Abstract
Background
Gentamicin (GEN) can have serious adverse effects including nephrotoxicity. Setarud (IMOD™) is a new herbal drug with beneficial immune effects, obtained by mixing Tanacetum vulgare (tansy), Rosa canina and Urtica dioica (nettle) extracts as well as selenium, flavonoids and carotenes. This novel study aims to evaluate the effectiveness of Setarud (IMOD™) in attenuating GEN-induced nephrotoxicity in male rats. Twenty-eight adult male Sprague Dawley rats (weighing 180–200 g) were randomly divided into four groups (7 rats in each group): Control, IMOD treated (20 mg/kg body weight), GEN treated (100 mg/kg body weight), and GEN + IMOD co-treated. Injections were done intraperitoneally for 12 days. Serum urea, creatinine (Cr), Cr clearance, malondialdehyde (MDA), reduced glutathione (GSH) level, and activities of antioxidant enzymes Peroxidase (POD), Catalase (CAT), and Glutathione peroxidase (GPx) were measured by the colorimetric method. Volume density of proximal convoluted tubule (PCT), tubular necrosis, tubular cast formation, and leukocytic infiltration were evaluated histopathologically.
Results
In the GEN group, there were significantly higher serum urea, Cr, and MDA levels with lower Cr clearance, GSH levels, POD, GPx and CAT activities, and PCT volume density with presence of tubular necrosis compared to the control and IMOD groups (P < 0.05). Treatment with IMOD significantly reduced the levels of urea, Cr and MDA, and increased Cr clearance and the activities of POD and CAT enzymes (P < 0.05). No significant differences in the activity of GSH and GPx were reported in the GEN + IMOD co-treated group compared to the GEN group. Moreover, IMOD significantly ameliorated PCT volume density and renal lesions caused by GEN.
Conclusion
IMOD (20 mg/kg body weight) can attenuate GEN-induced nephrotoxicity in rats by inhibition of oxidative stress or increasing the normal activity of antioxidant enzymes. Further studies are recommended on the effects of different doses of IMOD.
Article Highlights
-
IMOD can significantly reduce serum MDA and improve activity of antioxidant enzymes POD and CAT in the kidney of rats with GEN-induced nephrotoxicity;
-
IMOD can improve renal function and histopathological variables in rats with GEN-induced nephrotoxicity;
-
Although IMOD can attenuate the nephrotoxicity caused by GEN, it cannot return kidney function to normal level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Gentamicin (GEN) can be used for treating gram-negative bacterial infections (e.g. Pseudomonas, Proteus, etc.), causing less bacterial resistance compared to other antibiotics. However, its use is limited due to having potentially serious adverse effects including ototoxicity and nephrotoxicity [1,2,3]. Nephrotoxicity is a rapid decline in the renal function caused by toxic effect of chemicals and medications. It is manifested clinically as nonoliguric kidney failure, which is associated with increased serum creatinine and development of hypoosmolar urinary output after treatment [4]. There are different mechanisms that cause nephrotoxicity, including tubular cell toxicity, glomerular damage, inflammation, thrombotic microangiopathy, and crystal nephropathy [5, 6]. Since GEN is still used and is highly efficient in treating infections, it is important to find methods to reduce its nephrotoxicity. Recently, GEN-induced nephrotoxicity can be reduced by changing daily dosage and eliminating the risk factors [2]. Since oxidative stress plays an important role in causing GEN-nephrotoxicity, the use of various antioxidants compounds such as L-carnitine, melatonin, and vitamin E can attenuate nephrotoxicity [7]. Herbal plants such as olive leaves, garlic, Pistacia, Silymarin, Camphor, and Persian hogweed have also shown positive effects on the treatment of GEN-induced nephrotoxicity [8,9,10,11,12,13].
Setarud (IMOD™) is new herbal drug with beneficial immune effects [14]. It was first introduced for the treatment of human immunodeficiency virus infection in Iran. It is a naturally-derived immunomodulator [15], and is obtained by mixing Tanacetum vulgare (tansy), Rosa canina and Urtica dioica (nettle) extracts as well as selenium, flavonoids and carotenes [16]. Being rich in beta-carotene, polysaccharides and lectins, these herbal extracts are potentially useful for treatment of a wide range of complaints such as high blood cholesterol and sugar levels [17]. IMOD can act as an effective antihyperglycemic agent and can positively affect the liver and lipid metabolism [18]. It has been used in some animal studies in which the results have shown its positive effects on inflammatory bowel disease, stimulating angiogenesis, reducing the frequency of micronuclei in bone marrow erythrocytes, and reducing oxidative stress and increasing antioxidant enzymes activities in type 1 diabetes [19,20,21,22]. We found no study on the efficacy of IMOD in attenuating GEN-induced nephrotoxicity in rats. In this regard, and due to the mechanisms involved in the physiopathology of nephrotoxicity and considering antioxidant and anti-inflammatory effects of IMOD, and since males are more sensitive to GEN-induced nephrotoxicity [23], this novel study aims to evaluate the possible effectiveness of IMOD in attenuating GEN-induced nephrotoxicity in male rats.
