Abstract
Graphene derivative materials, such as graphene oxide (GO) and reduced graphene oxide (rGO), have garnered significant attention from scientists for over two decades due to their distinctive characteristics and versatile applications across various fields, particularly in biomedical applications. Incorporating gold nanoparticles (AuNPs) into rGO sheets as rGO-Au nanocomposites further enhances its performance in biomedical applications. This study presents a rapid and efficient method for synthesizing ultrasmall AuNPs anchored on reduced graphene oxide (rGO-Au) using microwave irradiation and ascorbic acid. The optimum microwave treatment was 4 min, ensuring the highest GO reduction degree. Structural characterization by TEM reveals a distinctive architecture with ultrasmall AuNPs (average size of 2.2 nm) distributed on the rGO sheets. Interestingly, while the synthesized rGO-Au did not exhibit any antibacterial activities against both Escherichia coli and Staphylococcus aureus in disk diffusion assays, it demonstrated bacteriostatic effect at remarkably low concentrations when assessed by optical density measurement. The effective concentration of rGO-Au to inhibit E. coli growth was determined to be 2.5 ppm, while for S. aureus, it was 5 ppm, resulting in growth inhibition of 53.1% and 50.0%, respectively. These findings provide a straightforward synthesis route for rGO-Au nanocomposites and underscore the importance of AuNPs’ size and quantity in modulating antibacterial properties.
Article Highlights
-
Microwave irradiation influenced the reduction degree of GO and the size of AuNPs in the rGO-Au nanocomposite.
-
The antibacterial properties of rGO-Au were determined by both the size and quantity of AuNPs present.
-
The mechanism of bacteria inhibition by rGO-Au mainly occurs through direct contact with the sharp edges of graphene sheets and wrapping around bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of graphene in 2003, the number of researches about the two-dimensional based materials/devices have steadily increased in various scientific, technological and industrial fields [1,2,3,4]. Graphene is a two-dimensional carbon material which exhibits remarkable properties such as high electrical conductivity, mechanical strength, and thermal stability [5, 6]. Graphene oxide (GO) and reduced graphene oxide (rGO) are chemically modified graphene that become very popular in laboratories due to the challenges and scalability issues producing pristine graphene [3, 7]. GO is obtained from graphite oxidation, introducing oxygen functional groups such as hydroxyl, carbonyl, carboxyl and epoxy groups on the surface [8, 9]. Removing the oxygen functional group in GO through a reduction process will produce rGO [10, 11]. This reduction process recovers sp2 carbon structure and improves the electronical properties of rGO. However, due to the limited amount of oxygen functional groups in rGO, it becomes less hydrophilic and difficult to disperse in water compared to GO [1, 5].
Theoretically, rGO has larger surface area than GO because of its surface's flatness like a pristine graphene [12]. This large surface area makes rGO very suitable as a scaffold for attaching nanoparticles and creating nanocomposites. Adding nanoparticles such as gold nanoparticles (AuNPs) into the surface of graphene materials could enhance their optical, electrical, and biomedical properties [13,14,15,16,17]. The existing conventional method for synthesizing rGO was time-consuming and involved hazardous reducing agents [18,19,20]. For this reason, microwave irradiation could be an alternative method, inducing thermal heating through molecular vibration and aligning with the electromagnetic field charge. This method offers a more energy-efficient approach and significantly reduces synthesis time [21]. Additionally, the hazardous reducing agent can be substituted with ascorbic acid, a green reducing agent that simultaneously acts as a capping agent in nanoparticle synthesis [22,23,24]. While depositing AuNPs on rGO surface as rGO-Au nanocomposite, the size of AuNPs influences the activity of that material, such as for antioxidant, antibacterial agent, and catalyst [21, 23, 25]. Smaller AuNPs are preferable because they provide a larger surface area to interact and contact with other materials [26]. Furthermore, synthesizing small AuNPs < 10 nm requires a stabilizer [20, 27]. Hence, we propose a straightforward and rapid approach employing microwave irradiation and ascorbic acid for synthesizing ultrasmall AuNPs anchored on rGO as rGO-Au nanocomposite. This study also explores the impact of microwave irradiation time on the reduction degree of rGO-Au nanocomposites and AuNPs size.
GO and its derivatives have been recognized for their antibacterial properties [28, 29]. Modifying the surface of GO or rGO with metal nanoparticles amplifies their antibacterial activity [30, 31]. Notably, rGO-Au nanocomposites have demonstrated superior antibacterial efficacy compared to GO [32, 33]. Nevertheless, in prior research, the AuNPs’ size was more than 10 nm [32, 33]. Notably, smaller AuNPs could enhance their biological activities due to the larger surface area [26]. Thus, we want to investigate the influence of ultrasmall AuNPs size in the rGO-Au nanocomposite on its antibacterial activity against E. coli and S. aureus. To the best of our knowledge, this is the first time rGO-Au nanocomposite with an average size of AuNPs < 5 nm has been tested for antibacterial assays. We also conducted a comparative analysis of two different antibacterial assay methods to provide a more comprehensive understanding, particularly concerning the inconsistency in antibacterial activity assessment that has been reported [34, 35].
