Abstract
The decision of surgical intervention for an aortic aneurysm is usually associated with an assessment of risk of its rupture. Global rupture risk assessment parameters like wall diameter and growth of the aneurysm over time often fail at predicting the risk of rupture with accuracy. This paper will investigate the hypothesis that the tissue’s microstructure determines its macroscopic failure. To this aim, two different testing protocols have been implemented. Human ascending thoracic aortic aneurysm (ATAA) tissue samples were subjected to bulge-inflation testing until rupture coupled with multi-photon microscopy (MPM) imaging. Image stacks of the sample were acquired at different pressure levels. Additionally, porcine aorta samples were tested under uniaxial tension until failure and their response was recorded. Prior to mechanical testing, MPM image stacks were acquired at four different zones on the sample. The image stacks acquired at the load free state were used to extract morphological information relating to collagen fibers. Then, an inverse random sampling approach was used to generate pseudomorphological parameters for network reconstruction. A discrete model of the collagen network signifying its stochastic nature was then developed, including both prefailure and post-failure mechanics. The model was able to replicate the mechanical response and failure of the tissue, and demonstrated that fiber-based damage can strongly shape the macroscopic failure response of the tissue. Identified values of collagen fiber failure strain were in the range of 8.8 to 29.3% in the case of aneurysmal samples, and 18.7 to 25.5% in the case of porcine samples. A statistical analysis enabled the characterization of correlation between fiber morphology and tissue failure. The model may serve as a useful tool for predicting macroscale failure of the aortic wall based on the variations in microscale morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
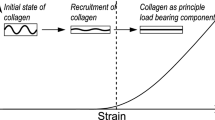
Many characteristic changes in aneurysmal aortas can be readily distinguished from healthy tissues. In terms of biomechanical behavior, tissues affected by aneurysms exhibit lower tensile strengths as compared to control specimens, along with an increase in stiffness[59]. In terms of microstructural changes, aneurysmal tissues show remarkable changes in organization [39]. This shows that aortic aneurysms are complex phenomena involving several constituents and processes, which is why the existing rupture risk indicators such as the maximum diameter or the growth rate tend to fail [26]. Furthermore, the abovementioned biomechanical and microstructural changes in the aneurysmal aortas are highly heterogeneous, leading to localized abnormalities. Hence, a better understanding of failure mechanics of the arterial tissue at the scale of its structural constituents is imperative for characterizing aortic rupture.
Uniaxial tensile testing is the most commonly used method for in vitro testing of soft tissues. Several researchers performed uniaxial tension tests on excised aneurysmal tissue samples in circumferential, longitudinal, and arbitrary (close to diagonal) directions to assess their failure properties [25, 28, 59]. A majority of these reports concluded that the aneurysmal arterial wall is slightly stronger in the circumferential direction [16, 35]. Similarly, bulge inflation testing allows for the investigation of mechanical behavior, simultaneously allowing the investigation of rupture. Bulge-inflation testing of porcine thoracic aortic samples revealed a mean failure strength of \(1.75 \pm 0.71\) MPa, with a mean failure stretch of \(1.523 \pm 0.178\) [33]. For aneurysmal tissues, the mean failure strength was reported to be just 0.94 MPa [12], with the failure strength showing no correlation with diameter of the aneurysm. The abovementioned experimental investigations reveal interesting aspects on the mechanics of the tissue, although the mechanical state of the micro-structural constituents cannot be inferred. Currently, we sternly believe that only numerical simulations could help in determining this crucial information.
Structurally motivated continuum models incorporate the contributions of individual microstructural constituents in their strain energy function. They have been employed to represent the elastic behavior [18] and later extended to model failure behavior of arterial walls [49]. From the results of these models, it was concluded that arterial wall rupture strongly depends on the strength of its fibers. In the continuum models, the state of damage of collagen fibers is defined by an internal scalar variable. Commonly used functions for the evolution of the damage variable include exponential [31, 57], sigmoidal [42], and polynomial form [43]. They fall into the homogenization modeling category and possess several advantages, including straightforward finite element formulations. They often provide an accurate representation of the tissue’s macroscopic mechanical state. The continuum damage models also capture tissue’s failure phenomena, but in most cases have data only corresponding to in vitro uniaxial tests. However, these models do not reveal any information on the mechanical state of the microstructural constituents such as their stress and strain profiles giving no regard to modeling the morphology and mechanics of the tissue in a multiscale context.
Stochastic fiber networks such as the ones found in arterial tissue, polymers, carbon nanotubes, and collagen gels have been investigated by directly incorporating their geometry in the model instead of homogenizing their contribution in the strain energy function [52, 62]. These models enable the investigation of multiscale mechanical processes leading up to damage initiation and failure of the tissue. They have been extensively implemented to study the failure properties of random fiber networks under tensile loading [20, 21]. More recently, this methodology has been extended to study the failure of lamellar unit of the aortic media [54] and the whole arterial wall [60]. Although they show good agreement with experimental observations, some limitations should be addressed. For the former, an affine deformation was assumed for the fibers which was shown not to be the case for network densities of such magnitude [44]. For the latter, the microstructure organization of collagen fibers was assumed to be a fully random process and not a result of microstructural investigation which further leads to a non-physiological rupture stretch value for collagen fibers. Nonetheless, [54, 60] do reinforce the hypothesis that tissue level failure is directly related to failure at the scale of fibrous microstructure.
In the present work, our main objective is to assess the applicability of a discrete model of collagen fibers including a failure mechanism in predicting tissue-level failure. This would further enhance the understanding of the influence of microstructural constituents on the arterial wall’s mechanical response and explore the applicability of microstructurally motivated biomechanical indicators to predict rupture. In order to accomplish this goal, collagen fiber network models were numerically generated from collagen fiber architecture experimentally obtained from second harmonic generation (SHG) images. Both uniaxial and biaxial loadings were analyzed, using experiments performed respectively on porcine healthy and ATAA tissues. The rest of the article is organized as follows: first, an overview of the experimental and numerical methods’ utilization is presented; later, the results of these analyses are presented; following which, discussions, challenges, and limitations of the current work are presented.