2 Materials and methods
2.1 Materials
In this experimental study, the GEN for induction of nephrotoxicity was obtained from DaruPakhsh Company and the IMOD drug was prepared from Pars Roos Company in Iran.
2.2 Animals
The animals were 28 male Sprague Dawley rats (2 months old, weighing 180–200 g). All rats were kept at 22 C and 50 ± 10% humidity, with adequate water and food in 12 h of daylight and 12 h of dark. After 1 week of adaptation with the new environmental conditions, they were randomly assigned to the following groups (7 rats in each group): Control (healthy rats receiving 0.5 mL normal saline), IMOD (receiving 20 mg/kg body weight), GEN (injection of 100 mg/kg body weight), and GEN + IMOD (receiving GEN at 100 mg/kg body weight plus IMOD at 20 mg/kg body weight). Injections were done intraperitoneally (IP) for 12 days. The dose of 100 mg/kg body weight for GEN in induction of nephrotoxicity was determined according to a previous study [24], and the dose of IMOD was determined based on a previous study [25]. After the last injection, all animals were kept in individual metabolic cages to collect 24 h urine samples.
At the end of experiment, the animals were anesthetized for 30–45 min with ketamine (80–120 mg/kg) IP + xylazine (5–10 mg/kg) IP, blood samples were taken from the animals’ hearts, poured into the test tubes, and its serum was isolated by centrifugation (D-78532 Tuttlingen, Germany) for 20 min at 3000 rpm and stored in a freezer at – 80 C. After blood sampling, to remove the kidney, the abdomen area was rinsed, the hair was removed, and the area was cut using a scissor and grasped by forceps (Fig. 1). One kidney was removed and fixed in formaldehyde solution (10%) for histopathological assessment. Other kidney was used for biochemical analysis to perform renal function tests. It was homogenized in 0.05 mol/L Tris hydrochloride (HCL; Merck Co., Germany) and 1.15% potassium chloride (Merck Co., Germany; pH = 7.4) buffer using a homogenizer (Wiggens, Germany). The homogenate was centrifuged at 18,000 ×g (4 C) for 30 min. The supernatant was then used for biochemical analysis of renal tissue [26].
2.3 Measurement of renal function markers
The kidney function markers including serum creatinine (Cr) and urea levels, were measured by an autoanalyzer (Olympus AU-600, Tokyo, Japan) using commercial kits (Pars Azmoon Company, Tehran, Iran). The Cr clearance was calculated and reported in mL/min/100 g body weight according to the method proposed by Goldman [27]
2.4 Malondialdehyde and glutathione assessment
Malondialdehyde (MDA) level assessment was based on the formation of the MDA-thiobarbituric acid (TBA) complex between one molecule of MDA and two molecules of TBA [28]. For its assessment, 1000 μl of 1% Trichloroacetic acid (TCA) and 1500 μl of 0.06% TBA were added to the test tube. Then, 100 μl of the serum sample was added. The tubes were placed in a bain-marie (100 C) for 30 min and, after cooling, centrifuged for 15 min at 1000 rpm. Some of the supernatant was removed and its absorbance was read at 535 nm using a spectrophotometer (UV-2100, UNICO, Japan) against a blank solution. The amount of molar absorption coefficient was reported as the content of MDA (nmol/L).
Glutathione (GSH) level was measured using the Ellman method [29]. Based on this method, 25 μl of supernatant sample obtained from the kidney tissue was mixed with 140 μl of 2 M Tris–EDTA (pH = 8) and 30 μl of 1 M 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB; Sigma-Aldrich), and the change in their absorbance was read by an ELISA reader (Stat Fax 2100, Awareness Technology, US) at 540 nm, and expressed as μmol/mg protein.
2.5 Antioxidant enzyme activity assessments in the kidney
The concentration of total protein in samples was measured by the Bradford protein assay [30]. For this purpose, 10 μl of samples were combined with 490 μl of Bradford reagent (containing 20 mg Coomassie brilliant blue G-250, 10 ml ethanol, 20 ml 85% phosphoric acid, and 10 ml distilled water, filtered through Whatman grade 1 filter). The UV absorption of samples at 595 nm was read by the spectrophotometer. Finally, the concentration of total protein was determined based on the standard curve. Based on its concentration, the specific activity of antioxidant enzymes was evaluated.