2 Material and methods
2.1 Materials
Graphite, potassium permanganate (KMnO4), gold standard solution (HAuCl4) 1000 ppm, hydrogen peroxide and ascorbic acid from Merck were used as received. The deionized (DI) water used was from Onemed. H2SO4 96% and HCl 37% were purchased from Mallinckrodt. Mueller Hinton Broth (MHB) and agarose media were obtained from Himedia. Two standard isolates of bacteria, E. coli and S. aureus from Microbiology Laboratory, Faculty of Biology, Gadjah Mada University were used in the study.
2.2 Synthesis of graphene oxide
The synthesis of GO was carried out using the Hummer method described by Chen [36] with some modification. Initially, 2 g of graphite was introduced into 50 mL of concentrated H2SO4 and stirred for 25 min in an ice bath. While vigorously stirring, 10 g of KMnO4 was slowly added to maintain the temperature below 20 °C. The temperature was then raised to 40 °C with continuous agitation and maintained for 2 h. Subsequently, 100 mL of DI water was gradually added, and the solution was stirred at 80 °C for 20 min. To halt the reaction, 300 mL of DI water and 1.4 mL of H2O2 were added. The resulting suspension was left overnight. The mixture was filtered using vacuum filtration and washed with 200 mL of 1.2 M HCl and 200 mL of ethanol. The obtained solid was washed twice with DI water and centrifuged for 15 min at 7000 rpm. Finally, the resulting GO was dried in an oven at 60 °C for 6 h.
2.3 Synthesis of rGO-Au nanocomposites
The synthesis of rGO-Au was conducted using the assistance of microwave irradiation. Initially, 25 mg of the synthesized GO was dispersed in 50 mL of DI water using sonication for 1.5 h. Three milliliters of HAuCl4 solution (500 ppm) were added to 50 mL of GO suspension (0.5 mg/mL) and stirred for 30 min. Afterwards, 87.5 mg of ascorbic acid was added to the GO suspension (3.5 times amount of GO) [37] and stirred for 10 min. The pH was then adjusted to 10 using NaOH 1 M. The mixture was positioned in the center of the microwave oven (Electrolux EMS2348X, frequency of 2450 MHz) for microwave irradiation at 800 W, conducted for 1–5 cycles. Each cycle comprises 1 min of microwave irradiation followed by a 30-s resting period. This process resulted in samples labeled as rGO-Au 1 min, rGO-Au 2 min, rGO-Au 3 min, rGO-Au 4 min, and rGO-Au 5 min. Subsequently, the solutions were cooled to room temperature. For comparison, one sample was not irradiated with microwaves, but it was only stirred for 10 min and labelled as rGO-Au NM. Next, the solutions were washed with deionized water and centrifuged at 4000 rpm for 20 min twice. Finally, the precipitated samples were freeze-dried to obtain solid rGO-Au.
2.4 Antibacterial assay by disk diffusion method
The antibacterial assay involved two species of human pathogenic bacteria, E. coli (gram-negative) and S. aureus (gram-positive). Initially, the bacteria were inoculated in sterile nutrient broth media and allowed to incubate for 24 h at 37 °C. Following incubation, the bacterial suspension underwent serial dilution until reaching a concentration of 105 cfu/mL. Subsequently, 100 µL of this diluted suspension was carefully applied to the surface of sterile agar media in a petri dish. A sterile cotton swab was then employed to ensure the even distribution of bacteria. On filter paper, 100 µL of GO, rGO-Au 4 min suspension (100 ppm), ciprofloxacin (positive control), and water (negative control) were methodically dripped. This loaded filter paper was then positioned on the petri dish containing agar media and the previously inoculated bacteria. The entire assembly was subjected to incubation at 37 °C for 24 h. Finally, the zones of inhibition of the samples were measured.
2.5 Antibacterial assay by OD measurement
To investigate impact of the synthesized nanocomposites on bacterial growth, optical density (OD) measurements were performed at 595 nm. S. aureus and E. coli bacteria underwent inoculation in Mueller Hinton Broth (MHB) media for 24 h at 37 °C. The bacterial suspension was then diluted to a concentration of 105 cfu/mL, determined by OD measurements. Following this, 100 µL suspensions of GO and rGO-Au were added to a 96-well plate, alongside MHB media, achieving a final concentration of 10 ppm. The prepared bacteria were then introduced into each well, and the plate was incubated at 37 °C. Subsequently, OD values were measured with an ELISA reader at 8, 10, 12, 14, 16, and 18 h. Sterile water served as the negative control, and due to the coloration of the nanocomposite suspensions, MHB media, and the nanocomposites suspension were also prepared to normalize OD values. The percentage bacterial growth inhibition was calculated as: Growth inhibition % = 100 × (ODnegative control−ODsample normalized)/(ODnegative control).
