Abstract
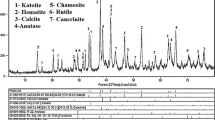
Gibbsite-type bauxite is the main material for alumina extraction by Bayer process globally, while the iron in red mud is difficult to use for the high alumina content. Therefore, the efficient separation of iron and alumina is the premise for the resource utilization of red mud. In this work, the separation of iron and alumina in red mud containing 47.45% Fe and 11.58% Al2O3 was studied through reduction roasting followed by magnetic separation. The analysis methods of XRD, VSM, SEM, and EDS were used to investigate the phase transformation of red mud during reduction roasting. Results show that hematite can be firstly reduced into magnetite, and alumogoethite into magnetite and alumina. Then, the magnetite reduction undergoes the process of Fe3O4→FeO→Fe, while alumina can react with FeO to form hercynite. The hercynite is ultimately reduced into metallic iron and alumina at elevated temperature. The specific saturation magnetization of reduced product is closely related to its main minerals, that is, the specific saturation magnetization of magnetite and metallic iron is higher than that of wustite and hercynite. The mass ratio of Fe to Al2O3 in magnetic concentrate increases with roasting temperature, from 4.55 at 600 °C to 10.27 at 1200 °C. Therefore, efficient separation of iron and alumina in red mud could be achieved through deep reduction-magnetic separation.








Similar content being viewed by others
References
Wang SH, Jin HX, Deng Y, Xiao YD (2020) Comprehensive utilization status of red mud in China: a critical review. J Clean Prod 289:125136. https://doi.org/10.1016/j.jclepro.2020.125136
Zhou GT, Wang YL, Zhang YG, Qi TG, Zhou QS, Liu GH, Li XB (2023) A clean two-stage Bayer process for achieving near-zero waste discharge from high-iron gibbsitic bauxite. J Clean Prod 405:136991. https://doi.org/10.1016/j.jclepro.2023.136991
Wei C, Pan X, Pei JN, Lyu ZY, Yu HY (2023) Preparation and characterization of unfired lightweight bricks using dealkalized calcium silicate residue from low-calcium sintering red mud. J Cent South Univ (Engl Ed) 30:1787–1180. https://doi.org/10.1007/s11771-023-5352-2
International Aluminum Institute (2023) Alumina production statistics reports. URL. https://www.world-aluminium.org/statistics/alumina-production
Mayes WM, Burke IT, Gomes HI, Anton ÁD, Molnár M, Feigl V, Ujaczki É (2016) Advances in understanding environmental risks of red mud after the Ajka spill, Hungary. J Sustain Metall 2:332–343. https://doi.org/10.1007/s40831-016-0050-z
Xue SG, Wang QL, Tian T, Ye YZ, Zhang YF, Zhu F (2019) Regional-scale investigation of salt ions distribution characteristics in bauxite residue: a case study in a disposal area. J Cent South Univ (Engl Ed) 26(2):422–429. https://doi.org/10.1007/s11771-019-4014-x
Pan XL, Wu HF, Lv ZY, Yu HY, Tu GF (2023) Recovery of valuable metals from red mud: a comprehensive review. Sci Total Environ 904:166686. https://doi.org/10.1016/j.scitotenv.2023.166686
Li LL, Wu Zg, Lv H, Liu FQ, Zhao HL (2018) Reaction behavior of aluminogoethite and Silica Minerals in Gibbsite Bauxite in high-temperature digestion. J Sustain Metall 175:257–265. https://doi.org/10.1007/s40831-022-00494-z
Zhang YY, Lu W, Qi YH, Zou ZS (2016) Recovery of iron and calcium aluminate slag from high-ferrous bauxite by high-temperature reduction and smelting process. Int J Min Metall Mater 23(8):881–890. https://doi.org/10.1007/s12613-016-1303-3