Peroxidase (POD) activity was measured using the method described by McAdam et al. [31], where 20 M Sodium phosphate (Merck Co.) buffer (pH = 6) and 200 mM Guaiacol were used as electron donors and 10 μl of H2O2 30% w/v as the electron acceptor. The change in the absorbance was finally read by the spectrophotometer at 575 nm in a 1 min period and expressed as unit/mg protein.
Glutathione peroxidase (GPx) activity was measured by modifying Paglia and Valentine’s method [32]. Based on this method, 200 μl of 0.4 M HCL buffer with pH = 7, 100 μL of 1 mM sodium azide (Sigma-Aldrich), 200 μL of serum sample, 200 μL of 2 mM glutathione (Sigma-Aldrich), and 100 μL of 0.2 mM H2O2 were added to the test tube and incubated using an incubator (Memmert, Germany) for 10 min at 37 C. Then, 0.4 ml of 10% TCA was added to the tubes and centrifuged at 2000 rpm for 3 min. Then, 25 μl of supernatant was transferred into ELISA microplates, and 140 μl of 0.2 M Tris–EDTA (Merck Co.) with pH = 8 and 30 μL of DTNB were added. After 30 min of incubation at room temperature, the samples were read at 420 nm using an ELISA reader (DTNB solvent in this test was sodium citrate). The amount of molar absorption coefficient was reported as the content of GPx (unit/mg protein).
Catalase (CAT) activity in serum samples was measured by the method described by Sinha et al. [33]. Based on this method, 1000 μl of 50 mM potassium phosphate (Sigma-Aldrich) buffer with pH = 7 and 50 μl of the serum sample was poured into the test tube. When the spectrophotometer was ready to read the absorption, 50 μl of hydrogen peroxide (H2O2; Sigma-Aldrich) was added and the absorption of the samples was read at 0, 30, and 60 s at 240 nm against Blank solution. Since the reaction begins with the addition of H2O2, it is better to add oxygenated water in the final step. The amount of molar absorption coefficient was reported as the content of CAT (unit/mg protein).
2.6 Histopathological and histological studies
After kidney sampling, the right kidney was fixed in 10% formaldehyde solution for at least 45 h. After processing, paraffin sections with 5 µm thickness were prepared and were stained by the periodic acid-Schiff (PAS) method. This method can show brush border of proximal convoluted tubules ((PCTs) clearly and is a specific staining method for tubular basement membrane and glomerular basement membrane [34]. The Caramel method [35] was used to assess histopathological variables such as leukocytic infiltration and tubular necrosis on tissue sections at × 400 magnification where the parameters were evaluated semi quantitatively based on a score from 0 to 4 as follows: 0 = Absence of necrotic cells in the PCTs in the field of view )no damage), 1 = Presence of one necrotic cell in the PCTs (Mild damage), 2 = Presence of more than one necrotic cell in the PCTs (Moderate damage), 3 = Presence of PCTs with complete necrosis scattered in the field of view (Severe damage), 4 = Presence of PCTs with complete necrosis (Very severe damage). The volume density (fraction) of the PCT was estimated by the point-counting rule. In this regard, random microscopic images of the kidney were taken by Leica DFC camera and transferred to a Computer. At × 300 linear magnification, a point probe (with a 12 × 13 cm frame containing 240 points on the transparent sheet) was superimposed onto the images of tissue sections on the monitor. Then, the points on normal PCTs (epithelium with PAS-positive brush border) were counted. For each animal, 10 kidney sections were studied and at least 60 microscopic fields per animal kidney sections were examined by the point-counting rule. The volume density of PCT per cortex was estimated as Vv (PCT/Cortex) = ΣPp/ΣPt, where ΣPp is the sum of points hitting on PCT epithelium and Pt is the number of probe points which is 240. For example, if 10 microscopic fields are evaluated in each kidney tissue section, ΣPt is obtained as 10 × 240 [36].
2.7 Statistical analysis
Data analysis was conducted in SPSS v.16 software. Mean and standard deviation were used for describing data. According to the Kolmogorov–Smirnov test results, the data distribution was normal (p > 0.05). One-way ANOVA was used to evaluate the differences between the study factors in the studied groups followed by Tukey’s post hoc test for multiple comparisons. The significance level was set as less than 0.05.