Using the same method, rGO-Au at various concentrations (2.5, 5, 10, 25, 50 ppm) was also evaluated to determine the effective concentration for antibacterial activity. The incubation time was extended to 24 h.
2.6 Characterization
The synthesized nanocomposites were characterized by Attenuated Total Reflection–Fourier Transform Infrared (ATR-FTIR, Shimadzu FTIR-8201 PC) to determine the functional groups. UV–Vis spectroscopy (Shimadzu 1800) also used to observed material electronic properties at 100 ppm concentration. Raman spectra were collected by Spectrometer QEP03513 using excitation wavelength of 532 nm. The crystallinity of nanocomposites was determined by X-ray Diffraction pattern (D8 Advance Bruker) on silicon substrate. Scanning electron microscope-energy dispersive x-ray spectroscopy (SEM–EDX, SM-6510LA) was used to analyze materials morphology and interaction with bacteria. The powder samples for SEM–EDX were placed on carbon tape substrate. The bacteria and rGO-Au were incubated 24 h, fixated with glutaraldehyde and hydrated gradually with ethanol (50–100%) before observed with SEM. Transmission electron microscope (TEM, JEOL JEM-1400) 120 kV, high resolution transmission electron microscope (HRTEM, Tecnai G2 20 S-TWIN) 200 kV were used to observe, distribution and size of AuNPs.
3 Results and discussion
3.1 UV–Vis analysis
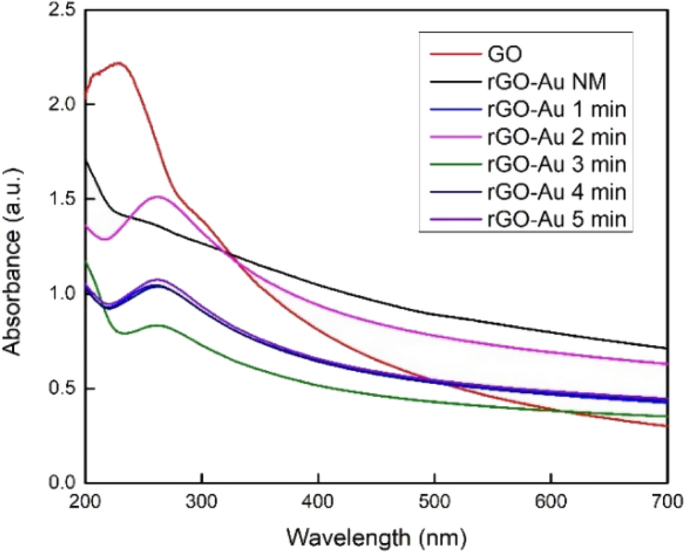
The UV–Vis spectra in Fig. 1 shows the successful of GO reduction. GO spectra shows maximum absorption peak at 230 due to π → π* transition of C=C bonds and a shoulder peak at 304 nm which attributed to n → π* electron transition of C=O bonds [29]. Ascorbic acid and microwave irradiation in rGO-Au (1–5 min) synthesis clearly reduced GO to rGO, which is indicated by the disappearance of GO peak at 230 nm and a new peak of rGO at 262 nm appears [22]. However, the spectra of rGO-Au NM neither exhibit the rGO peak nor the GO peak due to the incomplete reduction of GO sheets. The structure of rGO-Au NM lies between GO and rGO, making it challenging to observe the electron transition using UV–Vis. This result suggests a high degree of reduction in GO requires not only the presence of ascorbic acid as reductant but also a significant heat, such as that generated by microwave irradiation [38].
The UV–Vis spectra in Fig. 1 did not exhibit the typical plasmon resonance peak of AuNPs, which typically appears around 500–550 nm [39]. The damping effect presumably caused this phenomenon due to the size of AuNPs is very small [40, 41]. The highly conductive rGO surface is also probably channeling away the electrons of AuNPs and weakening the plasmon oscillation [42]. Some previous study also reported that depositing AuNPs on graphene sheets could drastically weaken and even eliminate the AuNPs peak [20, 43,44,45]. Therefore, the absence of the AuNPs peak in this UV–Vis spectra does not necessarily mean that AuNPs are not formed on the rGO surface.
