Abstract
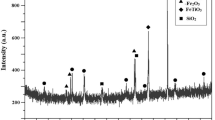
Today’s industrial technology offers to optimize and reutilize the resources available in terms of scum that remains after hot rolling in steel rerolling mills in Rajasthan, India. Vast reserves of low-grade coal exist and tons of mill scale are produced everyday after hot rolling in steel rerolling mills in Rajasthan, India. The present research work aimed to optimize the various parameters of solid-state reduction of mill scale using low-grade coal from Rajasthan, India. This study investigates the effect of reduction temperature (850–950 °C in steps of 50 °C), reduction time (60 to 180 min, in step of 60 min), mill scale size (800–300 μm), and mill scale to coal ratio (1:1, 1:2 and 1:3) on achievable metallic iron percentage and its metallization. A higher percentage of iron content is obtained at a lower particle size of 300 μm. The structural analysis is carried out on 300 μm mill scales using XRD analysis, which shows the percentage of iron is approximately 63%, and metallization above 80% can be obtained under the reduction temperature at 900 °C for 180 min, for 1:3 ratio (mill scale to coal ratio). The chemical composition of the mill scale is obtained using X-ray fluorescence (XRF), and particle sizes as well as distribution of mill scale are determined by scanning electron microscopy (SEM). The elements present in the mill scale are also verified using EDS, which indicates the presence of a major portion of Fe, followed by Fe2O3, Si, and C, respectively.













Similar content being viewed by others
References
Kou J, Sun T, Tao D, Cao Y, Xu C (2014) Coal-based direct reduction and magnetic separation of lump hematite ore. Mining Metallurgy Exploration 31(3):150–161
Hajidavalloo E, Alagheband A (2008) Thermal analysis of sponge iron preheating using waste energy of EAF. J Mater Process Technol 208(1–3):336–341
Manning CP, Fruehan RJ (2001) Emerging technologies for iron and steelmaking. Jom 53(10):36–43
Narcin N, Aydln S, Şeşen K, Dikec F (1995) Redaction of iron ore pellets with domestic lignite coal in a rotary tube furnace. Int J Miner Process 43(1–2):49–59
Man Y, Feng JX, Li FJ, Ge Q, Chen YM, Zhou JZ (2014) Influence of temperature and time on reduction behavior in iron ore–coal composite pellets. Powder Technol 256:361–366
Strezov V (2006) Iron ore reduction using sawdust: experimental analysis and kinetic modelling. Renew Energy 31(12):1892–1905
Iluţiu-Varvara DA, Aciu C, Pică EM, Sava C (2017) Research on the chemical characterization of the oily mill scale for natural resources conservation. Procedia Eng 181:439–443
Bugdayci M, Alkan M, Turan A, Yücel O (2018) Production of Iron based alloys from mill scale through Metallothermic reduction. High Temp Mater Process 37(9–10):889–898
Eissa M, Ghali S, Ahmed A, El-Faramawy H (2012) Optimum condition for smelting high carbon ferromanganese. IronMak SteelMak 39(6):419–430
Coetsee T, Pistorius PC, De Villiers EE (2002) Rate-determining steps for reduction in magnetite-coal pellets. Miner Eng 15(11):919–929
El-Faramawy H, Mattar T, Fathy A, Eissa M, Ahmed AM (2004) Silicomanganese production from manganese rich slag. IronMak SteelMak 31(1):31–36
El-Hussiny NA, Shalabi MEH (2011) A self-reduced intermediate product from iron and steel plants waste materials using a briquetting process. Powder Technol 205(1–3):217–223
Anis M, Makahanap BD, Manaf A (2019) Influence of particle size on reduction kinetics of natural lateritic Iron ores. Jusami| Indonesian J Mater Sci 13(1):30–33
Sinha KMK, Sharma T, Haldar DD (2014) Reduction of iron ore with non coking coal. Int J Eng Adv Technol 3:30–33