2 Materials and methods
2.1 Sample preparation
Two different types of aortic tissue specimens were utilized in this study.
-
Healthy porcine aortas (\(n = 4\)) were procured from a local butchery. The descending thoracic part of the aortas was dissected and the surrounding connective tissue was removed carefully. A cylindrical portion of length 5 cm was then excised and cut open along the longitudinal direction to be used for experimental testing. The medial layer of the aorta was peeled off with caution resulting in adventitial strips. Dog-bone shaped samples (length: \(23.0 \pm 0.9524\) mm; width: \(7.5\pm 0.1\) mm; thickness: \(0.270 \pm 0.075\) mm) were cut from the adventitial strips (4 circumferential and 2 longitudinal) and frozen until the day of the experiment [22, 30]. These samples were tested under uniaxial tensile loading.
-
Human ascending thoracic aneurysmal aortic (ATAA) tissue (\(n = 6\)) were procured from patients undergoing elective surgery to replace the diseased section with a graft. The surrounding connective tissue was removed and the diameter of the aorta was measured at multiple zones using a 0–150-mm range digital caliper with an accuracy of \(\pm 0.05\) mm. Following that the aortas were cut open longitudinally and 45-mm square samples with edges parallel to longitudinal and circumferential directions were excised. For human ATAA specimens, samples were collected from consenting patients undergoing surgical replacement following a protocol approved by the Institutional Review Board of the University Hospital Center of Saint Etienne, Saint Etienne, France. These samples were tested under bulge inflation loading, already presented in a previous paper [6].
2.1.1 Micro-structural imaging
Two different multi-photon microscopy setups were used in this study to image the collagen networks using their second harmonic generation (SHG) signal.
Porcine samples: Second harmonic generation (SHG) microscopy of collagen fibers was performed with a microscope consisting of a Biorad (MRC1024) scan head and an Olympus BX50WI upright microscope stand. An 810-nm excitation beam from a mode-locked femtosecond laser delivering up to 3W of power (COHERENT, CHAMALEON VISION II) was focused into the sample using a \(\times\)20 water immersion objective of NA = 1 (XLUMPLFN 20XW, Olympus). The samples were scanned in the x-y plane to acquire \(512 \times 512\) \(\mu m^{2}\) image window. The z-scan was realized by translation of the motorized objective. The incident laser intensity was varied by using a rotating half-wave plate and a polarizer in front of the microscope such that the average power delivered at the surface ranged from 10 to 100 mW. The SHG signal was collected in transmission through a 710-nm short-pass filter and a 405-nm interference filter by a photo-multiplier tube. Four different zones were imaged for each sample morphology in the tissue. While this was not based on any existing protocol, it helped us to have a better description of the microstructure morphology.
Human ascending thoracic aortic aneurysmal (ATAA) samples: A LEICA TCS SP2 upright confocal microscope equipped with a water immersion objective (HCX APO L UVI \(\times\) 20 NA0.5) was used to image human ATAA samples at zero pressure. The laser source was a Ti:sapphire femtosecond laser Chameleon Vision I from COHERENT, Inc. By setting the excitation wavelength 830 nm collagen second harmonic generation (SHG) signal was collected through a 375–425-nm band-pass filter. An imaging window of \(750 \times 750\) \(\mu\)m\(^{2}\) was set in the axial-circumferential plane with the depth of scan varying between 100 and 500 \(\mu\)m. SHG image stacks were recorded for each bulge inflation sample at three pressure levels until the point of rupture: zero pressure, physiological pressure (\(\approx 250\) mmHg), supra-physiological pressure (\(\approx 450\) mmHg). A representative SHG stack of a porcine and a human ATAA tissue is presented in Fig. 1.
2.1.2 Uniaxial testing
Uniaxial tensile tests until failure were performed on samples excised from porcine aortas after SHG imaging. On the day of testing, the samples were unfrozen by placing them in a saline solution for 15–30 min. The samples were then mounted onto the uniaxial tensile test apparatus while being submerged in saline solution throughout the test. The length (clamp to clamp) and width of each excised sample were measured with a vernier caliper with a precision of 0.01 mm. The thickness profile of each sample was measured with the help of two high spatial resolution line laser sensors (optoNCDT 1700BL, Micro-Epsilon Messtechnik GmbH & Co. KG, Germany) [6]. The average values of length, width, and thickness of each sample are presented in Table 1. The sample preparation workflow is presented in Fig. 2. A screw-driven high-precision tensile machine (Newport®, tension-compression stage) with a 22-N capacity load cell and a precision of 0.01 N was used to perform the test. In order to characterize the mechanical response of arterial tissue, it is important to account for the existence of a transient mechanical response: after a long-term resting of the tissue (absence of externally applied load), its mechanical response exhibits an important but transient hysteresis which is reduced after several load cycles. This is usually rid of by performing several preconditioning (loading–unloading) cycles. To ensure repeatability, a preconditioning test involving 5 loading and unloading cycles of the sample up to 1.30 stretch was conducted on the sample prior to testing, as this is sufficient to generate repeatable data [8, 40]. Following that, each sample underwent a quasi-static loading under displacement control until failure at a rate of 2 mm/min and the force-displacement data were recorded. The thickness and width of the samples enabled the computation of 1st Piola-Kirchhoff stress as \(\sigma\) = \(\frac{F}{A_{0}}\) for each sample, where F is the recorded force value and \(A_{0}\) is the initial cross-section area. The stretch was computed from the actual and reference inter-clamp lengths of the sample (l and \(l_0\) respectively) undergoing uniaxial tension as: \(\lambda\) = \(\frac{l}{l_0}\). The reference length of the strip was defined as the inter-clamp length of the strip after preconditioning. With this information, the recorded force-displacement data was then converted to 1st Piola-Kirchoff stress versus Green’s strain thus generating stress-strain curves for each sample.