3.2 XRD analysis
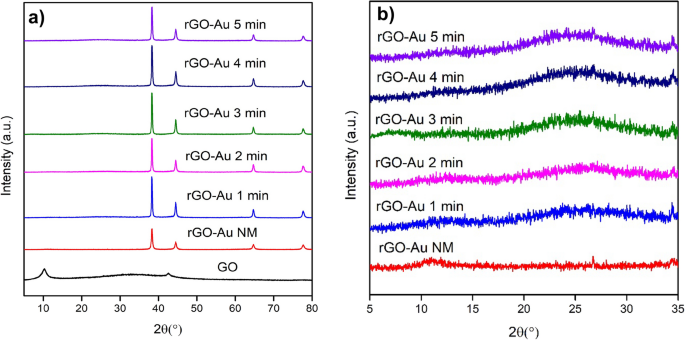
Different forms of crystallinity were observed among GO, rGO, and AuNPs using XRD as illustrated in Fig. 2. The unmodified GO exhibits a diffraction line at 2θ = 10.26°, attributed to the (001) plane [38]. After the reduction process, GO peak disappears and a new peak of rGO arises at 2θ = 25.04° for the (002) plane. This peak shift in rGO due to the removal of oxygen functional groups in GO, causing the interlayer space to shrink from 8.62 to 3.55 Å. In contrast, in the rGO-Au NM nanocomposite, the GO peak does not entirely vanish due to a partial reduction process; instead, it shifts to 10.97° (Fig. 2b). De Silva et al. [22] also reported a similar diffraction pattern in the reduction process of GO. Extended microwave treatment leads to a more pronounced reduction of GO sheets and increased rGO crystallinity, as indicated by the heightened peak at around 25° 2θ. However, extending the microwave irradiation to 5 min still gives similar peaks and patterns to those observed at 4 min. The reduction process likely reaches its maximum after 4 min s
Furthermore, the modification of the graphene surface with AuNPs introduces a new diffraction pattern for rGO-Au materials at 2θ = 38.28°, 44.51°, 64.73°, and 77.67°, corresponding to (111), (200), (220), and (311) planes of Au, respectively (Fig. 2a). This pattern match with JCPDS 4–784 data of Au and it confirms the presence of AuNPs on rGO surface.
3.3 Attenuated total reflection–infrared (ATR-IR) analysis
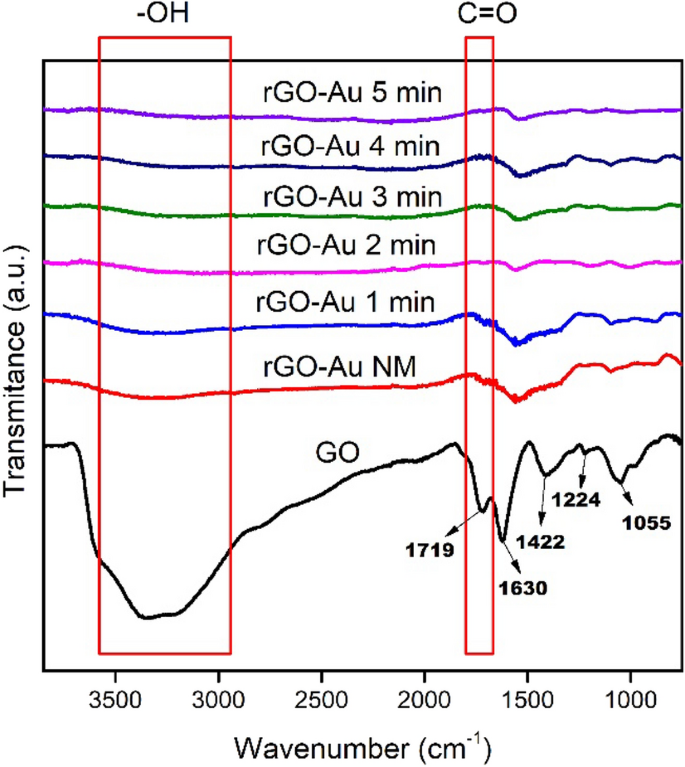
ATR-IR spectroscopy was used to investigate the changes in functional groups within both GO and rGO-Au nanocomposites. Figure 3 shows that GO exhibits several characteristic IR absorption bands which similar to synthesized rGO-Au such as C=C stretching vibration of aromatic group at ~ 1620 cm−1 and C–O stretching vibration at 1422, 1224 and 1055 cm−1 as carboxyl, epoxy and alkoxy group, respectively [20, 33]. However, ascorbic acid and microwave irradiation effectively remove oxygen functional groups in the synthesized rGO-Au materials, leading to a significant decrease intensity of all C–O peaks compared to GO. The GO reduction process also clearly observed at ~ 2800–3600 cm−1 and ~ 1700 cm−1 region in IR spectra. The broad peak around 2800–3600 cm−1 for both GO and rGO-Au materials in Fig. 3 is attributed to the O–H stretching vibration of hydroxyl groups and adsorbed water molecules [46]. This peak gradually diminishes with extended microwave irradiation, particularly evident in rGO-Au after 5 min irradiation, where the peak almost disappears due to the high degree of reduction. The reduction of GO to rGO-Au changes the material's characteristics from hydrophilic to hydrophobic [38], resulting in a significant decrease in adsorbed water molecules, as also reflected in 2800–3600 cm−1 region of the IR spectra. Additionally, the C=O stretching vibration of the carbonyl group in GO was initially observed at 1719 cm−1 (Fig. 3) and it vanishes in rGO-Au spectra due to the removal of carbonyl group in reduction process [46].
