Abstract
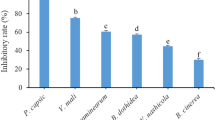
Sclerotium rolfsii, Rhizoctonia solani and Fusarium oxysporum are major soil-borne plant pathogens causing great economic losses in crop productivity. Although chemical fungicides reduce the severity of plant diseases they cause, their continuous use provoke a negative effect on environment and human health. Mosiera bullata is an endemic Cuban species belonging to Myrtaceae family. The objective of the present investigation was to evaluate the effect of M. bullata extract on mycelial growth and microscopic detection of morphology alteration in fungal structures of three species of plant pathogenic fungi. The extract obtained showed total phenolic compounds about 336.23 mg of chlorogenic acid/g and total flavonoid compounds about 91.52 mg of rutin/g of dry extract. M. bullata extract inhibited the mycelial growth of S. rolfsii (55.42%), F. oxysporum (54.63%) and R. solani (11.67%). This extract caused deformations at microscopic level in three fungi species evaluated. According with the in vitro antifungal activity of M. bullata observed against three major soil-borne plant pathogens, this plant species may be considered as a potential source of natural fungicides, but further studies at greenhouse and field are required to confirm it.



Similar content being viewed by others
Data availability
Not applicable.
References
Ajayi-Oyetunde OO, Bradley CA (2018) Rhizoctonia solani: taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol 67(1):3–17. https://doi.org/10.1111/ppa.12733
Al-Huqail AA, Behiry SI, Salem MZ, Ali HM, Siddiqui MH, Salem AZ (2019) Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill) HL Wendl flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 24(4):700
Asgarpanah J, Ariamanesh A (2015) Phytochemistry and pharmacological properties of Myrtus communis L. Indian J Tradit Knowl 14(1):82–87
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Bastos RG, Rosa CP, Oliver JC, Silva NC, Dias AL, Rocha CQ, Vilegas W, Silva GA, Silva MA (2016) Chemical characterization and antimicrobial activity of hydroethanolic crude extract of Eugenia florida DC (Myrtaceae) leaves. Int J Pharm Pharm Sci 8(6):110–115
Behiry SI, Okla MK, Alamri SA, El-Hefny M, Salem MZ, Alaraidh IA, Ali HM, Al-Ghtani SM, Monroy JC, Salem AZ (2019) Antifungal and antibacterial activities of Musa paradisiaca L peel extract HPLC analysis of phenolic and flavonoid contents. Processes 7(4):215
Billah KM, Hossain B, Prince MH, Sumon MP (2017) Pathogenicity of Sclerotium rolfsii on different host, and its over wintering survival; A mini review. Inter J Adv Agri Sci 2(7):1–6
Campaniello D, Corbo MR, Sinigaglia M (2010) Antifungal activity of eugenol against Penicillium, Aspergillus and Fusarium species. J Food Prot 73(6):1124–1128. https://doi.org/10.4315/0362-028X-73.6.1124
Cascaes MM, Guilhon GMSP, Andrade EHdA, Zoghbi MDGB, Santos LDS (2015) Constituents and pharmacological activities of Myrcia (Myrtaceae): a review of an aromatic and medicinal group of plants. Int J Mol Sci 16(10):23881–23904. https://doi.org/10.3390/ijms161023881
Chakrapani K, Sinha B, Chanu WT, Chakma T, Siram T (2020) Assessing in vitro antifungal activity of plant extracts against Rhizoctonia solani causing sheath blight of rice (Oryza sativa L). J Pharm Phytochem 9(1):1497–1501
Chávez AR, Jara ASA (2014) Control of soil fungi Rhizoctonia sp. Fusarium sp. and Sclerotium sp. with plant extracts. Investigación Agraria. 14(1):17–23
Cherkupally R, Kota SR, Amballa H, Reddy BN (2017) In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Annal Plant Sci. 6(9):1676–1680
Czaja K, Góralczyk K, Struciński P, Hernik A, Korcz W, Minorczyk M, Łyczewska M, Ludwicki JK (2015) Biopesticides-towards increased consumer safety in the European Union. Pest Manag Sci 71(1):3–6. https://doi.org/10.1002/ps.3829