2.1.3 Bulge-inflation testing
Bulge inflation testing until failure was performed on samples excised from human ATAA tissues using a protocol already presented in [6]. Two circular PVC supports of 30 mm each were used to constrain the samples with the adventitial side facing up. The sample was immersed in PBS (phosphate buffered saline) throughout the duration of the test. An automatic water pumping device (WPI®, NE-501 Multi-Phaser) coupled with a program developed in LabVIEW [3] was used to control the water volume injected on the intimal side, which was kept at a constant rate of 2 ml/min. The inflation was stopped by keeping the water volume constant when desired levels of pressure were attained for image acquisition. Furthermore, the pressure values were recorded simultaneously with a pressure transducer (Omega®) along with the corresponding radius of the inflated sample. For more detailed information on the experimental protocol, the reader is directed to a previously published work [5].
2.2 Numerical methods
2.2.1 Structural model of the tunica adventitia
We developed a structural model of the tunica adventitia of each experimentally tested sample by incorporating its collagen network organization [2]. The steps involved in reconstructing the collagen network from microstructural images are briefly described below:
Morphology estimation: The technique employed to extract relevant morphological parameters from multi-photon image stacks of collagen is similar to that described in [46]. The obtained image stacks were analyzed using an FFT analysis and the resulting orientation (axial-circumferential plane) intensity profile was recorded. For the case of the porcine samples, an average of 4 image stacks was taken as the representative orientation intensity. The resulting intensity of planar orientations was quantified using a finite mixture of von Mises distributions. Collagen fibers are undulated in their load-free configuration. These undulations can be computed on the images as the ratio of the actual length of the fiber to its end to end length. From a hierarchical standpoint, the network modelled includes collagen fibers, which have typically a radius of 1–10 \(\mu m\) [55]. Following a pore-size analysis of the image stacks, an average fiber diameter of 10 \(\mu\)m was considered for each individual collagen fiber. The volume fraction of collagen in each image stack was estimated using a semi-automatic segmentation technique in ScanIP®, a core image processing platform of the commercial software Synopsis’ SimplewareTM. Numerical network reconstruction: A 3-D volume of \(500 \times 500 \times t\) \(\mu\)m\(^{3}\) was defined for each sample, with t being the measured sample’s average thickness. The collagen fiber network of the adventitia was then reconstructed with each fiber assumed to be a worm-like chain with a uniform diameter. Each fiber was defined by a global orientation \(\theta\), which was inversely sampled from the quantified orientation distribution, ensured by a goodness of fit test (p-value \(< 0.05\)). The fiber is then reconstructed as a sinusoidal wave, whose curvature is determined by the observed crimp of the collagen fibers. The crimp of collagen was not measured on the image stacks used in this study, but was identified in an inverse manner. The length of the fiber was assumed to be larger than the area of interest probed by the microscope; and hence, each fiber was thought to have its ends intersect the edges of the defined volume. Fibers were added to the network until the desired volume fraction is reached. This numerical network generation schema is similar to the one described in detail in our previous work [2].
2.2.2 Constitutive model for matrix
The ground matrix was modeled as a single parameter isotropic, incompressible Neo-Hookean material [23, 24, 63] with the strain energy density \(\Psi = \frac{C}{2} (I_{1} - 3)\), where C is a material constant and \(I_1\) is the first invariant of the right Cauchy-Green deformation tensor.
2.2.3 Constitutive model for collagen
Collagen fibers were modeled as a linearly elastic material with Young’s modulus E until the point of critical stretch \(\lambda _{critical}\), beyond which the fiber is assumed to be completely damaged [4, 27, 38, 47]. The constitutive law is defined by Eq. 1 below.
2.2.4 Finite element model
A custom Matlab script was used to generate input files of the generated fiber networks to be exported to Abaqus® for finite element analysis. The fibers were modeled as beam elements in 3-D space with negligible but non-zero bending stiffness to aid with the stability of the model. The interaction between matrix and collagen fibers was assumed to be non-existent and the underlying fiber kinematics to be non-affine. The damage model for collagen as described by Eq. 1 was implemented in a user material subroutine (UMAT).
The boundary conditions imposed on the model in order to simulate the uniaxial and biaxial tension are shown in Fig. 3. The following notations apply: x axis corresponds to circumferential direction, y axis corresponds to longitudinal direction, L is the length of the representative element, \(u_{circ}\) is the applied displacement along the circumferential direction, \(u_{long}\) is the displacement applied along the longitudinal direction, \(u_{affine}\) is the computed displacement in longitudinal direction using affine transformation for a given displacement in circumferential direction.
In uniaxial simulations, the loading is applied by imposing a boundary condition for every node on the domain boundary. The desired axial stretch is achieved by displacing all the nodes on one axially symmetric surface, by a value \(u_{circ}\), while restraining the other end. In the transverse direction, all boundary nodes were kinematically coupled with a free pivot to control their transverse displacement. The pivot is part of a spring-like connector element aligned in transverse direction, whose other node is imposed with a transverse displacement according to the affine kinematics assumption. By varying the stiffness of this connector, the deformation in the transverse direction is controlled [2]. If the stiffness of the connector is too low, it induces high transverse deformations, emulating a fully non-affine behavior. Conversely, as the stiffness of the connector increases, the induced transverse deformation decreases, asymptotically approaching an affine behavior. The fully affine behavior was defined assuming the tissue to be incompressible. The reaction forces at each node on the loaded boundary were summed to obtain the total reaction force, which was then converted to first Piola-Kirchoff stress by dividing with the initial cross-section area.
For the biaxial case, displacement boundary conditions were imposed on two orthogonal surfaces. The amplitudes of imposed displacement in circumferential (\(u_{circ}\)) and longitudinal (\(u_{long}\)) directions were determined from the bulge inflation tests in the following manner. First, a geometrically accurate finite element model of each sample was constructed based on stereo-digital image correlation (s-DIC) and thickness measurements [6]. This model was then compared against the experimental s-DIC displacement to identify the anisotropic hyperelastic material parameters. These experimental data were obtained, for each sample, in a preliminary bulge inflation test performed in the elastic response domain, combined to s-DIC measurements. The identification procedure and stress/strain state in the region of interest were validated in [6] and further used in the present work as experimental results of the bulge inflation test. The stretch values for the discrete network model were taken as the average of the stretch values of all elements in the region of SHG imaging. Readers interested in further details of the finite element model and network reconstruction are directed to our previous article [2].
