Abstract
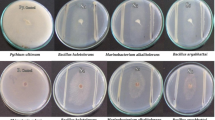
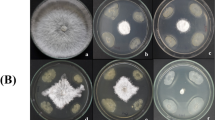
Biological control of soil-borne plant infections is sorely needed since they are challenging to treat with conventional fungicides. This fundamental research was aimed to find bacteria that live in saline soils (halophilic or halotrophic) and have antagonistic activity against Sclerotium oryzae and Ralstonia solani. A total of fifty bacterial strains were recovered from the saline soils of Gangavathi, Karnataka, India. Eight of the fifty bacterial strains in the dual culture approach demonstrated antibiosis against S. oryzae and R. solani. The isolates that produced HCN, siderophore, and hydrolytic enzymes strongly correlated with their biocontrol activity. Arnow’s and Csaky’s methods were used for testing the types of siderophores formed, and only a few isolates produced both catecholate and hydroxamate types. In Arnow’s and Csaky’s assays, the isolate Bacillus albus (HB-17) showed catechol and hydroxamate type siderophores. These prospective isolates’ identity was previously confirmed using 16 S rRNA analysis of Bacillus cereus, Bacillus albus, Bacillus safensis, Staphylococcus xylosus, Lysinibacillus sphaericus, and Pseudomonas stutzeri. Antibiotic resistance testing revealed that the isolates were resistant to Penicillin, Ofloxacin, and Vancomycin. Furthermore, the strains were tested for their plant growth-promoting properties, such as phosphorous (P) and zinc (Zn) solubilization, and potassium (K) release, at three NaCl concentrations (3, 6, and 10% w/v). At a concentration of 6% NaCl, the solubilization zones of zinc carbonate (ZnCO3), zinc oxide (ZnO), and phosphorous (Ca3PO4) were 5.20–9.0 mm, 6.06–13.20 mm, and 6.30–9.70 mm, respectively. Finally, we investigated the isolation of new bacterial strains with antifungal activity from saline soils in Gangavathi, Karnataka, India’s untapped agro-ecological habitats. This research also provides the path for further characterization of these isolates to maximize their antifungal potential.





Similar content being viewed by others
References
Andersson DI, Hughes D (2012) Evolution of antibiotic resistance at non-lethal drug concentration. Drug Resist Updat 15:162–172. doi: https://doi.org/10.1016/j.drup.2012.03.005
Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: https://doi.org/10.1038/nrmicro3270
Arnow LE (1937) Colorimetric determination of the components of 3,4-dihy droxyphenylalanine-tyrosine mixtures. J Biol Chem 118:531–537
Bahlm MI, Sørensen SJ, Hansen LH, Licht TR (2004) Effect of tetracycline on transfer and establishment of the tetracycline-inducible conjugative transposon Tn916 in the guts of gnotobiotic rats. Appl Environ Microbiol 70:758–764. doi: https://doi.org/10.1128/AEM.70.2.758-764.2004
Barthalomew JW, Mittewar T (1950) A simplified bacterial strain. Strain Tech 25:153
Berrada I, Anne W, Paul DV, ElMostafa EF, Jean S, Najib B, Marouane M, Mohamed A (2012) Diversity of culturable moderately halophilic and halotolerant bacteria in a marsh and two salterns a protected ecosystem of Lower Loukkos (Morocco). Afr J Microbiol Res 6(10):2419–2434
BhaskarRao T, Ramakrishna C, Ramesh M, Punniakotti E, Venkatesh V, Sailaja B, Raghurami RM, Arra Y, Laha GS, Sheshu M, Sundaram RM, Ladhalakshmi D, Balachandran SM, Satendra KM (2019) Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol Biol 100:59–71
Cappuccino JC, Sherman N (2013) Microbiology: a laboratory manual, 10th edn. Pearson Pub. Co., New York, Harlow, Essex
Castric KF, Castric PA (1983) Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol 45:700–702
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Coleman MB, Owen HH, Dacey HG (1930) Fermentation of monosaccharides by organisms of the Abortus melitensis group. J Lab Clin Med 15:641–642
Cother E, Nicol H (1999) Susceptibility of Australian rice cultivars to stem rot fungus Sclerotium oryzae. Austra Plant Path 28:85–91
Couillerot O, Prigent-Combaret C, Caballero-Mellado J, Moënne-Loccoz Y (2009) Pseudomonas fluorecens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett Appl Microbiol 48:505–512
Cowan ST, Steel KJ (1965) Manual for the identification of medical bacteria. J Clin Pathol 46:975
Cray JA, Connor MC, Stevenson A, Houghton JDR, Rangel DEN, Cooke LR, Hallsworth JE (2015) Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb Biotech 9:330–354. https://doi.org/10.1111/1751-7915.12349
Csaky TZ (1948) On the estimation of bound hydroxylamine in biological materials. Acta Chem Scand 2:450–454
