Abstract
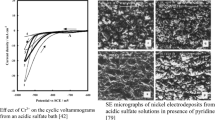
The indium recovery via electrowinning from sulfate baths has recently gained significant attention as it does not present toxic emissions, hermetically sealed system requirements, human health, and environmental hazards. Following previous works related to the optimization process for AISI 316L and Ni cathode, it was observed significant importance of the metal supports on the input and output parameters of the indium electrowinning process from sulfate solutions. Thus, comparing input and output parameters in both cases seems very interesting. Particularly, regarding the input parameters, the attention has been focused on the electrolyte composition, current density, and temperature, while productivity, morphology, and structure have been considered regarding the output parameters. Considering the productivity as the optimal output, the findings for Ni cathode were better than that for AISI 316L in most of the selected conditions studied. In the case of morphology, indium grains appear rounded and dendritic on the AISI 316L, while those on the Ni cathode show stratified lamellar grains. Anyway, the indium deposit obtained on Ni cathode shows bigger grains independently from the used operative conditions with respect to that on AISI 316L. Regarding the structure, it is clearly tetragonal, but preferential orientation, crystallinity, and deformation of the structure strongly depend on the metal support.












Similar content being viewed by others
References
Schulz KJ, DeYoung JH, Seal IIJRR, and Bradley DC (2017) Chapter I. Germanium and Indium, in: Crit. Miner. Resour. United States — Econ. Environ. Geol. Prospect. Futur. Supply, Reston, Virginia, I2–I27
Fontana D, Forte F, De Carolis R, Grosso M (2015) Materials recovery from waste liquid crystal displays: a focus on indium. Waste Manag 45:325–333. https://doi.org/10.1016/j.wasman.2015.07.043
Lupi C, Pilone D (2014) In(III) hydrometallurgical recovery from secondary materials by solvent extraction. J Environ Chem Eng 2:100–104. https://doi.org/10.1016/j.jece.2013.12.004
Ciacci L, Werner TT, Vassura I, Passarini F (2019) Backlighting the European indium recycling potentials. J Ind Ecol 23:426–437. https://doi.org/10.1111/jiec.12744
Zhang K, Wu Y, Wang W, Li B, Zhang Y, Zuo T (2015) Recycling indium from waste LCDs: a review, “Resources. Conserv Recycl 104:276–290. https://doi.org/10.1016/j.resconrec.2015.07.015
Zhang S, Ding Y, Liu B, Chang C (2017) Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag 65:113–127. https://doi.org/10.1016/j.wasman.2017.04.003
Rocchetti L, Amato A, Beolchini F (2016) Recovery of indium from liquid crystal displays. J Clean Prod 116:299–305. https://doi.org/10.1016/j.jclepro.2015.12.080
Kuong IH, Li J, Zhang J, Zeng X (2019) Estimating the evolution of urban mining resources in Hong Kong, up to the year 2050. Environ Sci Technol 53:1394–1403. https://doi.org/10.1021/acs.est.8b04063
Li Y, Liu Z, Li Q, Liu Z, Zeng L (2011) Recovery of indium from used indium – tin oxide (ITO) targets. Hydrometallurgy 105:207–212. https://doi.org/10.1016/j.hydromet.2010.09.006
Chou W, Huang Y (2009) Electrochemical removal of indium ions from aqueous solution using iron electrodes. J Hazard Mater 172:46–53. https://doi.org/10.1016/j.jhazmat.2009.06.119
Lee S, Lee S-Y, Swain B, Cho S-S, Lee CG (2016) A validation experiment on indium recovery by electrowinning of aqueous electrolytes: optimization of electrolyte composition. Mater Test 58(11–12):1001–1004
Lee M, Oh Y (2004) Analysis of ionic equilibria and electrowinning of indium from chloride solutions. Scand J Metall 33:279–285. https://doi.org/10.1111/j.1600-0692.2004.00693.x
Lupi C, Dell’Era A, Pasquali M (2017) Effectiveness of sodium citrate on electrodeposition process of Ni-Co-W alloys for hydrogen evolution reaction. Int J Hydrogen Energ 42:28766–28776
Walsh FC, Gabe DR (1979) The electrodeposition of indium. Surf Technol 8:87–99. https://doi.org/10.1016/0376-4583(79)90054-2
Walsh FC, Gabe DR (1978) Electrode reactions during the electrodeposition of indium from acid sulphate solutions. Surf Technol 6:425–436. https://doi.org/10.1016/0376-4583(78)90012-2
Dell’Era A, Ciro E, Pasquali M, Lupi C (2020) Process parameters affecting the efficiency of indium electrowinning results from sulfatebaths. Hydrometallurgy 193:105296. https://doi.org/10.1016/j.hydromet.2020.105296
Ciro E, Dell’Era A, Pasquali M, Lupi C (2020) Indium electrowinning study from sulfate aqueous solution using different metal cathodes. J Environ Chem Eng 8:103688. https://doi.org/10.1016/j.jece.2020.103688
Ciro E, Dell’Era A, Razzi M, Lupi C (2022) Optimization of indium electrowinning from sulfate solutions on Ni cathode. J Clean Prod 131309. https://doi.org/10.1016/j.jclepro.2022.131309
Pshenichnikov AG (1982) Electrocatalysts on the basis of nickel and its alloys in electrochemical energetic systems. J Res Inst Catal 30:137–154. http://hdl.handle.net/2115/25136
Savadogo O, Lavesque S (1991) The hydrogen evolution reaction in an acid medium on nickel electrodeposited with PMo12O40-3 or MoO4-2, J Appl. Electrochem 21:457–462. https://doi.org/10.1007/BF01024585
Tamm J, Tamm L, Arol’d J (2004) Cathodic hydrogen evolution on nickel in acidic environment. Russ J Electrochem 40(11):1152–1155. https://doi.org/10.1023/B:RUEL.0000048647.12787.bb
Zhao J, Deng Y, Wei H, Zheng X, Yu Z, Shao Y, Shield JE, Huang J (2017) Strained hybrid perovskite thin films and their impact on the intrinsic stability of perovskite solar cells. Sci Adv 3:1–8. https://doi.org/10.1126/sciadv.aao5616
Wang G, Fan Z, Murakami S, Lu Z, Hall DA, Sinclair DC, Feteira A, Tan X, Jones JL, Kleppe AK, Wang D, Reaney IM (2019) Origin of the large electrostrain in BiFeO3-BaTiO3 based lead-free ceramics. J Mater Chem A 7:21254–21263. https://doi.org/10.1039/c9ta07904a
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ciro, E., Dell’Era, A. & Lupi, C. Indium Electrowinning from Sulfate Solutions: the Influence of Cathodic Support on the Process Effectiveness. Mining, Metallurgy & Exploration 40, 1299–1309 (2023). https://doi.org/10.1007/s42461-023-00789-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00789-z