Abstract
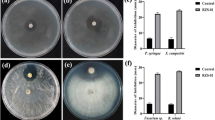
Sclerotinia sclerotiorum is an important devastating necrotrophic plant pathogen infecting various horticulture crops in India. The present study aimed to examine the variability among S. sclerotiorum isolates from different hosts by means of mycelial compatibility grouping (MCGs), production of oxalic acid and pathogenicity. The isolates were grouped into eight MCGs (MCG A to MCG H) and the MCG data was used to calculate Shannon’s diversity index (H) and Simpson index (S). High diversity was detected for the S. sclerotiorum isolates (H = 1.968, S = 0.845). Most of the isolates produced oxalic acid in potato dextrose agar and potato dextrose broth embedded with bromophenol blue, which was confirmed by changing the media colour from blue to yellow. A deep bright yellow colour with higher luminosity values (37.48, 36.64 and 36.84) was observed in SS1, SS3 (mustard) and SS6 (potato) isolates. The highest oxalic acid production was recorded in potato SS14 (43.25 mM) and mustard SS1 (41.11 mM) isolates, while potato isolate SS10 (10.88 mM) produced lowest oxalic acid. All isolates infect the tested plant leaves; however, lesion size and time required for infection were varied. Positive correlations were observed in S. sclerotiorum mycelial growth vs. luminosity values, mycelial dry weight vs. medium pH and mycelial dry weight vs. oxalic acid accumulation. This study indicates a high level of diversity among the S. sclerotiorum isolates from the different crops with respect to MCG, oxalic acid production and pathogenicity. Further, these results would be helpful in developing management strategies against white mold disease.





Similar content being viewed by others
References
Abán CL, Taboada G, Spedaletti Y, Aparicio M, Curti RN, Casalderrey NB, Maggio ME, Chocobar MO, Salgado M, Galván MZ (2018) Molecular, morphological and pathogenic diversity of Sclerotinia sclerotiorum isolates from common bean (Phaseolus vulgaris) fields in Argentina. Plant Pathol 67:1740–1748
Aldrich-Wolfe L, Travers S, Nelson BD Jr. (2015) Genetic variation of Sclerotinia sclerotiorum from multiple crops in the North Central United States. PLoS ONE 10:e0139188
Alkan N, Espeso EA, Prusky D (2013) Virulence regulation of phytopathogenic fungi by pH. Antioxid Redox Signal 19:1019–1025
Cessna SG, Sears VE, Dickman MB, Low PS (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 12:2191–2199
Chaudhary S, Lal M, Sagar S, Tyagi H, Kumar M, Sharma S, Chakrabarti SK (2020a) Genetic diversity studies based on morpho-pathological and molecular variability of the Sclerotinia sclerotiorum population infecting potato (Solanum tuberosum L). World J Microbiol Biotechnol 36:177
Chaudhary S, Sagar S, Lal M, Tomar A, Kumar V, Kumar M (2020b) Biocontrol and growth enhancement potential of Trichoderma spp. against Rhizoctonia solani causing sheath blight disease in Rice. J Environ Biol 41:1034–1045
Durman SB, Menendez AB, Godeas AM (2005) Variation in oxalic acid production and mycelial compatibility within field populations of Sclerotinia sclerotiorum. Soil Biol Biochem 37:2180–2184
El-Argawy E (2012) Oxalic acid production by Sclerotinia sclerotiorum and its relation to pathogenicity. J Plant Protect Pathol 3:211–225
Garg H, Kohn LM, Andrew M, Li H, Sivasithamparam K, Barbetti MJ (2010) Pathogenicity of morphologically different isolates of Sclerotinia sclerotiorum with Brassica napus and B. juncea genotypes. Eur J Plant Pathol 126:305–315
Godoy G, Steadman JR, Dickman MB, Dam R (1990) Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol 37:179–191
Guimaraẽs RL, Stolz HU (2004) Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol 136:3703–3711
Henson J, Butler MJ, Day AW (1999) The dark side of mycelium: melanins of phytopathogenic fungi. Annu Rev Phytopathol 37:447–471
Hunter RS, Harold RW (1987) The measurement of appearance, second edn. Wiley-interscience, New York, p 411
Kim KS, Min JY, Dickman MB (2008) Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol Plant Microbe Interact 21:605–612
Kohn LM, Carone I, Anderson JB (1990) Mycelial interaction in Sclerotinia sclerotiorum. Exp Mycol 14(3):255–267
Krishnamoorthy K, Sankaralinga A, Nakkeeran S (2016) Standardization of culture media and pH for the rapid growth of Sclerotinia sclerotiorum causing head rot disease of cabbage. Adv Life Sci 5:10659–10661
Kull LS, Vuong TD, Powers KS, Eskridge KM, Steadman JR, Hartman GL (2003) Evaluation of three resistance screening methods using six Sclerotinia sclerotiorum isolates and three entries of each soybean and dry bean. Plant Dis 87:1471–1476
Kull LS, Pedersen WL, Palmquist D, Hartman GL (2004) Mycelial compatibility grouping and aggressiveness of Sclerotinia sclerotiorum. Plant Dis 88:325–332