3.4 Elemental analysis by SEM–EDX
The percentage amounts of each element present in the synthesized materials were determined by SEM–EDX. As shown in Table 1, the reduction of GO to rGO-Au decrease the oxygen content significantly. This data aligns with earlier ATR-IR analysis and further clarifies the successful of GO reduction using ascorbic acid and microwave heating. The lower oxygen content in rGO-Au NM (29.54%) compared to GO (38.22%) also can be attributed to the presence of ascorbic acid, which known as reducing agent and could partially reduce the GO sheets [22]. Increasing the duration of microwave irradiation clearly enhances the reduction degree of rGO, resulting in a change in the C/O atomic ratio from 1.62 to 4.02. It appears that the optimum microwave irradiation time for achieving a significant reduction degree is 4 min (C/O = 4.11), which is consistent with the previous XRD analysis.
The amount of Au impregnated in rGO surface is also mentioned in Table 1. Interestingly, microwave irradiation lowers the Au content on the rGO surface. This phenomenon can be ascribed to the heat generated during microwave treatment, imparting high kinetic energy to molecules [47]. The heightened kinetic energy may induce collisions between gold ions (Au3+) and the rGO sheets, resulting in the dislodgment of some AuNPs from the surface. In contrast, rGO-Au NM has more oxygen functional groups that can retain Au strongly through electrostatic interaction and coordination by complexing during nucleation process [48]. However, microwave irradiation time is not correlated to Au content in rGO surface. The presence of sodium metal residues, as indicated in the EDX results (Table 1), is attributed to impurities introduced during the pH adjustment of the reaction using NaOH. Further detailed characterization analysis and antibacterial application will be focused on the rGO-Au 4 min, as it exhibits the highest reduction degree and Au content.
3.5 Raman analysis
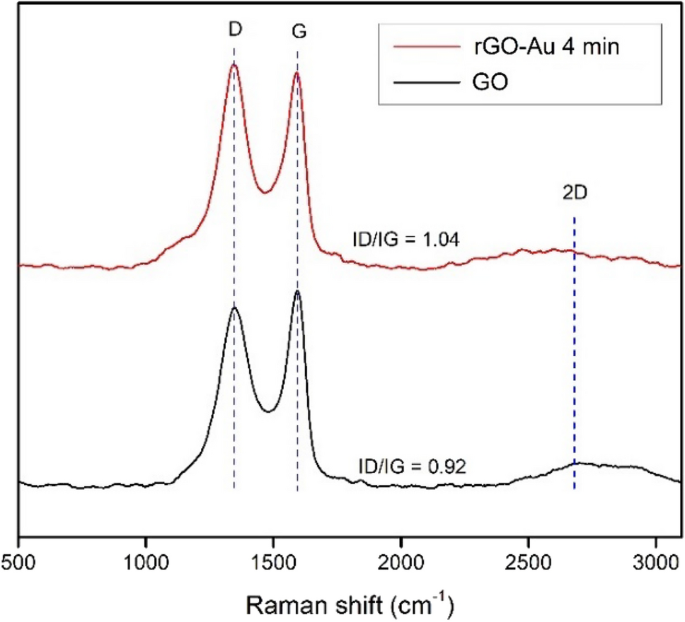
Raman spectroscopy is very useful to characterize carbon material structure [24]. Figure 4 shows sharp peaks at 1350 and 1590 cm−1 for both GO and rGO-Au 4 min were attributed to D band and G band, respectively. The G band represent sp2 carbon domain vibration while D band correspond to structural defect [49, 50]. In Fig. 4 the intensity of D band in both materials about the same as G band peak, indicating huge defect in the structure. The ID/IG ratio of GO found to be 0.92 while rGO-Au 4 min 1.04 suggesting that reduction process slightly increase the defect in graphene plane. The number of layers in synthesized graphene materials can be confirmed by 2D band around 2700 cm−1 [51]. Single-layer graphene produces sharp and intense peak 2D band region while the multilayer graphene sheets have broader peak [51, 52]. The weak intensity and broad 2D peak observed around ~ 2700 cm−1 for GO and rGO-Au 4 min represent the presence of multilayer rGO sheets.