3 Results
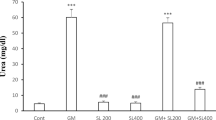
Table 1 presents the mean serum levels of Cr, urea, Cr clearance, MDA, and GSH. As can be seen, the urea, Cr and MDA levels were higher in rats with GEN-induced nephrotoxicity, while treatment with IMOD reduced their level. The GSH level and Cr clearance were lower in the GEN group. Co-treatment with IMOD caused an increase in Cr clearance, but no considerable change was reported in the GSH level compared to the GEN group. Results of ANOVA (Table 1) showed a significant difference among the groups in MDA, GSH, Cr, urea, and Cr clearance (p < 0.05). Based on the results of Tukey’s post hoc test for the renal function parameters, there was a significant increase in the serum levels of urea and Cr and a significant decrease in the Cr clearance in the GEN group compared to the control group (p < 0.001), a significant increase in the urea, Cr, and Cr clearance in the GEN + IMOD group compared to the control group (p < 0.001), a significant decrease in the urea and Cr levels and a significant increase in the Cr clearance in the IMOD group compared to the GEN group (p < 0.001), a significant increase in the urea and Cr levels and a significant decrease in the Cr clearance in the GEN + IMOD group compared to the IMOD group (p < 0.001), and a significant decrease in the urea and Cr levels and a significant increase in the Cr clearance in the GEN + IMOD group compared to the GEN group (p < 0.001). No significant difference was found between the control and IMOD groups (p > 0.05). In the MDA level, there was a significant increase in the GEN group compared to the control group (p = 0.001), a significant decrease in the IMOD group compared to the GEN group (p < 0.001), and a significant decrease in the GEN + IMOD group compared to the GEN group (p < 0.001). No significant difference was found between the control and IMOD groups (p = 0.421). In the GSH level, there was a significant decrease in the GEN group compared to the control group (p < 0.001), a significant decrease in the GEN + IMOD group compared to the control group (p < 0.001), a significant decrease in the IMOD group compared to the GEN group (p < 0.001), and a significant decrease in the GEN + IMOD group compared to the IMOD group (p < 0.001), No significant difference was found between the GEN and GEN + IMOD groups (p = 0.991).
Table 2 presents the mean score of the tubular necrosis and the mean volume density of PCT in four groups. As can be seen, in rats with GEN-induced nephrotoxicity, the tubular necrosis score was higher and the PCT volume density was lower. Treatment with IMOD reduced tubular necrosis score from 1.67 ± 0.19 to 0.87 ± 0.07 and increased volume density of PCT from 0.085 ± 0.01 to 0.12 ± 0.01. Results of ANOVA (Table 2) showed a significant difference among the groups in tubular necrosis and PCT volume density (p < 0.05). Based on the results of Tukey’s post hoc test, tubular necrosis was significantly higher in the GEN group than the control group (p < 0.001), higher in the GEN + IMOD group than in the control group (p < 0.001), lower in the IMOD group than in the GEN group (p < 0.001), higher in the GEN + IMOD group than in the IMOD group (p < 0.001), and lower in the GEN + IMOD group than in the GEN group (p < 0.001). No significant difference was found between the control and IMOD groups (p = 0.998). The PCT volume density was significantly higher in the IMOD group compared to the control group (p = 0.003), lower in the GEN group compared to the control group (p < 0.001), lower in the GEN + IMOD group compared to the control group (p < 0.001), higher in the IMOD group than in the GEN group (p < 0.001), lower in the GEN + IMOD group than in the IMOD group (p < 0.001), and higher in the GEN + IMOD group than in the GEN group (p < 0.001).
Figure 2 compares the specific activity of antioxidant enzymes in four groups. The GEN intoxicated rats showed a significant decrease in the activities of POD, GPX and CAT, but the co-treatment with IMOD showed a significant increase in the activities of POD, GPX, and CAT from 6.36 ± 2.26 to 21.36 ± 7.05, 721.64 ± 39.21 to 1453.7 ± 396.16, and 48.40 ± 8.35 to 64.86 ± 12.94 unit/mg protein, respectively. Regarding the POD level, there was a significant decrease in the GEN group compared to the control group (p < 0.001), a significant decrease in the GEN + IMOD group compared to the control group (p = 0.006), a significant increase in the IMOD group compared to the GEN group (p < 0.001), a significant decrease in the GEN + IMOD group compared to the IMOD group (p = 0.001), and a significant increase in the GEN + IMOD group compared to the GEN group (p < 0.001). Regarding the GPx activity, there was a significant decrease in the GEN group compared to the controls (p < 0.000), a significant decrease in the GEN + IMOD group compared to the controls (p = 0.011), a significant increase in the IMOD group compared to the GEN group (p < 0.001), and a significant decrease in the GEN + IMOD group than in the IMOD group (p = 0.007). No significant difference was found between the GEN and GEN + IMOD groups (p = 0.060). Regarding the CAT activity, a significant decrease was found in the GEN group compared to the control group (p < 0.001), a significant decrease in the GEN + IMOD group compared to the control group (p < 0.001), a significant increase in the IMOD group compared to the GEN group (p < 0.001), a significant decrease in the GEN + IMOD group compared to the IMOD group (p < 0.001), and a significant increase in the GEN + IMOD group compared to the GEN group (p = 0.017).