Dave BP, Kena A, Puja H (2006) Siderophores of halophilic archaea and their chemical characterization. Indian J Exp Biol 44:340–344
Eynard A, Lal R, Wiebe K (2005) Crop response in salt-affected soils. J Sustain Agric 27:5–50. https://doi.org/10.1300/J064v27n01_03
Fasim F, Ahmed N, Parsons R, Gadd GM (2002) Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett 213:1–6
Ferris H, Tuomisto H (2015) Unearthing the role of biological diversity in soil health. Soil Biol Biochem 85:101–109
Fich EA, Segerson NA, Rose JK (2016) The plant polyester cutin: biosynthesis, structure, and biological roles. Annu Rev Plant Biol 67:207–233
Franco N, Jayleen D, Jose R, Karlo ML (2018) Assessment of halophilic bacteria in the salt flats and wildlife refuge in Cabo Rojo, Puerto Rico and their antibiotic resistance pattern. EC Microbiol 14(10):707–714
Gillican CA (2008) Sustainable agriculture and plant diseases: An epidemiological perspective. Phil Trans R Soc B 27:741–756
Gopika K, Jagadeeswar R, Krishna rao V, Vijayalakshmi K (2017) Isolation, identification and evaluation of native antagonists microflora from stem rot infected rice plants against Sclerotium oryzae. Bull Env Pharmacol Life sci 6(2):349–353
Graham EB (2016) Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Front Microbiol 7:214
Guerin E, Cambray G, Sanchez-Alberola N, Campoy S, Erill I, Da Re S (2009) The SOS response controls integron recombination. Science 324:1034. doi: https://doi.org/10.1126/science.1172914
Hane JK, Anderson JP, Williams AH, Sperschneider J, Singh KB (2014) Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet 10:1004281
JL (1975) Powdered chitin agar as a selective medium for enumeration of Actinomycetes in water and soil. 29:422–426 3
Jackson LF, Webster RK, Wick CM, Bolstad J, Wilkerson JA (1977) Chemical control of stem rot of Rice in California. Phytopathology 67:1155–1158
Kushner DJ (1993) Growth and nutrition of halophilic bacteria. In: Vreeland RH, Hochstein L (eds) The biology of halophilic bacteria. CRC Press, Boca Raton, pp 87–103
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Path 132:1–41
Liu Y (2017) Ethylene signaling is important for isofavonoid-mediated resistance to Rhizoctonia solani in roots of Medicago truncatula. Mol Plant Microbe Interact 30:691–700
Maclean JL, Dawe DC, Hardy B, Hettel GP (2002) Rice Almanac. Los Banos, Phillippines. International Rice research Institute; West Africa Rice Development Association; International center for tropical agriculture; Food and Agriculture Organization, Bouake; Cali; Rome, p 253
Manwar AV, Khandelwal SR, Chaudhari BL, Meyer JM, Chincholkar SB (2004) Siderophore production by a marine Pseudomonas aeruginosa and its antagonistic action against phytopathogenic fungi. Appl Biochem Biotechnol 118:243–251
Martins JG, Martin C, Hernández-Apaolaza L, Barros MT, Soares HMVM, Lucena JJ (2018) Azotochelin and N-dihydroxy-N, N’-diisopropylhex anediamide as Fe sources to cucumber plants in hydroponic cultures. Emirates J Food Agric 30:65–76. https://doi.org/10.9755/ejfa.2018.v30. i1.1586
Menasria T, Monteoliva-Sánchez M, Benammar L, Benhadj M, Ayachi A, Hacène H, Gonzalez-Paredes A, Aguilera M (2019) Culturable halophilic bacteria inhabiting Algerian saline ecosystems: a source of promising features and potentialities. World J Microb Biotech 35(9):1–6
Nagaraju Y, Gundappagol RC, Mahadevaswamy (2020) Mining saline soils to manifest plant stress-alleviating halophilic bacteria. Curr Microbiol 77:2265–2278. https://doi.org/10.1007/s00284-020-02028-w
Nieto JJ, Fernandez CR, Garcia MT, Mellado E, Ventos A (1993) Survey of antimicrobial suceptability of moderately halophilic eubacteria and extremely halophilic aerobic arcahaeobacteria: utilization of antibiotic resistance as a genetic marker. Syst Appl Microbiol 16:352–360
Oren A (2010) Industrial and environmental applications of halophilic microorganisms. Environ Technol 31:825–834
Pan M, Chu LM (2016) Adsorption and degradation of five selected antibiotics in agricultural soil. Sci Total Environ 545–546:48–56. doi: https://doi.org/10.1016/j.scitotenv.2015.12.040
Payne SM (1994) Detection, isolation and characterization of siderophores. Methods Enzymol 235:329–344
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologiya 17:362–370
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie V Leeuwen 81:537–547
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014) Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in vertisols of central India. Appl Soil Ecol 73:87–96