3.6 Morphology analysis by scanning electron microscopy (SEM)
The SEM images depict the stacked layers' structure in both GO and rGO-Au materials (Fig. 5) which consistent with raman analysis. Notably, the removal of oxygen-containing groups in the reduction process results in a flatter surface of rGO-Au nanocomposites (Fig. 5b-d) than GO (Fig. 5a). The SEM images also confirms that AuNPs successfully anchored on rGO sheets (bright spots in Fig. 5b-d). This AuNPs deposition is based on electrostatic interaction between the negatively charged rGO sheets and positively charged Au(III) ions [5, 47]. In the absence of microwave heating, AuNPs shapes appear disordered, and agglomeration occurs, as shown in Fig. 5b. On the other hand, when subjected to microwave irradiation, a more ordered arrangement with spherical shapes is observed in rGO-Au (Fig. 5c and 5d). The shape and distribution of AuNPs also exhibit uniformity in both rGO-Au 2 min and rGO-Au 4 min, accompanied by a reduction in particle size. The SEM–EDX mapping image in Fig. S1 also reveals an even distribution of AuNPs on the graphene sheets of the rGO-Au 4 min nanocomposite. Apparently, in this case, ascorbic acid also plays a significant role as a capping agent, preventing the agglomeration of AuNPs and contributing to the uniformity [23, 53]. Compared to previous research, without ascorbic acid, AuNPs exhibit various shapes, and the reduction of the GO does not occur, although the materials were subjected to microwave irradiation [54]. This suggests the effectiveness of ascorbic acid combining with microwave treatment in enhancing the structural characteristics of the rGO-Au nanocomposite.
3.7 TEM analysis and synthesis mechanism
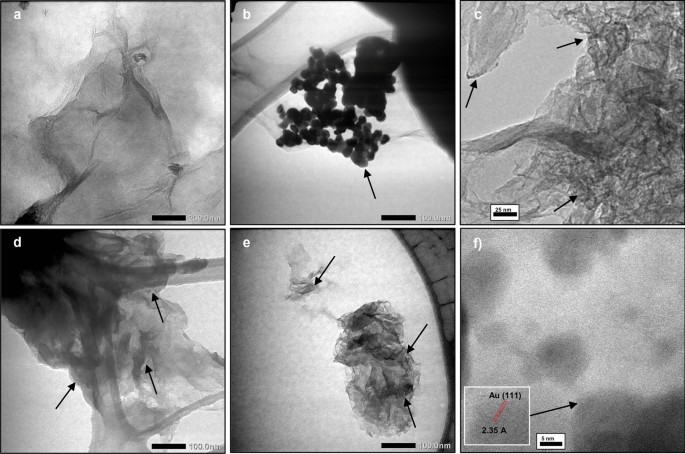
TEM analysis was conducted to investigate the particle size of AuNPs within the rGO-Au nanocomposite as shown in Fig. 6. AuNPs is confirmed in TEM images as a dark spots in the graphene sheet (pointed by the arrow) due to high electron density of gold material [55]. In the absence of microwave irradiation, AuNPs in the rGO-Au NM nanocomposite exhibit agglomeration (Fig. 6b) and the average size is 26.4 nm. In contrast, under microwave radiation, a significant decrease in AuNPs size is observed. Both rGO-Au 2 min and rGO-Au 4 min exhibit very small AuNPs size (< 5 nm) (Fig. 6c and 6d). The size of AuNPs was hardly seen in regular TEM. To validate this finding, TEM analysis was taken three times with different suspension of rGO-Au 4 min and the images were remained same without any agglomeration or larger particle of AuNPs observed. However, this result appears to contradict the larger size of AuNPs observed in previous SEM images (Fig. 5c and 5d). This discrepancy can be attributed to the SEM analysis being performed on solid samples that were not dispersed under sonication, leading to AuNPs agglomeration. Moreover, regular SEM is also unable to observe very small nanoparticles. The SEM–EDX mapping image (Fig. S1) also found that many AuNPs were spread on graphene sheets but remain unseen in SEM images.
High-resolution TEM analysis is necessary to accurately measure the size of AuNPs in rGO-Au 4 min nanocomposite. Figure 6e reveals that the average size of AuNPs is 2.2 nm on the rGO surface. The lattice spacing of 2.35 Å corresponds to (111) plane of AuNPs [56] is observed in Fig. 6f. To provide a comprehensive comparison, Table 2 summarizes various methods and findings from previous research on the fabrication of GO/rGO-Au. Previously, to synthesize AuNPs under 5 nm size, separate capping agents like cetyltrimethylammonium bromide (CTAB) and polyvinylpyrrolidone (PVP) were needed, in addition to the reducing agent [20, 27]. Relying on ascorbic acid alone was insufficient to produce ultrasmall gold nanoparticles [36]. Thus, our findings highlight the microwave-assisted synthesis method's simplicity, effectiveness, and rapidity in anchoring ultrasmall gold nanoparticles on the rGO surface using only ascorbic acid.