2.2.5 Parameter estimation
Parameters of the model that were unknown at the start of the simulation were identified in an inverse manner. To achieve this, a design of the experiment approach was used as described in [2] wherein multiple simulations (\(\approx\)2000) of each reconstructed network were conducted by varying the values of unknown parameters on a regularly gridded parameter space. A multi-dimensional gridded interpolant was built in Matlab using the recorded force-displacement responses. As a first step, the Neo-Hookean material parameter was identified on the initial part of the experimental testing curve. This is due to the assumption that collagen fibers are crimped initially and only contribute to load bearing beyond a certain stretch (identified on the curve as point with highest rate of change of slope). The parameters for collagen fibers such as elastic modulus and initial crimp were identified through a constrained optimization algorithm in Matlab. By fitting the peak of the elastic stress-stretch response, the tensile critical stretch for individual collagen fibers was identified. The values of identified parameters for each sample are reported as mean ± 95% confidence interval. Finally, in order to validate the developed model, the global stress-stretch response was compared to the experimental value for each sample.
3 Results
Experiments were performed on two different specimens: human ATAA tissues and healthy porcine aortas. In the case of ATAA specimens, a bulge inflation setup was used to pressurize the arterial wall until rupture. In the case of porcine specimens, adventitial strips were prepared and pulled in two different directions, with two samples being tested in the axial direction and four in the circumferential direction. The numerical model aimed at describing all of the different experiments, including both prefailure and failure behaviors.
3.1 Uniaxial tension
Dog-bone–shaped adventitial strips of porcine aortas aligned both circumferentially (\(n = 4\)) and longitudinally (\(n = 2\)) were loaded to failure. Figure 4a-f present the first Piola-Kirchoff stress (grip force divided by the undeformed cross section area) vs the stretch for each sample along with the simulated best fit tissue-specific model curve. The model was capable of reproducing the typical nonlinear response of the tissue along with the ultimate strength of the tissue. The specified geometrical and regressed constitutive model parameters as presented in Table 2 allowed the model to match the experimental prefailure and failure results to within 95% confidence intervals.
3.2 Bulge-inflation
For the case of bulge inflation samples, the total strain energy was compared between the simulated network model and the region of interest in the reconstructed finite element model. For each experimentally tested sample, the applied pressure was recorded until the point of rupture. Figure 5 presents the internal energy for each sample in the region of interest vs time along with a best fit tissue-specific model. The biaxial model results were in good agreement (red dotted line) with and remained within 95% confidence interval of the experiments. The average coefficient of determination between experiments and simulation had a minimum value of 0.972. Values of the regressed constitutive parameters are presented in Table 3. Figure 6a shows the percentage of collagen fibers that ruptured at the point of tissue-level rupture (identified as the point of maximum stress) in uniaxial samples. Figure 6c shows the percentage of collagen fibers that ruptured at the point of tissue-level rupture (identified as the time of maximum strain energy) in bulge inflation samples.
3.3 Fiber kinematics
SHG image stacks were recorded for each bulge inflation sample at intermediate pressure levels until the point of rupture as stated in section “Microstructural imaging.” For each sample, orientation distribution of collagen fibers was extracted at a supra-physiological pressure state loading state and compared to the distribution of fiber orientations in the representative model. Figure 7 shows the comparison of orientation distribution from experiments and simulations. All samples showed a good agreement with experimental data.
4 Discussion
4.1 Main contributions
From a biomechanics point of view, aortic aneurysm rupture is considered a local event where the wall stress exceeds the local tissue strength. While it is known that rupture of the tissue occurs at pressures much higher than the physiological rest levels, the exact mechanisms leading up to this event are unclear. Macroscopic characteristics of the tissue such as local strength and thickness have been shown to be not always in correlation with pressure at rupture [6]. These findings suggest that the intrinsic difference in microstructural characteristics could be the key determinants in predicting aortic rupture. Here, we investigate the hypothesis that rupture/damage at the scale of fibrous microstructure is responsible for the failure/rupture at the macroscopic level. For this purpose, we developed a finite element model of the adventitial microstructure by explicitly incorporating the morphology of the collagen fiber network. The capability of this modeling method is demonstrated by comparing the simulation results to two experimental testing methods: uniaxial tension and bulge inflation, with all the identified parameters in an acceptable range of values reported in the literature [7, 50]. However, the Neo-Hookean material parameter identified for the matrix was observed to be higher than the values reported in the literature. The reasons for this are not yet completely understood. One possibility could be the effect of freezing the samples at \(-20^{o}C\); which has an influence on both the recruitment stretch and the slope of stress-strain curve [1, 9]. The values obtained for collagen fiber stiffness, given that the diameter of the fibers considered in the model is 10 \(\mu m\), are within the values reported via experiments on individual extruded fibers [37].
4.2 Collagen rupture strain comparison
Important distinction has to be made while considering the failure properties of collagen as the parameters change with respect to its scale. At initial state, fibers tend to form large bundles which slowly separate into fibers as loading increases, which was confirmed by recent observations by our group [6]. These observations show that collagen fibers are the default structural units in the arterial tissue at physiological pressures, which possibly coalesce into fiber bundles when the loading is removed. Hence, relevant failure criteria corresponding only to collagen fibers (typical diameters of 1–10 \(\mu m\)) were considered in this study. Also, it should be noted that cohesion between the fibers in a bundle was assumed to be negligible at loads beyond the toe region. The current work utilized the simplest model for collagen fiber failure; that is, no damage is accumulated until a critical strain is reached, at which point the fiber is assumed to rupture. Direct comparisons could be made at this point to experimental studies conducted on individual collagen type I fibers, as they constitute a major portion of all collagen found in the vascular tree [15].