Yucel O, Demirci F, Turan A, Alkan M (2013) Determination of direct reduction conditions of mill scale. High Temp Mater Process 32(4):405–412
Sen R, Dehiya S, Pandel U, Banerjee MK (2015) Utilization of low grade coal for direct reduction of mill scale to obtain sponge Iron: effect of reduction time and particle size. Procedia Earth Planetary Sci 11:8–14
Ma N, Houser JB, Wood LA (2018) Production of cleaner mill scale by dynamic separation of the mill scale from the fast-moving flume water at a hot rolling mill. J Clean Prod 176:889–894
Gade N, Verma G, Sen R, Pandel U (2015) Effect of calcium carbonate on the reduction behaviour of mill scale. Procedia Earth Planetary Sci 11:319–324
Umadevi T, Kumar PP, Kumar MS, Kumar S, Ranjan M (2008) Influence of carbon addition via Corex sludge on pellet quality at JSW steel. IronMak SteelMak 35(6):421–429
Ahmed A, El-Fawakhry MK, Eissa M, Shahein S (2015) Direct chromium alloying by smelting reduction of mill scale and low grade chromite ore. IronMak SteelMak 42(9):648–655
Liu GS, Strezov V, Lucas JA, Wibberley LJ (2004) Thermal investigations of direct iron ore reduction with coal. Thermochim Acta 410(1–2):133–140
Bagatini MC, Zymla V, Osório E, Vilela ACF (2011) Characterization and reduction behavior of mill scale. ISIJ Int 51(7):1072–1079
Ye Q, Zhu H, Zhang L, Ma J, Zhou L, Liu P, Chen J, Chen G, Peng J (2014) Preparation of reduced iron powder using combined distribution of wood-charcoal by microwave heating. J Alloys Compd 613:102–106
Sen R, Dehiya S, Pandel U, Banerjee MK (2015) Utilization of low grade coal for direct reduction of mill scale to obtain sponge Iron: effect of reduction time and particle size. Procedia Earth Planetary Sci 11:8–14
Ahmed A, Ghali S, El-Fawakhry MK, El-Faramawy H, Eissa M (2014) Ironmaking Steelmaking. Process Prod Appl 41:310–320
Ünal Hİ, Turgut E, Atapek ŞH, Alkan A (2015) Direct reduction of ferrous oxides to form an Iron-rich alternative charge material. High Temp Mater Process 34(8):751–756
Joshi C, Dhokey NB (2015) Study of kinetics of mill scale reduction: for PM applications. Trans Indian Inst Metals 68(1):31–35
Gaballah NM, Zikry AF, Khalifa MG, Farag AB, El-Hussiny NA, Shalabi MEH (2013) Production of iron from mill scale industrial waste via hydrogen. Open JInorganic Non-Metallic Mater 3:23–28
Narcin N, Aydln S, Şeşen K, Dikec F (1995) Redaction of iron ore pellets with domestic lignite coal in a rotary tube furnace. Int J Miner Process 43(1–2):49–59
Martín MI, López FA, Torralba JM (2012) Production of sponge iron powder by reduction of rolling mill scale. IronMak SteelMak 39(3):155–162
Benchiheub O, Mechachti S, Serrai S, Khalifa MG (2010) Elaboration of iron powder from mill scale. J Mater Environ Sci 1(4):267–276
Sen R, Maan A, Goyal U, Birdhaniya A, Pandel U (2018) In crucible reduction of mill scale by lean grade coal: study of time, temperature and arrangement for optimum reduction conditions. Materials Today Proc 5(2):7256–7263
Acknowledgments
The authors would like to give sincere thanks to the Ministry of Steel, Government of India (F. No 11(11)/GBS/2013-TW DATED 28.09.2015) for financial support. Acknowledgement is also due to the Advanced research lab for Tribology, Mechanical Engineering Dept., MNIT, Jaipur, for providing the solid-state reduction facility and the Material Research Centre, MNIT Jaipur, for providing the characterization facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Kiragi, V.R., Kumar, S. et al. Synthesis and Characterization of Metallic Iron Reduced from Low-grade Coal in Rajasthan. Mining, Metallurgy & Exploration 37, 1741–1751 (2020). https://doi.org/10.1007/s42461-020-00281-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-020-00281-y