Comparing the activity level of POD, GPX, and CAT enzymes in the kidney of different groups. *Significant compared to the control group (P < 0.05), †Significant compared to the GEN group (P < 0.05); GEN = Gentamicin. Bars represent mean (SD). One-way ANOVA followed by a Tukey’s post hoc test for the comparison of groups
Histopathological study showed that in the control and IMOD groups, there were normal structure of glomeruli with no sign of intra-tubular casts, cell shedding, or tubular necrosis, and no leukocytic infiltration in the interstitial connective tissue (Figs. 3, 4), while we observed the degeneration of proximal tubules in the GEN group (Fig. 5). These tubules were without cuboidal epithelial cells and brush border, having large luminal membrane surface area. Cast formation and shedding in cells were also observed in damaged tubules. In the GEN + IMOD group, there was an amelioration in the kidney lesions in comparison with the GEN group as few casts, leukocytes and desquamated tubules were observed (Fig. 6).
Microscopic image of the kidney tissue section in the IMOD group (PAS staining × 400). It shows PCT epithelium (black arrows), PCT Lumen (black stars) with brush border (blue arrows), and there is no sign of intra-tubular casts, cell shedding, or tubular necrosis, and no leukocytic infiltration in the interstitial connective tissue
Microscopic image of the kidney tissue section in the GEN group (PAS staining × 400). It shows PCT with no cuboidal epithelial cells and no brush border (red arrows), having large PCT lumen (black stars), along with cast formation (yellow stars), leukocytic infiltration (area shown by red line), and cellular desquamation (black arrows) in injured tubules. In some regions of PCT, epithelium was replaced with simple squamous cells
4 Discussion
Gentamicin (GEN), by production of reactive oxygen species (ROS) and reactive nitrogen species, reduction of antioxidant defense mechanism, inflammatory processes, and mesangial cell contraction as well as reduction of renal blood flow can lead to tubular necrosis, leukocytic infiltration, cell damage, and finally decreased glomerular filtration rate, resulting in kidney dysfunction [4,5,6,7]. GEN can induce nephrotoxicity by inhibiting protein synthesis in renal cells that specifically causes necrosis of cells in the renal proximal tubule [24]. Studies have shown that the use of compounds that have antioxidant activity or antioxidant enzymes can improve the damage caused by GEN [37,38,39]. In this study, we aimed to investigate the antitoxic effect of Setaurd (IMOD™) (20 mg/kg B.Wt for 12 days), as a new herbal drug against GEN-induced nephrotoxicity in male rats. IMOD consists of a mixture of herbal extracts with anti-inflammatory, anti-viral and immunemodulating properties [15, 40], enriched with selenium [41]. Polyphenolic compounds and carotenes in this drug have an important role in adsorbing and neutralization of free radicals, quenching ROS, or decomposing peroxides [42, 43]. In addition, selenium content in this drug can protect cells from oxidative stress by inducing or activating cellular free-radical scavengers and enhancing the breakdown of hydrogen peroxides [44, 45]. Studies have shown that IMOD can reduce the level of pro-inflammatory cytokines such as TNF-α, IL-2, IL-6, IL-12p70, total thiol molecules, and INF-γ [41, 46, 47]. IMOD affects TLR4 signaling and thereby prevent pro-inflammatory cytokine induction [41]. Navaei-Nigjeh et al. concluded that IMOD reduce the toxic effects associated with Chlorpyrifos through free radical scavenging and protection from apoptosis and necrosis [48]. Baghaee et al. evaluated the effect of IMOD on inflammatory bowel disease in rats and reported that the drug significantly reduced TNF-α and IL-1β levels, resulting in a decrease in macroscopic tissue damage [19].
In our study, the rats with nephrotoxicity had significantly higher serum urea, Cr, and MDA levels, lower Cr clearance and GSH levels, as well as lower activity of POD, GPx, and CAT enzymes compared to healthy rats. Karahan et al. [49] also showed a significant increase in kidney MDA and lower GPx and CAT activities in rats treated with GEN alone, while GSH concentration was unaffected. In Khan et al.’s study [50], GSH content and activities of CAT and POD were also reduced by GEN in liver and kidney samples of rats. Apaydin Yildirim et al. [51], also reported that GEN significantly increased renal MDA in rats. In Yarijani et al.’s study, GEN also caused an increase in the Cr and urea levels and an increase in the MDA level in male Wistar rats [52]. Ghaznavi et al. [9] also showed a significant increase in the MDA level and a decrease in GSH, CAT, and GPx levels by GEN administration in rats. The co-treatment with IMOD in our study could significantly ameliorate the changes in the above-mentioned parameters, although it could not return them to the normal level.
