Abstract
Purpose
User involvement during medical device (MD) development and usability engineering techniques may help reduce serious adverse events due to human error during MD use. This paper reviews the scientific literature on MD usability and critically analyzes the MD design and development (MDDD) process.
Literature review
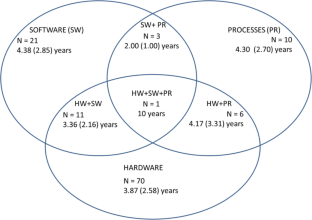
We searched 20 international databases for papers on usability and MDs. After applying exclusion criteria and removing duplicates, we analyzed 144 scientific papers regarding usability aspects and evaluated the target audience and study scope. Among hardware (HW), software (SW), and process (PR) evaluation methods, HW was the most evaluated (49% of papers), while the remainder analyzed HW + SW (15.2%), HW + PR (4.2%), and HW + SW + PR (0.7%). Task analysis, scenario simulation, and questionnaires were the most commonly used techniques (31.6%, 18.4%, and 12.8%, respectively). The target audiences were primarily patients/lay users (62%) and medical staff (14%). Gastroenterology (16.7%), nuclear medicine (13%), and nephrology/urology (9.3%) were the most referred specialties. We found that 48% of all papers did not mention any health facility or service analyzed, while 25.3% analyzed homecare services. Considering the usability scope, product evaluation (32%) and verification or validation trials (29%) were the most common.
Usability in MDDD
We present a brief review of the MDDD scenario and argue that better selection of usability methodologies in MDDD should be based around three factors: application of current technical standards on usability, usage of health technology assessment literature, consideration of ethics-related specificities of MD design.



Similar content being viewed by others
References
ABNT. Associação Brasileira de Normas Técnicas. ISO/IEC 9126–1: engenharia de software: qualidade de produto. Parte 1: modelo de qualidade. Rio de Janeiro: ABNT; 2003.
ABNT. Associação Brasileira de Normas Técnicas. ABNT NBR ISO 14971:2009: Produtos para a saúde — Aplicação de gerenciamento de risco a produtos para a saúde. Rio de Janeiro: ABNT; 2009.
ABNT. Associação Brasileira de Normas Técnicas. NBR ISO 9241-11: requisitos ergonômicos para o trabalho com dispositivos de interação visual. Parte 11: orientações sobre usabilidade. Rio de Janeiro: ABNT; 2011a.
ABNT. Associação Brasileira de Normas Técnicas. NBR ISO 9241-210: ergonomia da interação humano-sistema. Parte 210: projeto centrado no ser humano para sistemas interativos. Rio de Janeiro: ABNT; 2011b.
ABNT. Associação Brasileira de Normas Técnicas. ISO/TR 16982: ergonomia da interação humano-sistema: métodos de usabilidade que apoiam o projeto centrado no usuário. Rio de Janeiro: ABNT; 2014.
ABNT. Associação Brasileira de Normas Técnicas. ABNT NBR IEC 62366:2010 Emenda 1:2016 Produtos para a saúde - Aplicação da engenharia de usabilidade a produtos para a sáude. Rio de Janeiro: ABNT; 2016.
ANVISA. Manual de tecnovigilancia: abordagens de vigilância sanitária de produtos para a saúde comercializados no Brasil. Brasília: Ministério da Saúde; 2010.
ANVISA. Diretrizes metodológicas: elaboração de estudos para avaliação de equipamentos médicos assistenciais. Brasília: Ministério da Saúde; 2013a.
ANVISA. Relatório do Quantitativo de notificações/Número de notificações por mês de eventos adversos. Brasília: Ministério da Saúde; 2013b.
ANVISA. Resolução da Diretoria Colegiada – RDC n° 16, de 28 de março de 2013. Aprova o Regulamento Técnico de Boas Práticas de Fabricação de Produtos Médicos e Produtos para Diagnóstico de Uso In Vitro e dá outras providências. Brasília: Ministério da Saúde; 2013c.
ANVISA. Resolução da Diretoria Colegiada – RDC n° 15, de 20 de fevereiro de 2015. Dispõe sobre o regulamento para a realização de ensaios clínicos com dispositivos médicos no Brasil. Brasília: Ministério da Saúde; 2015.
Auer A, Jarmai K. Implementing responsible research and innovation practices in SMEs: insights into drivers and barriers from the Austrian medical device sector. Sustainability. 2017;10(1):17–35.
Bellido D, Leon A, Manas M, Marchan E, Esquinas G, Ros J. Adverse events in an internal medicine: a prospective study. Rev Calid Asist. 2017. https://doi.org/10.1016/j.cali.2017.02.003.