Miyazaki tested the tensile properties of individual collagen type I fibers (diameters of 1–10 \(\mu\)m) extruded from rabbit patellar tendons [37]. They have reported a failure strain of \(0.216 \pm 0.03\). A similar study conducted by Gentleman et al. on collagen fibers (diameters of \(59 - 125\) \(\mu\)m) extruded from rat tail tendons revealed similar findings with a fiber rupture strain of 0.20–0.25 [19].
Identified collagen rupture strain values showed a high variance in the case of ATAA samples, with a maximum of 0.293 and a minimum of 0.088. In as many as four samples, the identified collagen rupture strain value deviated strongly from those reported in the literature suggesting possible effects of remodeling in the aneurysmal pathology. On the contrary, the porcine samples indicated a very good comparison to experimental findings in terms of identified collagen rupture strain, with a maximum of 0.255 and a minimum of 0.187. This might just be due to the inherent differences in the two testing methods, with uniaxial testing more suitable for characterizing the failure of a material, although more data is required to reinforce these findings especially as the nature of human ATAA samples, being aged and aneurysmatic, may have influence compared to the mentioned studies on more standardized animal tissues. Also, the tests conducted in the literature being on extracted individual fibers might induce certain difference compared to embedded fibers. This might also indicate that discrete fiber failure might not be the only mechanism at play causing the rupture at the macroscopic level with mechanical sliding between fibers or bundles, or cross-link breakages being possible additional mechanisms. To this effect, further investigation by combining microstructural imaging and mechanical testing of the tissue (in multiple loading scenarios) is imperative.
4.3 Correlation between micro-structure and rupture
Based on the failure mechanism utilized in this work, we investigated the influence of structural parameters such as collagen fiber orientation and waviness on tissue rupture at macroscale. For this purpose, the orientation distribution is described by a single parameter called the orientation index (OI), which provides a measure of fiber alignment [41].
where \(\theta _i\) is the orientation of a fiber with respect to direction of load (for bulge inflation, this was chosen as the direction of principal stretch, as the observed stretch was different in the circumferential and longitudinal directions); n is the total number of fibers. A value of 1 for orientation index indicates that the fibers are perfectly aligned towards a preferred direction (for instance, in the case of uniaxial testing, it means that the fibers are predominantly aligned towards the loading direction), whereas a value of 0.5 indicates a random alignment.
In the case of bulge inflation tests, the orientation index did not show a significant correlation with rupture pressure, rather a weak negative trend was seen. The Pearson coefficient of correlation was observed to be −0.229 with a p-value of 0.56 as shown in Fig. 8c. Although at first sight this indicates no correlation between the two variables, under biaxial loading conditions (which is likely the case in vivo for aneurysms), the negative trend might indicate that rupture occurs in the direction perpendicular to predominant fiber orientation. On the contrary, orientation index showed significant correlation with respect to rupture stretch of the tissue in the case of uniaxial testing. A value of 0.853 for Pearson’s correlation coefficient with a p-value of 0.002 was recorded as shown in Fig. 8a. This, for instance, shows that for a given loading direction the strength of the tissue increases with an increase in fiber alignment with respect to that direction. This is likely the reason why in most cases the strength of the tissue in circumferential direction is higher than that of longitudinal direction [51], suggesting a predominant circumferential fiber alignment. This may also explain why the model performs better in uniaxial tests than bulge inflation: in uniaxial tests, the tensile response of fibers is likely to be the most influential on the macroresponse, while other mechanisms can be suspected in bulge inflation.
The relationship between collagen fiber waviness and tissue strength reveals a positive trend in both uniaxial and bulge inflation tests. The values of Pearson’s correlation coefficient and p-value are 0.932 and 0.002 for uniaxial tests and 0.244 and 0.65 for bulge inflation tests as shown in Fig. 8b and d. The correlation was observed to be non-existent in bulge inflation samples compared to uniaxially tested samples. The absence of correlation in the bulge inflation samples could be due to the fact that the fibers were already identified with low values of crimp in their initial state, thereby perhaps indicating that this parameter does not exert as much influence. The uniaxial samples showed that as the collagen crimp increases, the macroscopic failure stretch of the tissue increases. This finding also corroborates the usual hypothesis that aneurysmal tissue has lower failure stretch because the waviness of the network is lower [16].
Several parameters have been suggested to evaluate aneurysm rupture, including wall stress analysis [14] and wall strain analysis [58], and incorporating factors such as gender, thickness of the wall, and family history along with peak wall stress in computing the risk parameter [13, 17]. It has been shown that macroscopic mechanical indicators such as peak wall stress and strain have little correlation to rupture [6, 36]. These deterministic models of aortic aneurysms often do not take into account factors like local wall thickness and local biomechanical properties. In order to determine a probabilistic rupture risk indicator, Polzer et al. assumed a distribution for peak wall stress along the wall geometry based on local thickness distribution [45]. They concluded that taking into consideration the probabilistic nature of wall thickness and strength could enhance rupture risk assessments. Evidently, tissue-scale mechanical predictors for rupture risk have their limitations. The main problem surrounding this methodology is that there is a lack of knowledge on the intrinsic strength of the tissue, which seemingly is heterogeneous within a tissue and in-between tissues [53, 56]. This is the point at which a microstructure-level investigation could help in the assessment of the risk of aneurysm rupture in a superior way.
4.4 Ascending aorta vs descending aorta
An important distinction has to be made with regards to the current study. The normal aorta is made up of four sections: the ascending aorta rises up from the heart and is about 5 cm long; the aortic arch curves over the heart; the descending aorta travels down through the chest until the diaphragm; the abdominal aorta beginning at the diaphragm until it splits into iliac arteries in the lower abdomen. Given that, the diseased aortic segments procured from human subjects stem from the ascending thoracic region. On the other hand, porcine aortic segments were excised from the region overlapping the descending and abdominal sections. Even though all sections of the aorta have the same three layers and microstructural constituents (collagen, elastin, and smooth muscle), their content and morphological organization change with location along the wall [29]. Secondly, aneurysms developing in the ascending thoracic region tend to have a slower growth rate[34] and thus often develop dissections rather than rupture [10, 11]. Dissection and rupture are two fundamentally different phenomena; and therefore, the same failure criteria might not be applicable to them both. However, since the tissues loaded via bulge inflation in vitro deform at a rate much higher than that in vivo, the rupture information identified might not be fully ineffective. Nonetheless, it is to be noted that it is better to test abdominal aortic segments while studying rupture. Finally, the main idea of the current study is to link microstructure-based damage to macroscopic tissue failure. It can be concluded from the results of our study that multiscale models representing the overall tissue’s mechanics are powerful tools to predict tissue scale failure. Therefore, even though the difference in origin of tissues tested is noticeable, the model could still be adopted to study aortic rupture.
