Abstract
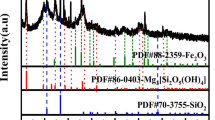
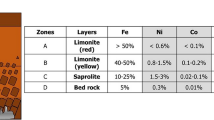
Silica was extracted from nickel laterite ore by hydrothermal process with sodium hydroxide as reaction assistant. The effects of reaction temperature, reaction time, and NaOH-to-ore mole ratio on the extraction rate of silica were investigated by a single-factor experiment. The optimal reaction conditions for the preparation of silica were obtained as follows: reaction temperature 250 °C, reaction time 2 h, NaOH-to-ore mole ratio 1.2:1. Under these conditions, the extraction rate of silica could reach more than 98%. The orthogonal experiment was used to investigate the main and secondary effects of each reaction condition on the extraction rate of silica. The results showed that the mole ratio of NaOH-to-ore was the most important factor affecting the extraction rate of silica, followed by reaction temperature and reaction time. XRD, SEM, and EDS were used to analyze the nickel laterite ore and the residue after reaction. The results show that the mechanism of extracting silica by this process is the Si in lizardite (Mg3Si2O5(OH)4) and uncombined quartz (SiO2) react with sodium hydroxide and enter into the leachate in the form of sodium silicate solution, and Mg, Fe, Ni, and other metal elements are enriched in the residue.












Similar content being viewed by others
References
Zhou SW, Wei YG, LiB WH, Ma BZ, Wang CY, Luo X (2017) Mineralogical characterization and design of a treatment process for Yunnan nickel laterite ore, China. Int J Miner Process 159:51–59. https://doi.org/10.1016/j.minpro.2017.01.002
Farrokhpay S, Cathelineau M, Blancher SB, Laugier O, Filippov L (2019) Characterization of Weda Bay nickel laterite ore from Indonesia. J Geochem Explor 196:270–281. https://doi.org/10.1016/j.gexplo.2018.11.002
Cameron RA, Yeung CW, Greer CW, Gould WD, Mortazavi S, Bédard PL, Morin L, Lortie L, Dinardo O, Kennedy KJ (2010) The bacterial community structure during bioleaching of a low-grade nickel sulphide ore in stirred-tank reactors at different combinations of temperature and pH. Hydrometallurgy 104(2):207–215. https://doi.org/10.1016/j.hydromet.2010.06.005
Cameron RA, Lastra R, Gould WD, Mortazavi S, Thibault Y, Bedard PL, Bedard L, Koren DW, Kennedy KJ (2013) Bioleaching of six nickel sulphide ores with differing mineralogies in stirred-tank reactors at 30 °C. Miner Eng 49:172–183. https://doi.org/10.1016/j.mineng.2011.03.016
Maree W, Kloppers L, Hangone G, Oyekola O (2017) The effects of mixtures of potassium amylxanthate (PAX) and isopropyl ethyl thionocarbamate (IPETC) collectors on grade and recovery in the froth flotation of a nickel sulfide ore. S Afr J Chem Eng 24:116–121. https://doi.org/10.1016/j.sajce.2017.07.001
Mu WN, Cui FH, Huang ZP, Zhai YC, Xu Q, Luo SH (2018) Synchronous extraction of nickel and copper from a mixed oxide-sulfide nickel ore in a low-temperature roasting system. J Clean Prod 77:371–377. https://doi.org/10.1016/j.jclepro.2017.12.260
Zhao KL, Yan W, Wang XH, Wang Z, Gao ZY, Wang CQ, He W (2020) Effect of a novel phosphate on the flotation of serpentine-containing copper-nickel sulfide ore. Miner Eng 150:106276. https://doi.org/10.1016/j.mineng.2020.106276
Garcia VB, Schutesky ME, Oliveira CG, Whitehouse MJ, Huhn SRB, Augustin CT (2020) The Neoarchean GT-34 Ni deposit, Carajás mineral Province, Brazil: An atypical IOCG-related Ni sulfide mineralization. Ore Geol Rev 127:103773. https://doi.org/10.1016/j.oregeorev.2020.103773
Ulrich M, Cathelineau M, Muñoz M, Boiron MC, Teitler Y, Karpoff AM (2019) The relative distribution of critical (Sc, REE) and transition metals (Ni Co, Cr, Mn, V) in some Ni-laterite deposits of New Caledonia. J Geochem Explor 197:93–113. https://doi.org/10.1016/j.gexplo.2018.11.017