BRASIL. Diretrizes metodológicas: elaboração de pareceres técnico-científicos. Brasília: Ministério da Saúde; 2014a.
BRASIL. Documento de referência para o Programa Nacional de Segurança do Paciente. Brasília: Fundação Oswaldo Cruz; 2014b.
Brown A, Dixon D, Eatock J, Meenan B, Young T. A survey of success factors in new product development in the medical devices industry. IEMCE. 2008. https://doi.org/10.1109/IEMCE.2008.4617987.
De Falco I, Tortora G, Dario P, Menciassi A. An integrated system for wireless capsule endoscopy in a liquid-distended stomach. IEEE Trans Biomed Eng. 2014. https://doi.org/10.1109/tbme.2013.2290018.
Doorn N, Fahlquist J. Responsibility in engineering: toward a new role for engineering ethicists. Bull Sci Technol Soc. 2010;30(3):222–30.
Dreyer M, Chefneux L, Goldberg A, von Heimburg J, Patrignani N, Schofield M, et al. Responsible innovation: a complementary view from industry with proposals for bridging different perspectives. Sustainability. 2017. https://doi.org/10.3390/su9101719.
Eindhoven D, Borleffs C, Dietz M, Schalij M, Brouwers C, de Bruijne M. Design and reliability of a specific instrument to evaluate patient safety for patients with acute myocardial infarction treated in a predefined care track: a retrospective patient record review study in a single tertiary hospital in the Netherlands. BMJ Open. 2017. https://doi.org/10.1136/bmjopen-2016-014360.
EngelbergCenter. Biomedical innovation: identifying challenges and prioritizing needs for medical device innovation. In: Engelberg Center for Health Care Reform. 2014. https://www.brookings.edu/events/biomedical-innovation-identifying-challenges-and-prioritizing-needs-in-medical-device-research-and-development/. Acessed 15 Dec 2018.
FDA. FDA Adverse Event Reporting System (FAERS) Statistics. 2014. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed 10 Nov 2014.
FDA. Framework for FDA’s Real-World Evidence Program. 2018. https://www.fda.gov/media/download&usg=AOvVaw0EVMNjhhH4ZQjqw1bLX3dv. Acessed 12 Apr 2019.
Flewwelling C, Easty A, Vicente K, Cafazzo J. The use of fault reporting of medical equipment to identify latent design flaws. J Biomed Inform. 2014. https://doi.org/10.1016/j.jbi.2014.04.009.
Freund Y, Goulet H, Leblanc J, Bokobza J, Ray P, Maignan M, et al. Effect of systematic physician cross-checking on reducing adverse events in the emergency department: the CHARMED cluster randomized trial. JAMA Intern Med. 2018;178(6):812–9.
Fries R. Reliable design of medical devices. New York: CRC Press; 2006.
Fries J, Spitz P, Kraines R, Holman H. Measurement of patient outcome in arthritis. Arthritis Rheum. 2005;23:137–45.
Fujita S, Iida S, Nagai Y, Shimamori Y, Koyano K, Moriyama Y, et al. Estimation of the number of patient deaths recognized by a medical practitioner as caused by adverse events in hospitals in Japan: a cross-sectional study. Medicine (Baltimore). 2017. https://doi.org/10.1097/md.0000000000008128.
Gurses A, Onzpk A, Pronovost P. Time to accelerate integration of human factors and ergonomics in patient safety. BMJ Qual Saf. 2011;21:347–51.
Hani S, Marcellis-Warin N. Open innovation and involvement of end-users in the medical device technologies design & development process: end-users perspectives. Technol Invest. 2016;7(3):73–85.
Hofmann B. Medicalization and overdiagnosis: different but alike. Med Health Care Philos. 2016;19(2):253–64.
Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Health Econ Health Policy. 2011;9(5):331–347. https://doi.org/10.2165/11593380-000000000-00000.
Jolly J, Hildebrand E, Branaghan R. Better instructions for use to improve reusable medical equipment (RME) sterility. Hum Factors. 2013;55(2):397–410.
Kangas M, Konttila A, Lindgren P, Winblad I, Jamsa T. Comparison of low-complexity fall detection algorithms for body attached accelerometers. Gait Posture. 2008;28(2):285–91.
L’Astorina A, Fiore MD. A new bet for scientists? Implementing the Responsible Research and Innovation (RRI) approach in the practices of research institutions. Relations. 2017. https://doi.org/10.7358/rela-2017-002-last.