4.5 Aortic aneurysms, rupture, and dissection
4.6 Limitations
The discrete computational model of the collagen fiber network described here has the following limitations.
-
1.
We do not take into account any physical interactions between the ground matrix and the fibers. Additionally, damage is considered only in the collagenous component, which may contribute to the overall tissue strength. However, we do not expect that accounting for damage to the ground matrix will change the major conclusions of the current work.
-
2.
The interactions between the fibers were assumed to be negligible. However, in the case of adventitial collagen, this requires extensive experimental investigations in order to characterize the nature of interactions.
-
3.
In predicting the tissue’s macroscopic failure, a brittle damage model was used for the fibers. This is a simplistic assumption as experimental evidence demonstrates more complex irreversible damage mechanisms, possibly originating at the fibril or molecular level [32].
-
4.
The described model for identifying macroscopic tissue rupture has no direct clinical application at the moment as the microstructure cannot be determined for a given patient. However, with the use of devices like endoscopic OCT [48, 61], this could be possible in the near future.
-
5.
In the presented model, collagen fibers were assumed to be oriented mostly in the axial-circumferential plane. Hence, the orientation distribution obtained from image stacks only corresponded to the azimuthal angle, neglecting the effect of a polar angle. For a more realistic representation of the microstructure, 3-D segmentation tools which can accurately render the fiber morphology are required. At this moment, the quality of the images prevents such analysis which would be beyond the scope of this study.
5 Conclusions
In conclusion, we have shown that a discrete fiber network computational model is capable of accurately representing prefailure and failure response of the aortic tissue in two different loading configurations. This model could provide new insights into failure mechanisms involved in aortic aneurysm rupture and is robust enough to incorporate patient-specific anatomical models given that patient-specific information could be obtained.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adham, M., Gournier, J.P., Favre, J.P., De La Roche, E., Ducerf, C., Baulieux, J., Barral, X., Pouyet, M.: Mechanical characteristics of fresh and frozen human descending thoracic aorta. Journal of Surgical Research 64(1), 32–34 (1996)
Ayyalasomayajula, V., Pierrat, B., Badel, P.: A computational model for understanding the micro-mechanics of collagen fiber network in the tunica adventitia. Biomechanics and modeling in mechanobiology pp. 1–22 (2019)
Bitter, R., Mohiuddin, T., Nawrocki, M.: LabVIEW: Advanced programming techniques. Crc Press (2006)
Cacho, F., Elbischger, P., Rodriguez, J., Doblaré, M., Holzapfel, G.A.: A constitutive model for fibrous tissues considering collagen fiber crimp. International Journal of Non-Linear Mechanics 42(2), 391–402 (2007)
Cavinato, C., Helfenstein-Didier, C., Olivier, T., Du Roscoat, S.R., Laroche, N., Badel, P.: Biaxial loading of arterial tissues with 3D in situ observations of adventitia fibrous microstructure: A method coupling multi-photon confocal microscopy and bulge inflation test. Journal of the mechanical behavior of biomedical materials 74, 488–498 (2017)
Cavinato, C., Molimard, J., Curt, N., Campisi, S., Orgéas, L., Badel, P.: Does the knowledge of the local thickness of human ascending thoracic aneurysm walls improve their mechanical analysis? Frontiers in bioengineering and biotechnology 7, 169 (2019)
Chen, H., Guo, X., Luo, T., Kassab, G.S.: A validated 3D microstructure-based constitutive model of coronary artery adventitia. Journal of Applied Physiology 121(1), 333–342 (2016)
Choudhury, N., Bouchot, O., Rouleau, L., Tremblay, D., Cartier, R., Butany, J., Mongrain, R., Leask, R.L.: Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovascular Pathology 18(2), 83–91 (2009)
Chow, M.J., Zhang, Y.: Changes in the mechanical and biochemical properties of aortic tissue due to cold storage. Journal of Surgical Research 171(2), 434–442 (2011)
Clouse, W.D., Hallett, J.W., Schaff, H.V., Spittell, P.C., Rowland, C.M., Ilstrup, D.M., Melton III, L.J.: Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. In: Mayo Clinic Proceedings, vol. 79, pp. 176–180. Elsevier (2004)
Davies, R.R., Goldstein, L.J., Coady, M.A., Tittle, S.L., Rizzo, J.A., Kopf, G.S., Elefteriades, J.A.: Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. The Annals of thoracic surgery 73(1), 17–28 (2002)
Duprey, A., Trabelsi, O., Vola, M., Favre, J.P., Avril, S.: Biaxial rupture properties of ascending thoracic aortic aneurysms. Acta Biomaterialia 42, 273–285 (2016). DOI: 10.1016/j.actbio.2016.06.028
Erhart, P., Hyhlik-Dürr, A., Geisbüsch, P., Kotelis, D., Müller-Eschner, M., Gasser, T.C., von Tengg-Kobligk, H., Böckler, D.: Finite element analysis in asymptomatic, symptomatic, and ruptured abdominal aortic aneurysms: In search of new rupture risk predictors. European Journal of Vascular and Endovascular Surgery 49(3), 239–245 (2015)
Fillinger, M.F., Marra, S.P., Raghavan, M.L., Kennedy, F.E.: Prediction of rupture risk in abdominal aortic aneurysm during observation: Wall stress versus diameter. Journal of vascular surgery 37(4), 724–732 (2003)