Daglia M (2012) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23(2):174–181. https://doi.org/10.1016/j.copbio.2011.08.007
Das M, Goswami S (2019) Antifungal and antibacterial property of guava (Psidium guajava) leaf extract: Role of phytochemicals. Intern J Health Sci Res 9(2):39–45
Das K, Tiwari RK, Shrivastava DK (2010) Techniques for evaluation of medicinal plant products as antimicrobial agents: Current methods and future trends. J Med Plants Res 4:104–111. https://doi.org/10.5897/jmpr09.030
dos Santos Rocha T, de Jesus ME, do Nascimento CM, Souza RRM, da Costa Silva M, de Souza Neta LC, Vale VLC (2021) Chemical and biological profile of Psidium bahianum landrum & funch (Myrtaceae). Brazilian J Botany 44(3):537–547. https://doi.org/10.1007/S40415-021-00727-7
Duarte-Leal Y, Martínez-Coca B, Café-Filho AC, Bassay-Blum LE (2020) Physiological and cultural characterization and mycelial compatibility of Sclerotium sp isolates from seven plant hosts. Revista De Protección Vegetal. 35(1):1–10
EL-Hefny M, Salem MZ, Behiry SI, Ali HM (2020) The potential antibacterial and antifungal activities of wood treated with Withania somnifera fruit extract, and the phenolic, caffeine, and flavonoid composition of the extract according to HPLC. Processes 8(1):113. https://doi.org/10.3390/pr8010113
Emara AR, Ibrahim HM, Masoud SA (2021) The role of storage on Mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Arab J Chem 14(10):103322. https://doi.org/10.1016/j.arabjc.2021.103322
Faria TDJ, Ferreira RS, Yassumoto L, Souza JRPD, Ishikawa NK, Barbosa ADM (2006) Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz Arch Biol Technol 49:867–871. https://doi.org/10.1590/S1516-89132006000700002
Freiesleben SH, Jäger AK (2014) Correlation between plant secondary metabolites and their antifungal mechanisms: a review. Med Aromatic Plants 3(2):154. https://doi.org/10.4172/2167-0412.1000154
González-Torres LR, Palmarola AL, González-Oliva L, Bécquer ER, Testé E, Barrios D (2016) Lista roja de la Flora de Cuba. Bissea 10:1–352
Goya-Jorge E, Fernández-Expósito O, Herrero-Martínez JM, Simó-Alfonso EF, Castañeda-Noa I, Jorge-Rodríguez ME (2022) Chemical composition of essential oils from the leaves of Mosiera bullata (Britton & P Wilson), an unexplored Cuban endemic species. J Essent Oil Res 34(2):123–130
Gurjar MS, Ali S, Akhtar M, Singh KS (2012) Efficacy of plant extracts in plant disease management. Agric Sci 3(3):425–433. https://doi.org/10.4236/as.2012.33050
Gurr S, McPherson J, Bowles D (1992) Lignin and associated phenolic acids in cell walls. In: Wilkinson DL (ed) Molecular Plant Pathology. Oxford Press, Oxford, pp 51–56
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429. https://doi.org/10.1016/j.foodcont.2014.05.047
Gyawali R, Hayek SA, Ibrahim SA (2015) Plant extracts as antimicrobials in food products: Mechanisms of action, extraction methods, and applications. In: Taylor TM (ed) Handbook of Natural Antimicrobials for Food Safety and Quality. Sawston, UK, Woodhead Publishing
Kavitha K, Murali M, Jayachandra K (2011) Preliminary phytochemical screening, anthelmintic activity of methanolic and aqueous extract of Syzygium cumini Linn. Bark (Myrtaceae). J Pharm Sci Res 3(9):1460–1465
Kim D, Jeong S, Lee C (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326. https://doi.org/10.1016/S0308-8146(02)00423-5
Kocić-Tanackov S, Dimić G, Lević J, Tanackov I, Tuco D (2011) Antifungal activities of basil (Ocimum basilicum L) extract on Fusarium species. Afr J Biotech 10(50):10188–10195
Kolhe Rohini C, Ghode Shweta P, ChaturVibhavari D, Ghode Prashant D, Chaudhari Rajesh Y, Patil Vijay R (2021) Preliminary phytochemical detection, quantitative estimation of total flavonoids, total phenols and antioxidant activity of leaves of Syzygium malaccense (Myrtaceae). Euro J Mol Clin Med 7(9):2651–2658
Lattanzio V, Kroon PA, Quideau S, Treutter D (2008) Plant phenolics - secondary metabolites with diverse functions. In: Daayf F, Lattanzio V (eds) Recent Advances in Polyphenol Research, vol 1. Blackwell Publishing Ltd, UK
