Abstract
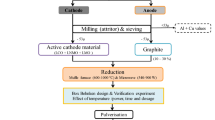
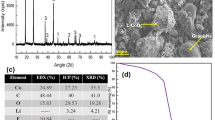
The limited lifespan, ever-growing demand, and significant lithium and cobalt content are responsible factors for the immediate recycling of discarded lithium-ion batteries. Discharged batteries were dismantled, segregated, and size reduced to recover cathode and anode material. The active cathode material comprises LiCoO2 (65.8%) and LiMn2O4 (34.2%) and was carbothermal reduced with recovered graphite in a microwave furnace followed by water leaching and magnetic separation. A Box Behnken statistical experimental design is used for process optimization. Both cobalt and manganese report to magnetic fraction, whereas graphite and lithium carbonate report to non-magnetic fraction and solution crystal respectively. The final product comprises of cobalt 73% and manganese of 14% with process yield of ̴ 36% and saturation magnetization of 76 emu/g.








Similar content being viewed by others
References
Meshram P, Pandey BD, Mankhand TR (2014) Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review. Hydrom 150:192–208. https://doi.org/10.1016/j.hydromet.2014.10.012
Dewulf J, Van der Vorst G, Denturck K, Van Langenhove H, Ghyoot W, Tytgat J, Vandeputte K (2010) Recycling rechargeable lithium ion batteries: critical analysis of natural resource savings. Resour Conserv Recycl 54:229–234. https://doi.org/10.1016/j.resconrec.2009.08.004
Li J, Wang G, Xu Z (2016) Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J Hazard Mater 302:97–104. https://doi.org/10.1016/j.jhazmat.2015.09.050
Vishvakarma S, Dhawan N (2019) Recovery of cobalt and lithium values from discarded Li-ion batteries. J Sustainable Metall 5:204–209. https://doi.org/10.1007/s40831-018-00208-4
Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutz M (2012) Development of a recycling process for Li-ion batteries. J Power Sources 207:173–182. https://doi.org/10.1016/j.jpowsour.2012.01.152
Pinegar H, Smith YR (2020) Recycling of end-of-life lithium ion batteries, Part II: Laboratory-scale research developments in mechanical, thermal, and leaching treatments. J Sustainable Metall 6:142–160. https://doi.org/10.1007/s40831-020-00265-8
Pindar S, Dhawan N (2019) Carbothermal reduction of spent mobile phones batteries for the recovery of lithium, cobalt, and manganese values. JOM 71:4483–4491. https://doi.org/10.1007/s11837-019-03799-9
Hu J, Zhang J, Li H, Chen Y, Wang C (2017) A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J Power Sources 351:192–199. https://doi.org/10.1016/j.jpowsour.2017.03.093
Wang F, Zhang T, He Y, Zhao Y, Wang S, Zhang G, Zhang Y, Feng Y (2018) Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J Clean Prod 185:646–652. https://doi.org/10.1016/j.jclepro.2018.03.069
Gao W, Song J, Cao H, Lin X, Zhang X, Zheng X, Zhang Y, Sun Z (2018) Selective recovery of valuable metals from spent lithium-ion batteries–process development and kinetics evaluation. J Clean Prod 178:833–845. https://doi.org/10.1016/j.jclepro.2018.01.040
Meng Q, Zhang Y, Dong P (2018) Use of electrochemical cathode-reduction method for leaching of cobalt from spent lithium-ion batteries. J Clean Prod 180:64–70. https://doi.org/10.1016/j.jclepro.2018.01.101
Chen Y, Liu N, Hu F, Ye L, Xi Y, Yang S (2018) Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 75:469–476. https://doi.org/10.1016/j.wasman.2018.02.024
Li L, Qu W, Zhang X, Lu J, Chen R, Wu F, Amine K (2015) Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. J Power Sources 282:544–551. https://doi.org/10.1016/j.jpowsour.2015.02.073
Maroufi S, Assefi M, Nekouei RK, Sahajwalla V (2019) Recovery of lithium and cobalt from waste lithium-ion batteries through a selective isolation-suspension approach. Sustain Mater Technol 23:e00139. https://doi.org/10.1016/j.susmat.2019.e00139