Our histological studies were confirmed by the histological findings, where the IMOD reduced tubular necrosis caused by GEN. Also, according to our results, IMOD inhibited oxidative stress and ameliorated the innate activity of enzymatic antioxidants. The reduction of tubular necrosis after administration of various antioxidants has also been reported in other studies [49, 53]. After tubular necrosis, the survived tubular cells dedifferentiate and proliferate, migrate and then differentiate, replacing the lost tubular cells [54]. The dedifferentiation and proliferation take place under influence of growth factors such as hepatocyte growth factor, epidermal growth factor, insulin-like growth factor-1, transforming growth factor-beta, platelet-derived growth factor [55] The amelioration of tubular necrosis by IMOD may be due to the inhibition of PCT cell death caused by inhibition of ROS, the modulation/stimulation of growth factors involved in tubular cell regeneration, or because of maintaining cell-to-cell and cell-to-basal lamina attachment. In our study, PCT volume density was significantly lower in rats with GEN-induced nephrotoxicity compared to other groups. After administration of IMOD, it was significantly improved; however, IMOD could not return it to the normal level. The absence of a PCT brush border was also reported in rats with nephrotoxicity. IMOD preserved the brush border of tubules. The improvement of PCT volume density by IMOD may contribute to the inhibition of tubular necrosis, maintenance of the apical membrane structure of PCT, or new brush border formation after tubular cell regeneration. GEN induced leukocytic infiltration in rats, but the administration of IMOD could significantly reduce it. Similar results have been reported in other studies after administration of different antioxidants [38, 56]. Superoxide anion, as ROS, reacts with nitric oxide and produces peroxynitrite which can activate NFκβ. The NFκβ can induce inflammation and leukocytic infiltration [38]. Due to the antioxidant compounds in IMOD, it seems that this drug reduced leukocytic infiltration by inhibition of superoxide anion or peroxynitrite. In our study, after injection of GEN, necrotic tubular cells and the proteins secreted by the tubular cells formed a tubular pattern that may lead to tubular obstruction. After administration of IMOD, tubular cast formation was significantly reduced. This is consistent with the results of other studies [57, 58]. The reduction in tubular cast formation after injection of IMOD can be related to the antioxidant activity of the compounds of IMOD, as discussed above.
There were some limitations in conducting this study such as financial constraints, setting up of the experiments, low sample size, lack of a follow-up phase, and no study of gene expression. Therefore, more studies are recommended using larger sample sizes, a follow-up phase, other lab rats, or assessing the expression level of study enzymes. We tested the effect of a 20 mg/kg B.Wt dose of IMOD. Further studies are recommended on the effects of different doses of IMOD.
5 Conclusion
IMOD (20 mg/kg body weight) can significantly reduce serum MDA (as a marker of oxidative stress) and improve activity of antioxidant enzymes of POD and CAT, renal function tests (reduction of serum Cr and urea levels, and increase of Cr clearance), and histopathological variables (reduction of PCT volume density, tubular necrosis, tubular cast formation, and leukocytic infiltration) in rats with GEN-induced nephrotoxicity. Although IMOD can reduce the side effects of GEN, it cannot return kidney function to normal level.
Data availability
The datasets used and analyzed during the current study are not publicly available (due to individual privacy), but are available from the corresponding author on reasonable request.
References
Hayward RS, Harding J, Molloy R, et al. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol. 2018;84(2):223–38.
Randjelovic P, Veljkovic S, Stojiljkovic N, Sokolovic D, Ilic I. Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. EXCLI J. 2017;16:388–99.
Pessoa EA, Convento MB, Silva RG, Oliveira AS, Borges FT, Schor N. Gentamicin-induced preconditioning of proximal tubular LLC-PK1 cells stimulates nitric oxide production but not the synthesis of heat shock protein. Braz J Med Biol Res. 2009;42(7):614–20.
Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003–12.
Al-Naimi MS, Rasheed HA, Hussien NR, Al-Kuraishy HM, Al-Gareeb AI. Nephrotoxicity: role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res. 2019;10(3):95–9.
Al-Kuraishy HM, Al-Gareeb AI, Hussien NR. Betterment of diclofenac-induced nephrotoxicity by pentoxifylline through modulation of inflammatory biomarkers. Asian J Pharm Clin Res. 2019;12(3):433–7.
Dennis JM, Witting PK. Protective role for antioxidants in acute kidney disease. Nutrients. 2017;9(7):718.
Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2012;6(1):25–32.
Ghaznavi H, Fatemi I, Kalantari H, et al. Ameliorative effects of gallic acid on gentamicin-induced nephrotoxicity in rats. J Asian Nat Prod Res. 2018;20(12):1182–93.
Ehsani V, Amirteimoury M, Taghipour Z, et al. Protective effect of hydroalcoholic extract of Pistacia vera against gentamicin-induced nephrotoxicity in rats. Ren Fail. 2017;39(1):519–25.