Lal M, Chaudhary S, Sharma S, Subhash S, Kumar M (2022) Bio-intensive management of fungal diseases of potatoes. In: Chakrabarti SK, Sharma S, Shah MA (eds) Sustainable management of potato pest and diseases. Springer, Singapore, pp 452–493
Li YH, Wang H, Li JC, Wang DJ, Li DR (2005) Infection of Sclerotinia sclerotiorum to rapeseed, soybean and sunflower and its virulence differentiation. Acta Phytopathol Sinica 35:486–492
Li Z, Zhang M, Wang Y, Li R, Fernando WD (2008) Mycelial compatibility group and pathogenicity variation of Sclerotinia sclerotiorum populations in sunflower from China, Canada and England. Plant Pathol J 7:131–139
Li J, Zhang Y, Yu PL, Pan H, Rollins JA (2018) Introduction of large sequence inserts by CRISPR-Cas9 to create pathogenicity mutants in the multinucleate filamentous pathogen Sclerotinia sclerotiorum. mBio 9:e00567–e00518
Liang Y, Xiong W, Steinkellner S, Feng J (2018) Deficiency of the melanin biosynthesis genes SCD1 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity, in Sclerotinia sclerotiorum. Mol Plant Pathol 19(6):1444–1453
Liu J, Zhang J, Meng Q, Shi F, Ma L, Li Y, Zuo Y (2016) Differentiation in pathogenicity of Sclerotinia sclerotiorum on sunflower in Heilongjiang province (in chinese). Plant Protect 42:119–124
Liu J, Meng Q, Zhang Y, Xiang H, Li Y, Shi F, Ma L, Liu C, Liu Y, Su B, Li Z (2018) Mycelial compatibility group and genetic variation of sunflower Sclerotinia sclerotiorum in Northeast China. Physiol Mol Plant Pathol 102:185–192
Lujan P, Sanogo S, Puppala N, Randall J (2016) Factors affecting mycelium pigmentation and pathogenicity of Sclerotinia sclerotiorum on Valencia peanut. Can J Plant Sci 96:461–473
Morrall RAA, Duczek LT, Sheard JW (1972) Variations and correlations within and between morphology, pathogenicity and pectolytic enzyme activity in Sclerotinia from Saskatchewan. Can J Bot 50:767–786
Nahar MS, Naher N, Alam MJ, Hossain MS, Mian MY, Miller SA (2019) Variation in isolates of Sclerotinia sclerotiorum (Lib.) De Bary causing white mold disease in Bangladesh crops. Crop Protect 124:104849
Pannullo A, Kamvar ZN, Miorini TJJ, Steadman JR, Everhart SE (2019) Genetic variation and structure of Sclerotinia sclerotiorum population from soybean in Brazil. Trop Plant Pathol 44:53–64
Rollins JA (2003) The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol Plant Microbe Interact 16:785–795
Steadman JR, Marcinkowska J, Rutledge S (1994) A semi-selective medium for isolation of Sclerotinia sclerotiorum. Can J Plant Pathol 16:68–70
Tok FM, Dervis S, Arslan M (2016) Analysis of genetic diversity of Sclerotinia sclerotiorum from eggplant by mycelial compatibility, random amplification of polymorphic DNA (RAPD) and simple sequence repeat (SSR) analyses. Biotechnol Biotechnol Equip 30:921–928
Wu BM, Subbarao KV (2006) Analyses of lettuce drop incidence and population structure of Sclerotinia sclerotiorum and S. minor. Phytopathology 96:1322–1329
Xia S, Xu Y, Hoy R, Zhang J, Qin L, Li X (2020) The notorious soilborne pathogenic fungus Sclerotinia sclerotiorum: an update on genes studied with mutant analysis. Pathogens 9:27
Xu XQ, Zhang ZQ (2000) Kinetic spectrophotometric determination of oxalic acid based on the catalytic oxidation of bromophenol blue by dichromate. Mirochimica Acta 135:169–172
Xu L, Xiang M, White D, Chen W (2015) pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum. Environ Microbiol 17:2896–2909
Xu L, Li G, Jiang D, Chen W (2018) Sclerotinia sclerotiorum: An evaluation of virulence theories. Annu Rev Phytopathol 56:15.1-15.28
Zhan J, Pettway RE, McDonald BA (2003) The global genetic structure of the wheat pathogen Mycosphaerella graminicola is characterized by high nuclear diversity, low mitochondrial diversity, regular recombination, and gene flow. Fungal Genet Biol 38:286–297
Acknowledgements
The authors are grateful to Director, ICAR- Central Potato Research Institute, Shimla, Himachal Pradesh, India for providing research facilities to conduct this study.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
SC and ML contributed by conceptualization and designing the methodology and the experiments. SC collected isolates from diseased samples and along with SS performed the mycelial compatibility and pathogenicity assay. SS and ALM performed oxalic acid production assay. SC and SS wrote the original draft of the manuscript and MK and ML reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhary, S., Lal, M., Sagar, S. et al. Variation in oxalic acid production, mycelial compatibility and pathogenicity amongst isolates of Sclerotinia sclerotiorum causing white mold disease. Vegetos (2023). https://doi.org/10.1007/s42535-023-00676-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-023-00676-4