Landman AB, Redden L, Neri P, Poole S, Horsky J, Raja AS, et al. Using a medical simulation center as an electronic health record usability laboratory. J Am Med Inform Assoc. 2014;21(3):558–63.
Lehoux P, Miller F, Hivon M, Demers-Payette O, Urbach D. Clinicians as health technology designers: two contrasting tales about user involvement in innovation development. Health Policy Technol. 2013;2:122–30.
Leite C, Reis C, Binsfeld P, Rosa S. Novas Tecnologias Aplicada à Saúde: Desenvolvimento de Sistemas Dinâmicos – Conceitos, Aplicações e Utilização de Técnicas Inteligentes. Mossoró-RN:EDUERN; 2019.
Lynch R, Farrington C. Quantified lives and vital data. Exploring health and technology through personal medical devices. London: Palgrave Macmillan; 2018.
Markiewicz K, Til J, IJzerman M. Medical devices early assessment methods: systematic literature review. Int J Technol Assess Health Care. 2014;30(2):137–46.
Markiewicz K, Til J, IJzerman M. Early assessment of medical devices in development for company decision making: an exploration of best practices. J Commer Biotechnol. 2017. https://doi.org/10.5912/jcb780.
Martin JL, Clark DJ, Morgan SP, Crowe JA, Murphy E. A user-centred approach to requirements elicitation in medical device development: a case study from an industry perspective. Appl Ergon. 2012;43(1):184–90.
Matsumoto K, Nagahara A, Ueyama H, Konuma H, Morimoto T, Sasaki H, et al. Development and clinical usability of a new traction device “medical ring” for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2013;27:3444–51. https://doi.org/10.1007/s00464-013-2887-6.
McCrory B, Lowndes BR, Lagrange CA, Miller EE, Hallbeck MS. Comparative usability testing of conventional and single incision laparoscopic surgery devices. Hum Factors. 2013;55(3):619–31.
Mendes W, Pavao ALB, Martins M, Travassos C. The application of Iberoamerican study of adverse events (IBEAS) methodology in Brazilian hospitals. Int J Qual Health Care. 2018;30(6):480–5.
Money A, Barnett J, Kuljis J, Craven M, Martin J, Young T. The role of the user within the medical device design and development process: medical device manufacturers’ perspectives. BMC Med Inform Decis Mak. 2011. https://doi.org/10.1186/1472-6947-11-15.
Santos ICT, Gazelle GS, Rocha LA, Tavares JMRS. Modeling of the medical device development process. Expert Rev Med Devices. 2012;9(5):537–43.
Sarah S, Jennifer M, Alexandra L, Michael C, Sonja ON, Julie B. Medical device design in context: a model of user–device interaction and consequences. Displays. 2012. https://doi.org/10.1016/j.displa.2011.12.001.
Shah SGS, Robinson I. Benefits of and barriers to involving users in medical device technology development and evaluation. Int J Technol Assess Health Care. 2007;23(1):131–7.
Tarricone R, Torbica A, Drummond M. Challenges in the assessment of medical devices: the MedtecHTA project. Health Econ. 2017a. https://doi.org/10.1002/hec.3469.
Tarricone R, Torbica A, Drummond M. Key recommendations from the MedtecHTA project. Health Econ. 2017b. https://doi.org/10.1002/hec.3468.
Turchetti G, Spadoni E, Geisler E. Health technology assessment. IEEE Eng Med Biol Mag. 2010;29(3):70–6.
Van de Poel I, Asveld L, Flipse S, Klaassen P, Scholten V, Yaghmaei E. Company strategies for Responsible Research and Innovation (RRI): a conceptual model. Sustainability. 2017;9(11):2045.
Vincent C, Li Y, Blandford A. Integration of human factors and ergonomics during medical device design and development: It’s all about communication. Appl Ergon. 2014;45:413–9.
Walton M, Harrison R, Kelly P, Smith-Merry J, Manias E, Jorm C, et al. Patients reports of adverse events: a data linkage study of Australian adults aged 45 years and over. BMJ Qual Saf. 2017;26(9):743–50.
WHO. Health technology assessment of medical devices, Geneva. 2012.
Wiklund M, Kendler J, Strochlic A. Usability testing of medical devices. 2a ed. Boca Ration: CRC Press; 2011.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roma, M.S.G., de Vilhena Garcia, E. Medical device usability: literature review, current status, and challenges. Res. Biomed. Eng. 36, 163–170 (2020). https://doi.org/10.1007/s42600-019-00037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-019-00037-8