Xiao J, Li J, Xu Z (2017) A novel approach for in situ recovery of lithium carbonate from spent lithium-ion batteries using vacuum metallurgy. Environ Sci Technol 51:11960–11966. https://doi.org/10.1021/acs.est.7b02561
Xiao J, Li J, Xu Z (2017) Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J Hazard Mater 338:124–131. https://doi.org/10.1016/j.jhazmat.2017.05.024
Sunil SR, Vishvakarma S, Barnwal A, Dhawan N (2019) Processing of spent Li-ion batteries for recovery of cobalt and lithium values. JOM 71:4659–4665. https://doi.org/10.1007/s11837-019-03540-6
Sunil SR, Dhawan N (2019) Thermal processing of spent Li-ion batteries for extraction of lithium and cobalt manganese values. Trans Indian Inst Metals 72:3055–3044. https://doi.org/10.1007/s12666-019-01769-y
Zhao Y, Liu B, Zhang L, Guo S (2019) Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling. J Hazard Mater 121487:121487. https://doi.org/10.1016/j.jhazmat.2019.121487
Huang B, Pan Z, Su X, An L (2018) Recycling of lithium-ion batteries: Recent advances and perspectives. J Power Sources 399:274–286. https://doi.org/10.1016/j.jpowsour.2018.07.116
Zheng X, Zhu Z, Lin X, Zhang Y, He Y, Cao H, Sun Z (2018) A mini-review on metal recycling from spent lithium ion batteries. Eng 4:361–370. https://doi.org/10.1016/j.eng.2018.05.018
Xu J, Thomas HR, Francis RW, Lum KR, Wang J, Liang B (2008) A review of processes and technologies for the recycling of lithium-ion secondary batteries. J Power Sources 177:512–527. https://doi.org/10.1016/j.jpowsour.2007.11.074
Zeng X, Li J, Shen B (2015) Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J Hazard Mater 295:112–118. https://doi.org/10.1016/j.jhazmat.2015.02.064
Meshram P, Pandey BD, Mankhand TR, Deveci H (2016) Acid baking of spent lithium-ion batteries for selective recovery of major metals: a two-step process. J Ind Eng Chem 43:117–126. https://doi.org/10.1016/j.jiec.2016.07.056
Nayaka GP, Pai KV, Santhosh G, Manjanna J (2016) Recovery of cobalt as cobalt oxalate from spent lithium-ion batteries by using glycine as leaching agent. J Enviro Chem Eng 4:2378–2383. https://doi.org/10.1016/j.jece.2016.04.016
Xiaowei L, Jean-Charles R, Suyuan Y (2004) Effect of temperature on graphite oxidation behavior. Nucl Eng Des 227:273–280. https://doi.org/10.1016/j.nucengdes.2003.11.004
Yue Y, Wei S, Yongjie B, Chenyang Z, Shaole S, Yuehua H (2018) Recovering valuable metals from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain Chem Eng 6:10445–10453. https://doi.org/10.1021/acssuschemeng.8b01805
Fan Y, Yang H, Li M, Zou G (2009) Evaluation of the microwave absorption property of flake graphite. Mater ChemPhys 115:696–698. https://doi.org/10.1016/j.matchemphys.2009.02.010
Pindar S, Dhawan N (2020) Recycling of mixed discarded lithium-ion batteries via microwave processing route. Sustain Mater Technol 25:e00157. https://doi.org/10.1016/j.susmat.2020.e00157
Gratz E, Sa Q, Apelian D, Wang Y (2014) A closed loop process for recycling spent lithium ion batteries. J. Power Sources 262:255–262. https://doi.org/10.1016/j.jpowsour.2014.03.126
Acknowledgments
Thanks are due to Rahul Kumar Singh for his help during the experimental setup.
Funding
The authors acknowledge the financial assistance provided by the Indian Institute of Technology, Roorkee (Faculty Initiation Grant-100714).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pindar, S., Dhawan, N. Microwave Processing of Electrode Active Materials for the Recovery of Cobalt, Manganese, and Lithium. Mining, Metallurgy & Exploration 37, 1285–1295 (2020). https://doi.org/10.1007/s42461-020-00230-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-020-00230-9