Supriyatna YI, Sihotang IH, Sudibyo (2019) Preliminary study of smelting of Indonesian nickel laterite ore using an electric arc furnace. Mater Today Proc 13:127–131. https://doi.org/10.1016/j.matpr.2019.03.201
Wang XD, McDonald RG, Hart RD, Li J, Riessen AV (2013) Acid resistance of goethite in nickel laterite ore from Western Australia. Part I. The relationship between goethite morphologies and acid leaching performance. Hydrometallurgy 140:48–58. https://doi.org/10.1016/j.hydromet.2013.09.005
Wang XD, McDonald RG, Hart RD, Li J, Riessen AV (2014) Acid resistance of goethite in nickel laterite ore from Western Australia. Part II. Effect of liberating cementations on acid leaching performance. Hydrometallurgy 141:49–58. https://doi.org/10.1016/j.hydromet.2013.10.015
Tupaz CAJ, Watanabe Y, Sanematsu K, Echigo T (2020) Mineralogy and geochemistry of the Berong Ni-Co laterite deposit, Palawan, Philippines. Ore Geol Rev 125:103686. https://doi.org/10.1016/j.oregeorev.2020.103686
Li B, Wang H, Wei YG (2011) The reduction of nickel from low-grade nickel laterite ore using a solid-state deoxidisation method. Miner Eng 24(14):1556–1562. https://doi.org/10.1016/j.mineng.2011.08.006
Lv XM, Lv W, You ZX, Lv XW, Bai CG (2018) Non-isothermal kinetics study on carbothermic reduction of nickel laterite ore. Powder Technol 340:495–501. https://doi.org/10.1016/j.powtec.2018.09.061
Sarbishei S, Khajavi LT (2019) Kinetic analysis on nickel laterite ore calcination using model-free and model-fitting methods. Miner Eng 136:129–139. https://doi.org/10.1016/j.mineng.2019.03.010
Sadykhov GB, Anisonyan KG, Goncharov KV (2019) Kop’ev DY, Olyunina TV, Mikhailova AB (2019) Reducing roasting of hematite laterite nickel ores with the formation of granulated nickel-containing cast iron. Russ Metall 7:659–664. https://doi.org/10.1134/S0036029519070139
Mu WN, Zhai YC, Liu Y (2010) Leaching of magnesium from desiliconization slag of nickel laterite ores by carbonation process. T Nonferr Metal Soc 20:s87–s91. https://doi.org/10.1016/S1003-6326(10)60018-0
Mubarok MZ, Lieberto J (2013) Precipitation of nickel hydroxide from simulated and atmospheric-leach solution of nickel laterite ore. Proc Earth Planet Sci 6:457–464. https://doi.org/10.1016/j.proeps.2013.01.060
Lu J, Liu SJ, Shangguan J, Du WG, Du F, Yang S (2013) The effect of sodium sulphate on the hydrogen reduction process of nickel laterite ore. Miner Eng 49:154–164. https://doi.org/10.1016/j.mineng.2013.05.023
Meng L, Qu JK, Guo Q, Xie KQ, Zhang PY, Han LX, Zhang GZ, Qi T (2015) Recovery of Ni Co. Purif Technol 143:80–87. https://doi.org/10.1016/j.seppur.2015.01.012
Wang LY, Lee MS (2017) Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with Cyanex 301. Int J Miner Process 166:45–52. https://doi.org/10.1016/j.minpro.2017.07.004
Mu WN, Lu XY, Cui FH, Luo SH, Zhai YC (2018) Transformation and leaching kinetics of silicon from low-grade nickel laterite ore by pre-roasting and alkaline leaching process. T Nonferr Metal Soc 28(1):169–176. https://doi.org/10.1016/S1003-6326(18)64650-3
Giese EC, Carpen HL, Bertolino LC, Schneider CL (2019) Characterization and bioleaching of nickel laterite ore using Bacillus subtilis strain. Biotechnol Prog 35(6). https://doi.org/10.1002/btpr.2860
Jiang M, Sun TC, Liu ZG, Kou J, Liu N, Zhang SY (2013) Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int J Miner Process 123:32–38. https://doi.org/10.1016/j.minpro.2013.04.005