Lee H, Lee D (2015) Mode of action of bioactive phytochemicals, plant secondary metabolites, possessing antimicrobial properties. In: Méndez A (ed) The Battle against Microbial Pathogens Basic Science. Technological Advances and Educational Programs, Formatex Research Center
López-Zapata SP, Castaño-Zapata J (2019) Integrated management of Panama disease Fusarium oxysporum Schlechtend Fr f sp cubense (EF SM) WC Snyder & HN Hansen: a review Revista UDCA. Actualidad Divulgación Científica. 22(2):1240
Martín MC, Leyva L, Suárez MA, Pichardo T, Caraballoso IB, Capó YA (2021) Antifungal activity of Bacillus amyloliquefaciens against Fusarium oxysporum f sp cubense race 1. Agronomía Mesoamericana. 32:466–478
Mesa VAM, Marín P, Ocampo O, Calle J, Monsalve Z (2019) Fungicidas a partir de extractos vegetales: una alternativa en el manejo integrado de hongos fitopatógenos. RIA Revista De Investigaciones Agropecuarias 45(1):23–30
Morcia C, Malnati M, Terzi V (2012) In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Additi Conta 29(3):415–422. https://doi.org/10.1080/19440049.2011.643458
Ngbolua KN, Lufuluabo LG, Moke LE, Bongo GN, Liyongo CI, Ashande CM, Sapo BS, Zoawe BG, Mpiana PT (2018) A review on the phytochemistry and pharmacology of Psidium guajava L. (Myrtaceae) and future direction. Discov Phytomed. 5(2):7–13
Nguyen NT, Vicet-Muro L, Siverio-Mota D, Jorge-Rodriguez ME, González-Mosquera DM, Castañeda-Noa I (2016) Tamizaje fitoquímico y evaluación de la actividad sobre el sistema nervioso central del extracto etanólico de Eugenia clarensis Britton & P Wilson. J Pharm Pharm Res. 4(1):39–48
Nugroho C, Mirnia E, Cumagun CJR (2019) Antifungal activities of sweet basil (Ocimum basilicum L) aqueous extract against Sclerotium rolfsii, causal agent of damping-off on tomato seedling. AGRIVITA, J Agri Sci. 41(1):149–157
Olea AF, Bravo A, Martínez R, Thomas M, Sedan C, Espinoza L, Zambrano E, Carvajal D, Silva-Moreno E, Carrasco H (2019) Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 24(7):1239. https://doi.org/10.3390/molecules24071239
Paiva PMG, Gomes FS, Napoleão TH, Sá RA, Correia MTS, Coelho LCBB (2010) Antimicrobial activity of secondary metabolites and lectins from plants. In: Mendez Vilas A (ed) Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology 1(2). Badajoz, Formatex Research Center
Paparu P, Acur A, Kato F, Acam C, Nakibuule J, Nkuboye A, Musoke S, Mukankusi C (2020) Morphological and pathogenic characterization of Sclerotium rolfsii, the causal agent of southern blight disease on common bean in Uganda. Plant Dis 104(8):2130–2137. https://doi.org/10.1094/PDIS-10-19-2144-RE
Parwanayoni NMS, Suprapta DN, Temaja IGRM, Swantara IMD, Khalimi K (2018) Synergistic activity of leaves extracts of Mansoaalliacea, L and Allamanda cathartica L to inhibit Athelia rolfsii, the cause of stem rot disease in peanut plants. J Biol 8(4):29–35
Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM (2013) Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem 20(7):932–952
Rafiq M, Javaid A, Shoaib A (2021) Antifungal activity of methanolic leaf extract of Carthamus oxycantha against Rhizoctonia solani. Pak J Bot 53(3):1133–1139
Ramirez-Mares MV, Hernandez-Carlos B (2015) Plant-derived natural products from the American continent for the control of phytopathogenic fungi: A review. J Global Inn Agri Social Sci. 3(4):96–118
Rodríguez LL, Cruz-Martín M, Acosta-Suárez M, Pichardo T, Bermúdez-Caraballoso I, Alvarado-Capó Y (2017) Antagonismo in vitro de cepas de Bacillus spp frente a Fusarium oxysporum f sp cubense. Biotecnología Vegetal. 17(4):229–236
Salazar-González M, Sánchez-Cuevas M, Silva-Acuña R, Romero-Marcano G (2019) Evaluation of aqueous extracts from nine tropical plants in the in vitro growth of the phytopathogenic fungus Sclerotium rolfsii Sacc. SABER 31:271–282