Shoppert A, Valeev D, Diallo MM, Loginova I, Beavogui MC, Rakhmonov A, Ovchenkov Y, Pankratov D (2022) High-iron bauxite residue (red mud) valorization using hydrochemical conversion of goethite to magnetite. Materials 15(23):8423. https://doi.org/10.3390/ma15238423
Li XB, Zhou ZY, Wang YL, Zhou QS, Qi TG, Liu GH, Peng ZH (2020) Enrichment and separation of iron minerals in gibbsitic bauxite residue based on reductive bayer digestion. T Nonferr Metal Soc 30(7):1980–1990. https://doi.org/10.1016/S1003-6326(20)65355-9
Wang K, Dou ZH, Liu Y, Li XF, Lv GZ, Zhang TA (2022) Summary of research progress on separation and extraction of valuable metals from Bayer red mud. Environ Sci Pollut Res 29(60):89834–89852. https://doi.org/10.1007/s11356-022-23837-5
Yuan S, Wang RF, Zhang H, Li YJ, Liu L, Fu YF (2022) Investigation of mineral phase transformation technology followed by magnetic separation for recovery of iron values from red mud. Sustainability 14(21):13787. https://doi.org/10.3390/su142113787
Yu JW, Li YF, Lv Y, Han YX, Gao P (2022) Recovery of iron from high-iron red mud using suspension magnetization roasting and magnetic separation. Min Eng 178:107394. https://doi.org/10.1016/j.mineng.2022.107394
Sadangi JK, Das SP, Tripathy A, Biswal SK (2018) Investigation into recovery of iron values from red mud dumps. Sep Sci Technol 53(14):2186–2191. https://doi.org/10.1080/01496395.2018.1446984
Wei D, Xiao JH, Peng Y, Shen SY, Chen T (2019) Iron extraction from red mud using roasting with sodium salt. Min Process Extr Metall Rev 42(3):153–161. https://doi.org/10.1080/08827508.2019.1706049
Xu WZ, Li CH, Wang KJ (2022) Iron recovery from red mud by reduction roasting-magnetic separation. Non-ferrous Met 12(01):57–63. https://doi.org/10.3696/j.issn.2095-1744.2022.01.009
Zinoveev D, Grudinsky P, Zakunov A, Semenov A, Panova M, Valeev D, Kondratiev A, Dyubanov V, Petelin (2019) Influence of Na2CO3 and K2CO3 addition on iron grain growth during carbothermic reduction of red mud. Metals 9(12):1313. https://doi.org/10.3390/met9121313
Wan JY, Chen TJ, Zhou XL, Luo YH, Liu W, Lu QC (2021) Efficient improvement for the direct reduction of high-iron red mud by co-reduction with high-manganese iron ore. Min Eng 174:107024. https://doi.org/10.1016/j.mineng.2021.107024
Luo LQ, Zhang XX, Wang HY, Zheng BT, Wei CX (2021) Comparing strategies for iron enrichment from Zn- and Pb-bearing refractory iron ore using reduction roasting-magnetic separation. Powder Technol 393:333–341. https://doi.org/10.1016/jpowtec.2021.07.085
Liu YJ, Zuo KS, Yang G, Shang Z, Zhang JB (2016) Recovery of ferric oxide from Bayer red mud by reduction roasting-magnetic separation process. J Wuhan Univ Technol 31(2):404–407. https://doi.org/10.1007/s11595-016-1383-y
Funding
This work was financially supported by the National Natural Science Foundation of China (52004194), the University Synergy Innovation Program of Anhui Province (GXXT-2022-083), “Xiaohe” Young Talent Innovation Project in Changsha in 2022, and the Scientific Research Foundation for High-level Talents of Anhui University of Science and Technology (2022yjrc25).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Zhao, Y., Lin, Z. et al. Efficient Separation of Iron and Alumina in Red Mud Using Reduction Roasting and Magnetic Separation. Mining, Metallurgy & Exploration (2024). https://doi.org/10.1007/s42461-024-00990-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42461-024-00990-8