midgut epithelium. Microbiol Res 242:126642. https://doi.org/10.1016/J.MICRES.2020.126642
Singab ANB, Mostafa NM, Elkhawas YA, Al-Sayed E, Bishr MM, Elissawy AM, Chang FR (2022) Cyclodepsipeptides: Isolation from endophytic fungi of Sarcophyton ehrenbergi and verification of their larvicidal activity via in-vitro and in-silico studies. Mar Drugs 20(5):331. https://doi.org/10.3390/MD20050331
Sobhy HM, Abdel-Bary NA, Harras FA, Faragalla FH, Husseinen HI (2020) Efficacy of entomopathogenic nematodes against Spodoptera littoralis (Boisd.) and Agrotis ipsilon (H.)(Lepidoptera: Noctuidae). Egypt J Biol Pest Control 30(1):1–8. https://doi.org/10.1186/S41938-020-00265-6/TABLES/4_422
Soliman M, Mohanna KM, Abdel-Fattah TT, Moustafa OR, El-Sheikh W (2019) Efficiency of agroecosystem compounds against the desert locust Schistocerca gregaria (Forskal) and the African migratory locust Locusta migratoria migratorioides (Reiche and Fairmaire). Sci J Agric Sci 1(1):30–37. https://doi.org/10.21608/sjas.2019.54196
Subbanna ARNS, Rajasekhara H, Stanley J, Mishra KK, Pattanayak A (2018) Pesticidal prospectives of chitinolytic bacteria in agricultural pest management. Soil Biol Biochem 116:52–66. https://doi.org/10.1016/J.SOILBIO.2017.09.019
SPSS Inc (2020) IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp
Tang B, Zhang L, Xiong X, Wang H, Wang S (2018) Advances in trehalose metabolism and its regulation of insect chitin synthesis. Sci Agric Sinica 51(4):697–707. https://doi.org/10.3864/J.ISSN.0578-1752.2018.04.009
van der Linden CF, Fatouros NE, Kammenga JE (2022) The potential of entomopathogenic nematodes to control moth pests of ornamental plantings. Biol Control 165:104815. https://doi.org/10.1016/J.BIOCONTROL.2021.104815
van Sambeek J, Wiesner A (1999) Successful parasitation of locusts by entomopathogenic nematodes is correlated with inhibition of insect phagocytes. J Invertebr Pathol 73(2):154–161. https://doi.org/10.1006/jipa.1998.4823
Wang QY, Nangong ZY, Yang J, Song P, Wang Y, Cui L, Cui L (2012) Toxic activity of a protein complex purified from Xenorhabdus nematophila HB310 to Plutella xylostella larvae. Insect Science 19(3):329–336. https://doi.org/10.1111/j.1744-7917.2011.01472.x
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66(1709):302–303. https://doi.org/10.1126/science.66.1709.302-a
Wright DJ, Peters A, Schroer S, Fife JP (2005) Application technology. Nematodes as Biocontrol Agents. (pp. 91–106). Wallingford UK: CABI Publishing. https://doi.org/10.1079/9780851990170.0091
Yao W, Liu K, Liu H, Jiang Y, Wang R, Wang W, Wang T (2021) A valuable product of microbial cell factories: Microbial lipase. Front Microbiol 12:743377. https://doi.org/10.3389/FMICB.2021.743377/BIBTEX_448
Zhang L, Hunter DM (2017) Management of locusts and grasshoppers in China. J Orthoptera Res 155–159. https://doi.org/10.3897/JOR.26.20119
Acknowledgements
The authors are grateful for the relentless efforts and support provided by Hanan El-Sadawy, Professor Researcher of Biological Control at the National Research Center, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors contributed equally in all parts of this study. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fathy, Z., El-Rahman, R.M.A. Effect of entomopathogenic nematodes Steinernema species (steinernematidae: rhabditida) and Heterorhabditis bacteriophora (heterorhabditidae: rhabditida) on the digestive enzymes and midgut histology of the African migratory locust Locusta migratoria migratorioides (acrididae: orthoptera). Int J Trop Insect Sci 43, 727–736 (2023). https://doi.org/10.1007/s42690-023-00979-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-00979-8