Abstract
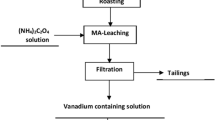
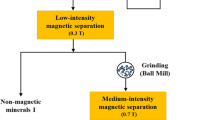
The study focused on the recovery of vanadium from a Korean vanadium-bearing titaniferous magnetite (VTM) ore, examining the behaviors of magnetic separation and Na2CO3 roasting-water leaching. Two concentrates were prepared using single-stage dry magnetic separation and three-step wet magnetic separation, resulting in V2O5 grades of 0.8% and 1.0% and major gangue mineral SiO2 contents of 6.5% and 1.7%, respectively. These concentrates were subsequently roasted at 1050 °C with the addition of Na2CO3 and then water-leached at 25 °C. Various stoichiometric ratios of V2O5 to Na2CO3 were tested, ranging from 1:1 to 1:40 of the theoretically required amount. As the amount of Na2CO3 increased, the vanadium leaching efficiency showed fluctuations between 4 and 35% for ratios of 1:1 to 1:10, while it linearly increased from 4 to 82% for ratios of 1:10 to 1:40 in the leaching of the low-grade concentrate. In contrast, the leaching efficiency increased from 47 to 74% for ratios of 1:1 to 1:8 and then sharply rose to 84% at a ratio of 1:10 in the leaching of the high-grade concentrate. Subsequently, it reached a plateau of 92 to 94% for ratios of 1:20 to 1:40. The difference in leaching efficiency was primarily due to the reaction of gangue minerals with Na2CO3, which hindered vanadium leaching from the VTM concentrates. This hindrance was notably more pronounced in the low-grade concentrate with the lower salt ratio, as the gangue minerals exhibited a preference for consuming Na2CO3 over vanadium. The mineral formation was analyzed in detail using XRD and MLA to shed light on the mechanism of different vanadium leaching behavior depending on the concentrate grade and vanadium to salt ratio. Based on the results of this study, it is evident that applying a proper magnetic separation procedure is significant to achieving satisfactory vanadium recovery with less salt amount by removing gangue minerals ahead.












Similar content being viewed by others
References
Shi Y, Eze C, Xiong B, He W, Zhang H, Lim TM, Ukil A, Zhao J (2019) Recent development of membrane for vanadium redox flow battery applications: a review. Appl Energy 238:202–224
Australian Government (2022) 2022 Critical minerals strategy. https://www.industry.gov.au/sites/default/files/2022-09/2022-critical-minerals-strategy_0.pdf
European Commission (2023) Critical raw materials. https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en
Government of Canada (2022) The Canadian critical minerals strategy. https://www.canada.ca/en/campaign/critical-minerals-in-canada/canadian-critical-minerals-strategy.html
Government of the United Kingdom (2022) Resilience for the future: the United Kingdom’s critical minerals strategy. https://www.gov.uk/government/publications/uk-critical-mineral-strategy/resilience-for-the-future-the-uks-critical-minerals-strategy#what-is-a-critical-mineral
Korean Government (2023) Measures for securing critical minerals supply. https://www.korea.kr/common/download.do?fileId=197226696&tblKey=GMN
USGS (2022) 2022 Final list of critical minerals. Federal Register 87(37):10381–10382
Gao F, Du H, Wang S, Chen B, Li J, Zhang Y, Li M, Liu B, Olayiwola AU (2022) A comparative study of extracting vanadium from vanadium titano-magnetite ores: calcium salt roasting vs sodium salt roasting. Miner Process Extr Metall Rev. 44(5):352–364. https://doi.org/10.1080/08827508.2022.2069105
Lee J, Kurniawan KE, Chung KW, Kim R, Jeon H (2021) A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production. J Mater Res Technol 12:343–364
Moskalyk RR, Alfantazi AM (2003) Processing of vanadium: a review. Miner Eng 16:793–805
Taylor PR, Shuey SA, Vidal EE, Gomez JC (2006) Extractive metallurgy of vanadium-containing titaniferous magnetite ores: a review. Miner Metall Process 23(2):80–86
Li HY, Wang C, Lin M, Guo Y, Xie B (2020) Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag. Powder Technol 360:503–508