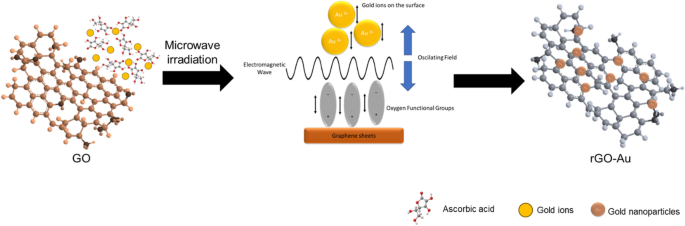
The key benefit of microwave-assisted synthesis is the quick heating of the reaction, allowing for faster synthesis. Conventional heating relies on conduction and convection heat transfer, which is very slow and creates a thermal gradient in the reaction vessel. On the contrary, microwave irradiation provides much faster energy transfer directly to the materials, creating uniform and rapid heating throughout the entire reaction vessel [47, 60]. Figure 7 illustrates how microwave irradiation affects rGO-Au synthesis in this work. Microwave irradiation directly supplies energy to gold ions in the solution and functional groups on the rGO surface through electromagnetic waves. The ions and functional groups oscillate to align with the electromagnetic field, leading to vigorous vibration and collisions [47, 61]. These dynamics might prevent AuNPs from agglomerating during the nucleation process, contributing to a more controlled and uniform synthesis. In this scenario, ascorbic acid also plays a critical role as a reducing and capping agent. Without it, the reduction of GO will not occur, and the size of AuNPs become larger [54]. Apparently, microwave heating serves not merely as an alternative heating method but potentially provides a different reaction mechanism in nanoparticle synthesis, resulting in different nanostructured materials. Although microwave synthesis method provides fast synthesis and distinct nanomaterial properties, scale up to industrial level could be very challenging. The depth of microwave radiation penetration is very limited (only few centimeters), thus large vessel in batch production will be ineffective [62].
3.8 Antibacterial properties
For the following discussion, the rGO-Au nanocomposite, subjected to 4 min of microwave treatment, was employed for the antibacterial properties assay and will be referred to as rGO-Au. Antibacterial assay by disk diffusion method did not reveal any observed antibacterial activity using GO and rGO-Au at 100 ppm against human pathogens E. coli and S. aureus, as demonstrated in Fig. S2. Positive control ciprofloxacin is the only substance that shows antibacterial activity. In this case, it seems that the lack diffusion of GO and rGO-Au prevent these materials to spread in agar media. Some researcher also reported that graphene-based materials demonstrated no inhibition zone with the disk diffusion method [63, 64]. However, this result contradicts Shalini’s [65] and Kadiyala’s [33] findings where GO, GO-Au and rGO-Au nanocomposite demonstrate > 10 mm inhibition zone in disk diffusion method against E. coli and S. aureus [65]. This inconsistency may be attributed to differences GO characteristic [35]. In Shalini's experiment [65], a distinct GO synthesis method was used, which may resuliting GO that exhibits better dispersibility in water and agar media. Improved dispersibility of GO can maximize the contact between the material and bacteria [66].
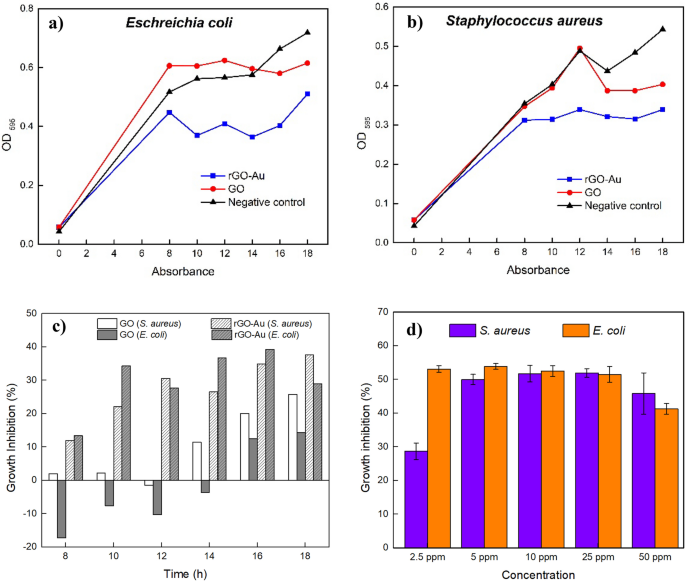
On the other hand, the OD assay demonstrates that both GO and rGO-Au has bacteriostatic effect that suppress E. coli and S. aureus growth at a low concentration of 10 ppm (see Fig. 8a and 8b). By comparing OD of control and treated bacteria suspension, the relative growth inhibition ability of GO and rGO-Au can be determined. However, the synthesized nanocomposites did not kill the bacteria completely, explaining the lack of observed antibacterial activity in the disk diffusion method. These results suggest that the disk diffusion method is inadequate for accurately evaluating the antibacterial properties of graphene-based materials, which may demonstrate bacteriostatic activity rather than bactericidal effects.
As illustrated in Fig. 8a and 8b, the bacterial number increased with prolonged incubation time, as observed in the negative control's OD increase. From the beginning rGO-Au showed higher bacterial growth inhibition than GO for both E. coli and S. aureus. Interestingly, GO enhanced E. coli growth in the beginning, and finally inhibit the bacterial growth 14 h incubation. The bacterial growth enhancement by GO due to the GO itself could act as framework for bacterial to attach and proliferate [63]. After 18 h incubation rGO-Au nanocomposite exhibited superior bacteriostatic activity compared to unmodified GO, inhibiting 29.0% of E. coli and 37.6% of S. aureus growth, whereas GO only inhibited 14.3% of E. coli and 25.8% of S. aureus growth (Fig. 8c).