Reetha S, Bhuvaneswari G, Thamizhiniyan P, Mycin TR (2014) Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim cepa. L). Int J Curr Microbiol App Sci 3:568–574
Richa K, Tiwari IM, Kumari M, Devanna BN, Sonah H, Kumari A, Nagar R, Sharma V, Botel JR, Tilakr STR (2016) Functional characterization of novel chitinase genes present in the sheath blight resistance QTL: qSBR11-1in rice line tetep. Front Plant Sci 7:1–10
Sambrook J, Fritsch ER, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Saravanan VS, Subramoniam SR, Raj SA (2003) Assessing in vitro solubilization of different zinc solubilizing bacterial (ZBS) strains. Braz J Microbiol 34:121–125
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3:430–439
Schwyn B, Neilands J (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sengupta S, Chattopadhyay MK, Grossart HP (2013) The multifaceted roles of antibiotics and antibiotic resistance in nature. Front Microbiol 4:47. doi: https://doi.org/10.3389/fmicb.2013.00047
Shahab S, Ahmed N (2008) Effect of various parameters on the efficiency of zinc phosphate solubilization by indigenous bacterial isolates. Afr J Biotechnol 7(10):1543–1549
Shawish R, Tarabees R (2017) Prevalence and antimicrobial resistance of Bacillus cereus isolated from beef products in Egypt Reyad. O Vet J 7(4):337–341
Shirling ET, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Simine CDD, Sayer JA, Gadd GM (1998) Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol Fertil Soils 28:87–94
Skidmore AM, Dickinson CH (1976) Colony interaction and hyphal interference between Septoria nodorum and phylloplane fungi. Trans J Br Ceramic Soc 66:57–74
Smibert R, Krieg N, Gerhardt P, Murray R, Wood W (1994) Methods for general and molecular bacteriology. American Society for Microbiology, Washington DC
Song F, Goodman RM (2001) Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol 59:1–11. DOI:https://doi.org/10.1006/pmpp.2001.0353
Tan WZ, Zhang W, Ou ZQ, Li CW, Zhou GJ, Wang ZK, Yin LL (2011) Analyses of the temporal development and yield losses due to sheath blight of Rice (Rhizoctonia solani AG1IA). Agric Sci China 6:1074–1081. https://doi.org/10.1016/S1671-2927(07)60149-7
Tango MSA, Islam MR (2002) Potential of extremophiles for biotechnological and petroleum applications. Energy Sourc 24:543–559
Tank N, Saraf M (2009) Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. J Basic Microbiol 49:195–204. doi: https://doi.org/10.1002/jobm.200800090
Thamthiankul S, Suan-ngay S, Tantimavanich S, Panbangered W (2001) Chitinase from Bacillus thuringiensis subsp. pakistani. Appl Microbi Biotech 56:395–401 https://doi.org/10.1007/s002530100630
VanderHeijden MGA, de Bruin S, Luckerhoff L, van Logtestijn RSP, Schlaeppi KA (2016) widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J 10:389–399
Vora JU, Jain NK, Modi HA (2014) Extraction, characterization and application studies of red pigment of halophile Serratia marcescens KH1R KM035849 isolated from Kharaghoda soil. Int J Pure App Biosci 2(6):160–168
Yandigeri MS, Meena KK, Singh D, Malviya N, Singh DP, Solanki MK, Yadav AK, Arora DK (2012) Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul 68(3):411–420. https://doi.org/10.1007/s10725-012-9730-2
Yellareddygari SK, Reddy MS, Kloepper JW, Lawrence KS, Fadamiro H (2014) Rice sheath blight: a review of disease and pathogen management approaches. J Plant Pathol Microbiol 5(4):241. https://doi.org/10.4172/2157-7471.1000241
Zhao LJ, Liang H, Huang J, Chen Z, Nie Y (2018) Manipulation of the rhizosphere microbial community through applying a new bio-organic fertilizer improves watermelon quality and health. PLoS ONE 13(2):e0192967. doi: https://doi.org/10.1371/journal.pone.0192967
Author information
Authors and Affiliations
Contributions
YN experimented with M, and SBG helped prepare the manuscript and synthesize the hypothesis.
Corresponding author
Ethics declarations
Conflict of interest
The subject matter and materials presented in this work are entirely free of any financial or other conflicts of interest, according to all of the writers. They do not belong to or participate in any groups or businesses that have a stake in the topics or resources covered in this work, whether such interests be pecuniary or not.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagaraju, Y., Mahadevaswamy & Gowder, S.B. Harnessing the saline soil-inhabiting bacteria for antagonistic, antibiotic resistance, and plant growth-promoting attributes. Vegetos 36, 907–919 (2023). https://doi.org/10.1007/s42535-022-00466-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00466-4