Luo Y, Che X, Cui X, Zheng Q, Wang L (2021) Selective leaching of vanadium from V-Ti magnetite concentrates by pellet calcification roasting-H2SO4 leaching process. Int J Min Sci Technol 31:507–513
Wen J, Jiang T, Liu Y, Xue X (2019a) Extraction behavior of vanadium and chromium by calcification roasting-acid leaching from high chromium vanadium slag: optimization using response surface methodology. Miner Process Extr Metall Rev 40(1):56–66
Wen J, Jiang T, Xu Y, Cao J, Xue X (2019b) Efficient extraction and separation of vanadium and chromium in high chromium vanadium slag by sodium salt roasting-(NH4)2SO4 leaching. J Ind Eng Chem 71:327–335
Zhang S, Li G, Xiao R, Luo J, Yi L, Rao M (2021) Extraction of vanadium from low-vanadium grade magnetite concentrate pellets with sodium salt. J Mater Res Technol 15:5712–5722
Choi IH, Kim HR, Moon G, Jyothi RK, Lee JY (2018) Spent V2O5-WO3/TiO2 catalyst processing for valuable metals by soda roasting-water leaching. Hydrometallurgy 175:292–299
Navarro R, Guzman J, Saucedo I, Revilla J, Guibal E (2007) Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manage 27:425–438
Nazari E, Rashchi F, Saba M, Mirazimi SMJ (2014) Simultaneous recovery of vanadium and nickel from power plant fly-ash: optimization of parameters using response surface methodology. Waste Manage 34:2687–2696
Tan H, Fan B, Zheng S, Zhang Y (2023) Recovery and separation of vanadium, nickel, and molybdenum from the industrial waste of a petroleum refinery by a complexation method. ACS Sustain Chem Eng 11:4894–4902
Roskill (2019) Vanadium outlook to 2028. Roskill, London
Kim KJ, Choi S, Park YR, Lee JH, Park JY, Kim SJ (2007) Magnetic and electronic properties of vanadium-substituted magnetite VxFe3-xO4 thin films. J Magn Magn Mater 310:e876–e877
Van Vuuren CPJ, Stander PP (2001) The oxidation of FeV2O4 by oxygen in a sodium carbonate mixture. Miner Eng 14(7):803–808
Gilligan R, Nikoloski AN (2020) The extraction of vanadium from titanomagnetites and other sources. Miner Eng 146:106106
Largo Inc (2021) An updated life of mine plan (“LOMP”) for Campbell pit and pre-feasibility study for Nan and Gan deposits: Maracas Menchen Project, Bahia, Brazil Independent NI 43-101 Technical Report
Bushveld Vametco Alloys (Pty) Ltd (2020) Competent persons’ report on the Vametco vanadium mine. North West Province, South Africa
Kim Y, Yoo J, Park H, Han Y (2022) Evaluation of variables determining leaching efficiency in vanadium extraction using alkali roasting. Metall Mater Trans B 53:3680–3689
Brady PV, Walther JV (1989) Controls on silicate dissolution rates in neutral and basic pH solutions at 25°C. Geochim Cosmochim Acta 53:2823–2830
Nath M, Song S, Garbers-Craig AM, Li Y (2018) Phase evolution with temperature in chromium-containing refractory castables used for waste melting furnaces and Cr(VI) leachability. Ceram Int 44:20391–20398
Tole MP, Lasaga AC, Pantano C, White WB (1986) The kinetics of dissolution of nepheline (NaAlSiO4). Geochim Cosmochim Acta 50:379–392
Han Y, Kim S, Go B, Lee S, Park S, Jeon H (2021) Optimized magnetic separation for efficient recovery of V and Ti enriched concentrates from vanadium-titanium magnetite ore: Effect of grinding and magnetic intensity. Powder Technol 391:282–291
Funding
This research was supported by the Basic Research Project (GP2022-010, 24-3212-1) of the Korea Institute of Geoscience and Mineral Resources (KIGAM), funded by the Ministry of Science and ICT of the Republic of Korea. This work was also supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (No. 20216110100040/IP2023-011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 292 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, R., Eom, Y., Ahn, Y. et al. The Effect of Different Magnetic Separation Procedures of a Korean VTM Ore on Combined Pyro-hydrometallurgical Vanadium Recovery Behavior. Mining, Metallurgy & Exploration 41, 547–558 (2024). https://doi.org/10.1007/s42461-024-00943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-024-00943-1