Zhang JH, Gao LH, He ZJ, Hou XM, Zhan WL, Pang QH (2020) Separation and recovery of iron and nickel from low-grade laterite nickel ore by microwave carbothermic reduction roasting. J Mater Res Technol 9(6):12223–12235. https://doi.org/10.1016/j.jmrt.2020.08.036
Yuan S, Zhou WT, Li YJ, Han YX (2020) Efficient enrichment of nickel and iron in laterite nickel ore by deep reduction and magnetic separation. T Nonferr Metal Soc 30(3):812–822. https://doi.org/10.1016/S1003-6326(20)65256-6
Liu YJ, Lv XM, You ZX, Lv XW (2020) Kinetics study on non-isothermal carbothermic reduction of nickel laterite ore in presence of Na2SO4. Powder Technol 362:486–492. https://doi.org/10.1016/j.powtec.2019.11.103
Zhang YY, Cui KK, Wang J, Wang XF, Qie JM, Xu QY, Qi YH (2020) Effects of direct reduction process on the microstructure and reduction characteristics of carbon-bearing nickel laterite ore pellets. Powder Technol 376:496–506. https://doi.org/10.1016/j.powtec.2020.08.059
MacCarthy J, Nosrati A, Skinner W, Addai-Mensah J (2016) Atmospheric acid leaching mechanisms and kinetics and rheological studies of a low grade saprolitic nickel laterite ore. Hydrometallurgy 160:26–37. https://doi.org/10.1016/j.hydromet.2015.11.004
Santos ALA, Becheleni EMA, Viana PRM, Papini RM, Silvas FPC, Rocha SDF (2021) Kinetics of Atmospheric Leaching from a Brazilian Nickel Laterite Ore Allied to Redox Potential Control. Min Metall Explor 38(1):187–201. https://doi.org/10.1007/s42461-020-00310-w
Sui YL, Hao YY, Zhang XP, Li JP, Wen GY, Zhong SK, Zhang ZW, Wu L (2021) Improved electrochemical properties of vanadium substituted Na0.67Fe0.5Mn0.5O2 cathode material for sodium-ion batteries. Ceram Int 47(4):5227–5234. https://doi.org/10.1016/j.ceramint.2020.10.102
Wen GY, Sui YL, Sui XP, Li JP, Zhang ZW, Zhong SK, Tang SB, Wu L (2021) Mn3O4 anchored polypyrrole nanotubes as an efficient sulfur host for high performance lithium sulfur batteries. J Colloid Interface Sci 589:208–216. https://doi.org/10.1016/j.jcis.2021.01.006
Li JH, Li DS, Xu ZF, Liao CF, Liu Y, Zhong B (2018) Selective leaching of valuable metals from laterite nickel ore with ammonium chloride-hydrochloric acid solution. J Clean Prod 179:24–30. https://doi.org/10.1016/j.jclepro.2018.01.085
MacCarthy J, Nosrati A, Skinner W, Addai-Mensah J (2015) Acid leaching and rheological behaviour of a siliceous goethitic nickel laterite ore: Influence of particle size and temperature. Miner Eng 77:52–63. https://doi.org/10.1016/j.mineng.2014.12.031
Zhu DQ, Cui Y, Hapugoda S, Vining K, Pan J (2012) Mineralogy and crystal chemistry of a low grade nickel laterite ore. T Nonferr Metal Soc 22(4):907–916. https://doi.org/10.1016/S1003-6326(11)61264-8
Funding
This work was financially supported by National Natural Science Foundation of China (No. 51674068, 51704064, 51874079, 51804035), Natural Science Foundation of Liaoning Province (No. 2019-ZD-0507), Natural Science Foundation of Hebei Province (No. E2018501091), Training Foundation for Scientific Research of Talents Project, Hebei Province (No. A2016005004), Hebei Province Higher Education Science and Technology Research Project (No. QN2017403), Fundamental Research Funds for the Central Universities (No. N172302001, N182312007, N182304015), Qinhuangdao City University Student of Science and Technology Innovation, Entrepreneurship Project (No. PZB1810008T-46, PZB1810008T-14), and Department of Education Project of Liaoning Province (No. QN2020012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, S., Chang, L., Bi, X. et al. The Extraction of Silica from Nickel Laterite Ore by Alkaline Hydrothermal Process. Mining, Metallurgy & Exploration 39, 1245–1253 (2022). https://doi.org/10.1007/s42461-022-00597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00597-x