Mahi-Birjand M, Karimzadeh I, Zarban A, Abdollahpour-Alitappeh M, Saadatjoo SA, Ziaee M. Protective effects of silymarin on gentamicin-induced nephrotoxicity in infectious patients: a randomized double blinded placebo-controlled clinical trial. Pharm Sci. 2020;26(3):287–95.
Valibeik A, Naderi N, Amini A, Tavakoli Dastjerd N, Rahimi Monfared S, Jafaripour L, et al. Effect of camphor on biochemical factors and gene expression of antioxidant enzymes, inflammatory and apoptotic factors against gentamicin-induced nephrotoxicity in rats. J Renal Inj Prev. 2021;10(3): e21.
Akbaribazm M, Goodarzi N, Rahimi M, Naseri L, Khazaei M. Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L extract on rats with gentamicin-induced nephrotoxicity. Asian Pac J Trop Biomed. 2021;11:47–58.
Zabihollahi R, Namazi R, Aghasadeghi MR, Esfahani AF, Sadat SM, Modarressi MH. The in vitro anti-viral potential of Setarud (IMOD™), a commercial herbal medicine with protective activity against acquired immune deficiency syndrome in clinical trials. Indian J Pharmacol. 2012;44:448–53.
Novitsky YA, Madani H, Gharibdoust F, Farhadi M, Farzamfar B, Mohraz M. European Patent Application No.087825, 2007.
Paydary K, Emamzadeh-Fard S, Khorram Khorshid HR, Kamali K, SeyedAlinaghi S, Mohraz M. Safety and efficacy of Setarud (IMOD™) among people living with HIV/AIDS: a review. Recent Pat Antiinfect Drug Discov. 2012;7(1):66–72.
Khairandish P, Mohraz M, Farzamfar B, Abdollahi M, Shahhosseiny MH, Madani H, et al. Preclinical and phase 1 clinical safety of Setarud (IMOD™), a novel immunomodulator. DARU J Pharm Sci. 2015;17(3):148–56.
Azonov JA, Khorram-Khorshid HR, Novitsky YA, Farhadi M, Ghorbanoghli Z, Shahhosseiny MH. Protective effects of Setarud (IMOD™) on development of diet-induced hypercholesterolemia in rabbits. DARU J Pharm Sci. 2015;16(4):218–22.
Baghaei A, Esmaily H, Abdolghaffari AH, Baeeri M, Gharibdoost F, Abdollahi M. Efficacy of Setarud (IMod), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian J Biochem Biophys. 2010;47(4):219–26.
Hormozi M, Talebi S, Khorram Khorshid HR, Zarnani AH, Kamali K, Jeddi-Tehrani M, Soltangoraee H, Akhondi MM. The effect of Setarud (IMOD(TM)) on angiogenesis in transplanted human ovarian tissue to nude mice. Iran J Reprod Med. 2015;13(10):605–14.
Shakeri-Boroujeni A, Mozdarani H, Mahmmoudzadeh M, Faeghi F. Potent radioprotective effect of herbal immunomodulator drug (IMOD) on mouse bone marrow erythrocytes as assayed with the micronucleus test. Int J Radiat Res. 2016;14(3):221–8.
Ali-Aghdam A, Karimi-Sales E, Mahmoudi J, Ghiasi R, Alipour MR. Immunomodulator drug (IMODTM) and exercise improve cardiac oxidative stress and antioxidant balance in diabetic rats. Jundishapur J Nat Pharm Prod. 2020;15(3): e62898.
Miri S, Safari T, Komeili GR, Nematbakhsh M, Niazi AA, Jahantigh M, Bagheri H, Maghool F. Sex difference in gentamicin-induced nephrotoxicity: influence of l-arginine in rat model. Int J Prev Med. 2018;24(9):108.
Udupa V, Prakash V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol Rep. 2018;6:91–9.
Ali-Aghdam A, Karimi-Sales E, Mahmoudi J, Ghiasi R, Alipour M. Immunomodulator drug (IMODTM) and exercise improve cardiac oxidative stress and antioxidant balance indiabetic rats. Jundishapur J Nat Pharm Prod. 2020;15(3): e62898.
Li J, Li QX, Xie XF, Ao Y, Tie CR, Song RJ. Differential roles of dihydropyridine calcium antagonist nifedipine, nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats. Eur J Pharmacol. 2009;620:97–104.
Goldman R. Endogenous creatinine clearance by rats. Proc Soc Exp Biol Med. 1967;125(4):1021–4.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
McAdam JW, Nelson CJ, Sharp RE. Peroxidase activity in the leaf elongation zone of tall fescue: I. spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992;99(3):872–8.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Lab Clin Med. 1967;70:158–69.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94.
Suvarna KM, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. Elsevier: Churchill Livingstone; 2013.
Lamarca B. Endothelial dysfunction; an important mediator in the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2012;64(4):309–20.