Fratzl, P.: Collagen: Structure and mechanics, an introduction. In: Collagen, pp. 1–13. Springer (2008)
García-Herrera, C.M., Atienza, J., Rojo, F., Claes, E., Guinea, G., Celentano, D.J., García-Montero, C., Burgos, R.: Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Medical & biological engineering & computing 50(6), 559–566 (2012)
Gasser, T.C., Nchimi, A., Swedenborg, J., Roy, J., Sakalihasan, N., Böckler, D., Hyhlik-Dürr, A.: A novel strategy to translate the biomechanical rupture risk of abdominal aortic aneurysms to their equivalent diameter risk: Method and retrospective validation. European Journal of Vascular and Endovascular Surgery 47(3), 288–295 (2014)
Gasser, T.C., Ogden, R.W., Holzapfel, G.A.: Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3(6), 15–35 (2006). https://doi.org/10.1098/rsif.2005.0073
Gentleman, E., Lay, A.N., Dickerson, D.A., Nauman, E.A., Livesay, G.A., Dee, K.C.: Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 24(21), 3805–3813 (2003)
Hadi, M.F., Barocas, V.H.: Microscale fiber network alignment affects macroscale failure behavior in simulated collagen tissue analogs. Journal of biomechanical engineering 135(2), 021026 (2013)
Hadi, M.F., Sander, E.A., Barocas, V.H.: Multiscale model predicts tissue-level failure from collagen fiber-level damage. Journal of biomechanical engineering 134(9), 091005 (2012)
Hill, M.R., Duan, X., Gibson, G.A., Watkins, S., Robertson, A.M.: A theoretical and non-destructive experimental approach for direct inclusion of measured collagen orientation and recruitment into mechanical models of the artery wall. Journal of biomechanics 45(5), 762–771 (2012)
Holzapfel, G.A., Gasser, T.C., Ogden, R.W.: A new constitutive framework for arterial wall mechanics and a comparative study of material models. Journal of elasticity and the physical science of solids 61(1–3), 1–48 (2000)
Holzapfel, G.A., Weizsäcker, H.W.: Biomechanical behavior of the arterial wall and its numerical characterization. Computers in biology and medicine 28(4), 377–392 (1998)
Iliopoulos, D.C., Kritharis, E.P., Giagini, A.T., Papadodima, S.A., Sokolis, D.P.: Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. The Journal of thoracic and cardiovascular surgery 137(1), 101–109 (2009)
Isselbacher, E.M.: Thoracic and abdominal aortic aneurysms. Circulation 111(6), 816–828 (2005)
Kato, Y., Christiansen, D.L., Hahn, R.A., Shieh, S.J., Goldstein, J.D., Silver, F.H.: Mechanical properties of collagen fibres: A comparison of reconstituted and rat tail tendon fibres. Biomaterials 10(1), 38–42 (1989). https://doi.org/10.1016/0142-9612(89)90007-0
Khanafer, K., Duprey, A., Zainal, M., Schlicht, M., Williams, D., Berguer, R.: Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. The Journal of Thoracic and Cardiovascular Surgery 142(3), 682–686 (2011). https://doi.org/10.1016/j.jtcvs.2010.09.068
Komutrattananont, P., Mahakkanukrauh, P., Das, S.: Morphology of the human aorta and age-related changes: anatomical facts. Anatomy & cell biology 52(2), 109–114 (2019)
Krasny, W., Morin, C., Magoariec, H., Avril, S.: A comprehensive study of layer-specific morphological changes in the microstructure of carotid arteries under uniaxial load. Acta biomaterialia 57, 342–351 (2017)
Li, K., Holzapfel, G.A.: Multiscale modeling of fiber recruitment and damage with a discrete fiber dispersion method. Journal of the Mechanics and Physics of Solids 126, 226–244 (2019)
Marino, M., Converse, M.I., Monson, K.L., Wriggers, P.: Molecular-level collagen damage explains softening and failure of arterial tissues: A quantitative interpretation of CHP data with a novel elasto-damage model. Journal of the mechanical behavior of biomedical materials 97, 254–271 (2019)
Marra, S.P., Kennedy, F.E., Kinkaid, J.N., Fillinger, M.F.: Elastic and rupture properties of porcine aortic tissue measured using inflation testing. Cardiovascular Engineering 6(4), 123–131 (2006)
Mathur, A., Mohan, V., Ameta, D., Gaurav, B., Haranahalli, P.: Aortic aneurysm. Journal of translational internal medicine 4(1), 35–41 (2016)
Matsumoto, T., Fukui, T., Tanaka, T., Ikuta, N., Ohashi, T., Kumagai, K., Akimoto, H., Tabayashi, K., Sato, M.: Biaxial tensile properties of thoracic aortic aneurysm tissues. Journal of Biomechanical Science and Engineering 4(4), 518–529 (2009)
Miller, K., Mufty, H., Catlin, A., Rogers, C., Saunders, B., Sciarrone, R., Fourneau, I., Meuris, B., Tavner, A., Joldes, G.R., et al.: Is there a relationship between stress in walls of abdominal aortic aneurysm and symptoms? Journal of Surgical Research 252, 37–46 (2020)
Miyazaki, H., Hayashi, K.: Tensile tests of collagen fibers obtained from the rabbit patellar tendon. Biomedical Microdevices 2(2), 151–157 (1999)
Morgan, F.: Mechanical properties of raw collagen fibers. J Soc Leather Trade’s Chemists 44, 2–23 (1960)
Niestrawska, J.A., Viertler, C., Regitnig, P., Cohnert, T.U., Sommer, G., Holzapfel, G.A.: Microstructure and mechanics of healthy and aneurysmatic abdominal aortas: Experimental analysis and modelling. Journal of The Royal Society Interface 13(124), 20160620 (2016)
Okamoto, R.J., Xu, H., Kouchoukos, N.T., Moon, M.R., Sundt III, T.M.: The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. The Journal of thoracic and cardiovascular surgery 126(3), 842–850 (2003)