Samada LH, Tambunan USF (2020) Biopesticides as promising alternatives to chemical pesticides: A review of their current and future status On Line. J Biol Sci 20(2):66–76. https://doi.org/10.3844/ojbsci.2020.66.76
Seo J, Lee S, Elam ML, Johnson SA, Kang J, Arjmandi BH (2014) Study to find the best extraction solvent for use with guava leaves (Psidium guajava L) for high antioxidant efficacy. Food Sci Nutr 2(2):174–180
Shaikh JR, Patil MK (2020) Qualitative tests for preliminary phytochemical screening: An overview. Inter J Chem Stud. 8(2):603–608
Shukla R, Singh P, Prakash B, Dubey NK (2012) Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm) Sweet essential oil and its major component 1, 8-cineole against fungal isolates from chickpea seeds. Food Control 25(1):27–33
Siddique S, Parveen Z, Firdaus-e-Bareen MA, Akram M (2021) In vitro antifungal activities of essential oils from selected species of family Myrtaceae. PhOL PharmacologyOnLine 3:1612–1625
Singha IM, Unni BG, Kakoty Y, Das J, Wann SB, Singh L, Kalita MC (2011) Evaluation of in vitro antifungal activity of medicinal plants against phytopathogenic fungi. Arch Phytopathol Plant Prot 44(11):1033–1040. https://doi.org/10.1080/03235401003672913
Souza-Moreira TM, Severi JA, Rodríguez ER, de Paula MI, Freitas JA, Vilegas W, Pietro RC (2019) Flavonoids from Plinia cauliflora (Mart) Kausel (Myrtaceae) with antifungal activity. Nat Prod Res 33(17):2579–2582
Takó M, Kerekes EB, Zambrano C, Kotogán A, Papp T, Krisch J, Vágvölgyi C (2020) Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 9:165. https://doi.org/10.3390/antiox9020165
Urquiola-Cruz AJ, Acosta-Ramos Z (2008) Cuban novelties in the genus Mosiera (Myrtaceae). Willdenowia 38(2):533–544. https://doi.org/10.3372/wi.38.38213
Wang C, Zhang J, Chen H, Fan Y, Shi Z (2010) Antifungal activity of eugenol against Botrytis cinerea. Tropical Plant Pathol 35:137–143. https://doi.org/10.1590/S1982-56762010000300001
Yang JY, Chung KR, Huang JW (2020) A combined effect of Bacillus sp, tobacco extracts and plant oils on the control of cruciferous vegetable anthracnose. Arch Phytopathol Plant Prot. 53(1–2):48–69
Yoon MY, Cha B, Kim JC (2013) Recent trends in studies on botanical fungicides in agriculture. Plant Pathol J 29(1):1–9. https://doi.org/10.5423/PPJ.RW.05.2012.0072
Yu D, Wang J, Shao X, Xu F, Wang H (2015) Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J Appl Microbiol 119(5):1253–1262. https://doi.org/10.1111/jam.12939
Zaynab M, Fatima M, Abbas S, Sharif Y, Umair M, Zafar MH, Bahadar K (2018) Role of secondary metabolites in plant defense against pathogens. Microb Pathog 124:198–202. https://doi.org/10.1016/j.micpath.2018.08.034
Acknowledgements
This research was supported by the Bioplantas Centre, Universidad de Ciego de Ávila. Authors are also grateful to Plant Biotechnology Institute (IBP) for their collaboration and donating microorganisms. Authors are grateful to Dr. Yanier Acosta and Mr. Gustavo Lorente for their important scientific suggestions.
Author information
Authors and Affiliations
Contributions
LPG, JMR, YQM, RTS and ATPM designed the research; LPG, JMR, RTS and ATPM conducted the experiments; LPG, JMR, YQM, MLM, MEMM, MAS, AFS and ATPM analyzed the data; LPG, JMR, MLM, MEMM, AFS and ATPM wrote the paper; LPG, JMR, MLM, MEMM, RTS and ATPM had primary responsibility for the final content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors do not have any conflict of interests.
Human and animal rights
This research did not involve experiments with human or animal participants.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez Gómez, L., Mendoza Rodríguez, J., Quirós Molina, Y. et al. Antifungal activity of Mosiera bullata (Britton & P. Wilson) extract against phytopathogenic fungi. Vegetos 36, 1295–1304 (2023). https://doi.org/10.1007/s42535-022-00540-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00540-x