Carlos A. Stereological tools in biomedical research. Acad Bras Ciênc. 2003;75(4):413–23.
Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223(1):86–98.
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45.
Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19(3):159–70.
Mehri A, Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of efficacy and safety of Urtica dioica in the treatment of diabetes. Int J Pharmacol. 2001;7:161–70.
Mirzaee S, Drewniak A, Sarrami-Forooshani R, Kaptein TM, Gharibdoost F, Geijtenbeek TB. Herbal medicine IMOD suppresses LPS-induced production of proinflammatory cytokines in human dendritic cells. Front Pharmacol. 2015;27(6):64.
Zhang YJ, Gan RY, Li S, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–56.
Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49(11):5165–70.
Ren F, Chen X, Hesketh J, Gan F, Huang K. Selenium promotes T-cell response to TCR-stimulation and ConA, but not PHA in primary porcine splenocytes. PLoS ONE. 2012;7(4): e35375.
Schüller P, Püttmann S, Micke O, Senner V, Schäfer U, Willich N. Selenium-a novel radio sensitizer? Increased radiation sensitivity in C6 rat glioma cells incubated with different concentrations of selenite. Trace Elements Electrolytes. 2005;22(3):201–6.
Farhoudi M, Najafi-Nesheli M, Hashemilar M, Mahmoodpoor A, Sharifipour E, Baradaran B, Taheraghdam A, Savadi-Oskouei D, Sadeghi-Bazargani H, Sadeghi-hokmabadi E, Akbari H, Rikhtegar R. Effect of IMOD™ on the inflammatory process after acute ischemic stroke: a randomized clinical trial. Daru. 2013;21(1):26.
Mahmoodpoor A, Eslami K, Mojtahedzadeh M, et al. Examination of Setarud (IMOD™) in the management of patients with severe sepsis. Daru. 2010;18(1):23–8.
Navaei-Nigjeh M, Asadi H, Baeeri M, et al. In vitro protection of human lymphocytes from toxic effects of chlorpyrifos by selenium-enriched medicines. Iran J Basic Med Sci. 2015;18(3):284–91.
Karahan I, Atessahin A, Yilmaz S, Cerbisat O, Sakin F. Protective effect of lycopene in—induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215:198–204.
Khan MR, Badar I, Siddiquah A. Prevention of hepatorenal toxicity with Sonchus asper in gentamicin treated rats. BMC Complement Altern Med. 2011;11:113.
Apaydin Yildirim B, Kordali S, Terim Kapakin KA, Yildirim F, Aktas Senocak E, Altun S. Effect of Helichrysum plicatum DC. Subsp plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. J Zhejiang Univ Sci B. 2017;18(6):501–11.
Mohamadi Yarijani Z, Najafi H, Shackebaei D, Madani SH, Modarresi M, Jassemi SV. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed Pharmacother. 2019;112: 108635.
Farombi EO, Ekor M. Curcumin attenuates gentamicin induced renal oxidative damage in rats. Food Chem Toxicol. 2006;44:1443–8.
Lombardi D, Becherucci F, Romagnani P. How much can the tubule regenerate and who does it? An open question. Nephrol Dial Transplant. 2016;31(8):1243–50.
Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006;17(1):188–98.
Yaman I, Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–90.
Teslariu O, Pasca AS, Mititelu-Tartau L, Schiriac CE, Gales C, Saftencu PM. The protective effects of zinc in experimental gentamicin induced acute renal failure in rats. J Physiol Pharmacol. 2016;67:751–7.
Abd-Elhamid TH, Elgamal DA, Ali SS, Ali FEM, Hassanein EHM, El-Shoura EAM, et al. Reno-protective effects of ursodeoxycholic acid against gentamicin-induced nephrotoxicity through modulation of NF-κB, eNOS and caspase-3 expressions. Cell Tissue Res. 2018;374:367–87.
Acknowledgements
The authors would like to thank the Lorestan University of Medical Sciences for financial support and Dr. Hamidreza Khorram-Khorshid and Dr. Kurosh Kamali for donating IMOD.
Funding
This study was funded by Lorestan University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization, data collection, and writing initial draft: MH; data analysis and project administration: PB; supervision: HA, MT. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures for the care and use of animals were in accordance with the ethical standards of the local committee on animal experimentation and the 1964 Helsinki Declaration. The study was approved by the Research Ethics Committee of Lorestan University of Medical Sciences (ID: IR.LUMS.REC.1399.176).
Consent for publication
Not applicable.
Competing interests
The authors of this paper report no conflicts of interest in connection with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hormozi, M., Ahmadvand, H., Tavafi, M. et al. Effectiveness of setarud (IMOD™) in attenuating gentamicin-induced nephrotoxicity in male rats. Discov Appl Sci 6, 372 (2024). https://doi.org/10.1007/s42452-024-06071-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06071-1