Pasta, S., Phillippi, J.A., Tsamis, A., D’Amore, A., Raffa, G.M., Pilato, M., Scardulla, C., Watkins, S.C., Wagner, W.R., Gleason, T.G.: Constitutive modeling of ascending thoracic aortic aneurysms using microstructural parameters. Medical engineering & physics 38(2), 121–130 (2016)
Peña, E.: Damage functions of the internal variables for soft biological fibred tissues. Mechanics Research Communications 38(8), 610–615 (2011)
Peña, E., Calvo, B., Martínez, M., Doblaré, M.: On finite-strain damage of viscoelastic-fibred materials. Application to soft biological tissues. International Journal for Numerical Methods in Engineering 74(7), 1198–1218 (2008)
Picu, R.: Mechanics of random fiber networks—a review. Soft Matter 7(15), 6768–6785 (2011)
Polzer, S., Gasser, T.C.: Biomechanical rupture risk assessment of abdominal aortic aneurysms based on a novel probabilistic rupture risk index. J. R. Soc. Interface 12(113), 20150852 (2015). https://doi.org/10.1098/rsif.2015.0852
Polzer, S., Gasser, T.C., Forsell, C., Tichy, M.: Automatic Identification and Validation of Planar Collagen Organization in the Aorta Wall with Application to Abdominal Aortic Aneurysm. Microscopy and Microanalysis 19(06), 1395–1404 (2013). https://doi.org/10.1017/S1431927613013251
Sasaki, N., Odajima, S.: Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. Journal of biomechanics 29(9), 1131–1136 (1996)
Sergeev, A.M., Gelikonov, V., Gelikonov, G., Feldchtein, F.I., Kuranov, R., Gladkova, N., Shakhova, N., Snopova, L., Shakhov, A., Kuznetzova, I., et al.: In vivo endoscopic oct imaging of precancer and cancer states of human mucosa. Optics express 1(13), 432–440 (1997)
Shah, S.B., Witzenburg, C., Hadi, M.F., Wagner, H.P., Goodrich, J.M., Alford, P.W., Barocas, V.H.: Prefailure and failure mechanics of the porcine ascending thoracic aorta: Experiments and a multiscale model. Journal of biomechanical engineering 136(2), 021028 (2014)
Sherman, V.R., Yang, W., Meyers, M.A.: The materials science of collagen. Journal of the Mechanical Behavior of Biomedical Materials 52, 22–50 (2015). https://doi.org/10.1016/j.jmbbm.2015.05.023
Sommer, G., Regitnig, P., Költringer, L., Holzapfel, G.A.: Biaxial mechanical properties of intact and layer-dissected human carotid arteries at physiological and supraphysiological loadings. American Journal of Physiology-Heart and Circulatory Physiology 298(3), H898–H912 (2010). https://doi.org/10.1152/ajpheart.00378.2009
Spanos, P., Esteva, M.: Effect of stochastic nanotube waviness on the elastic and thermal properties of nanocomposites by fiber embedment in finite elements. Journal of Computational and Theoretical Nanoscience 6(10), 2317–2333 (2009)
Takamizawa, K., Hayashi, K.: Strain energy density function and uniform strain hypothesis for arterial mechanics. Journal of biomechanics 20(1), 7–17 (1987)
Thunes, J.R., Phillippi, J.A., Gleason, T.G., Vorp, D.A., Maiti, S.: Structural modeling reveals microstructure-strength relationship for human ascending thoracic aorta. Journal of biomechanics 71, 84–93 (2018)
Ushiki, T.: Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Archives of histology and cytology 65(2), 109–126 (2002)
Vallabhaneni, S.R., Gilling-Smith, G.L., How, T.V., Carter, S.D., Brennan, J.A., Harris, P.L.: Heterogeneity of tensile strength and matrix metalloproteinase activity in the wall of abdominal aortic aneurysms. Journal of Endovascular Therapy 11(4), 494–502 (2004)
Volokh, K.: Hyperelasticity with softening for modeling materials failure. Journal of the Mechanics and Physics of Solids 55(10), 2237–2264 (2007)
Volokh, K.: Comparison of biomechanical failure criteria for abdominal aortic aneurysm. Journal of biomechanics 43(10), 2032–2034 (2010)
Vorp, D.A., Schiro, B.J., Ehrlich, M.P., Juvonen, T.S., Ergin, M., Griffith, B.P.: Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. The Annals of Thoracic Surgery 75(4), 1210–1214 (2003). https://doi.org/10.1016/S0003-4975(02)04711-2
Witzenburg, C.M., Barocas, V.H.: A nonlinear anisotropic inverse method for computational dissection of inhomogeneous planar tissues. Computer methods in biomechanics and biomedical engineering 19(15), 1630–1646 (2016)
Zagaynova, E., Gladkova, N., Shakhova, N., Gelikonov, G., Gelikonov, V.: Endoscopic oct with forward-looking probe: clinical studies in urology and gastroenterology. Journal of biophotonics 1(2), 114–128 (2008)
Zhang, L., Lake, S.P., Lai, V.K., Picu, C.R., Barocas, V.H., Shephard, M.S.: A coupled fiber-matrix model demonstrates highly inhomogeneous microstructural interactions in soft tissues under tensile load. Journal of biomechanical engineering 135(1), 011008 (2013)
Zulliger, M.A., Fridez, P., Hayashi, K., Stergiopulos, N.: A strain energy function for arteries accounting for wall composition and structure. Journal of biomechanics 37(7), 989–1000 (2004)
Acknowledgements
This work was funded by the European Research Counsil, starting grant no.638804, AArteMIS. We would like to thank Irene Wang, PhD, LIPHy Lab, CNRS, University of Grenoble Alps, France, for her assistance in acquiring SHG image stacks of porcine samples. We would like to thank Dr. Jamaleddin Mousavi for the general discussions in writing the umat.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayyalasomayajula, V., Pierrat, B. & Badel, P. An evaluation of fiber-based damage for assessing the failure of aortic tissue: comparison between healthy and aneurysmal aortas. Mech Soft Mater 4, 4 (2022). https://doi.org/10.1007/s42558-022-00041-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42558-022-00041-6