The presence of AuNPs on the rGO-Au nanocomposite clearly enhances bacteriostatic ability, suggesting the crucial role of AuNPs in the observed effects. rGO-Au was found to be more effective than GO in inhibiting bacterial growth against E. coli and S. aureus. After monitoring the bacterial growth at intervals, rGO-Au was retested at various concentrations to determine its effectiveness. Figure 8d illustrates that rGO-Au displayed 53.1% inhibition on E. coli at just 2.5 ppm concentration, while S. aureus showed 28.7% inhibition. The optimal concentrations of rGO-Au were determined to be 2.5 ppm for E. coli and 5 ppm for S. aureus. At these concentrations, bacterial growth was suppressed by 53.1% and 50.0%, respectively. These number indicate that the synthesized rGO-Au was not an antibacterial agent, but has good potential as a bacteriostat since it could inhibit half of bacterial growth. The synthesized rGO-Au nanocomposite demonstrated greater efficacy against E. coli, a Gram-negative strain, than the Gram-positive strain S. aureus. Gram-negative bacteria like E. coli have a thinner peptidoglycan layer in their cell wall, making them more prone to damage. On the other hand, a higher concentration of rGO-Au is required to kill Gram-positive bacteria such as S. aureus because the cell wall structure is thicker [26, 64].
In general, increasing rGO-Au concentration above 5 ppm did not result in a significant difference and could even slightly decrease the antibacterial activity at 50 ppm concentration (Fig. 9d). The growth inhibition stagnancy of E. coli and S. aureus was likely due to the limited amount of gold on rGO sheets. Compared to other studies (as depicted in Table 3), the GO-AuNPs and rGO-AuNPs showed high antibacterial activity at around 40–60 ppm, but AuNPs content was around 2%, more than three times AuNPs amount in our rGO-Au nanocomposite. Although AuNPs are crucial for enhancing graphene-based material antibacterial properties, their amount should be considered and optimized. The AuNPs content of 0.62% in the rGO-Au material proved insufficient to completely kill all E. coli and S. aureus bacteria even at 50 ppm concentration. Furthermore, the ineffectiveness of high concentrations of rGO-Au in our study can be attributed to agglomeration and precipitation occurring as the concentration of rGO-Au increases. Despite the minimal amount of AuNPs content, our rGO-Au exhibits remarkable effectiveness even at low concentrations. Table 3 shows that the synthesized rGO-Au with very low Au content at a concentration of 2.5 ppm exhibits comparable bacteriostatic performance to bare AuNPs with a particle size of 20–40 nm against E. coli. The tiny size of the AuNPs attached to the rGO sheets, could be the reason behind its efficacy by providing a larger surface area for direct contact and interaction with bacteria [67]. In summary, AuNPs’ size and quantity on graphene based-materials have a vital role in bacterial growth inhibition. Nevertheless, silver nanoparticles, as well-known antibacterial nanomaterials, completely inhibit bacterial growth of E. coli and S. aureus at a concentration of 1 ppm [68], surpassing the antibacterial properties of rGO-Au nanocomposites.
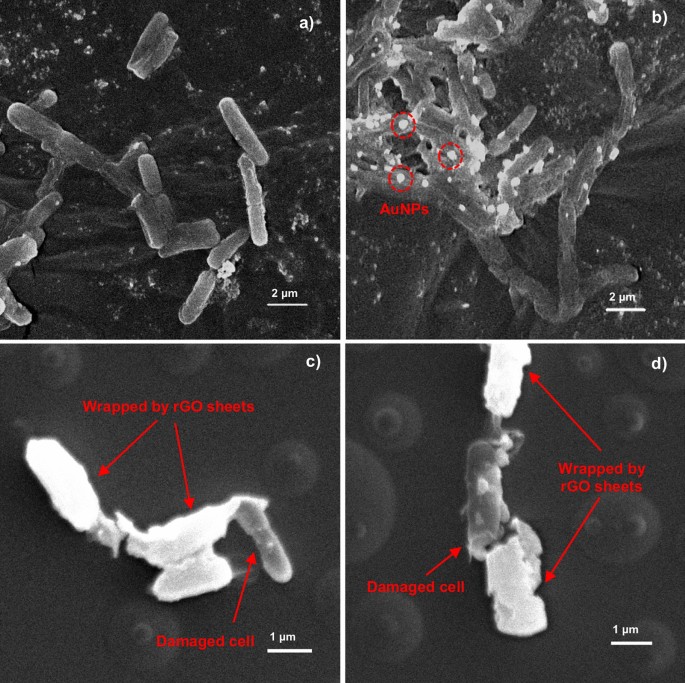
The known mechanisms involved in the antibacterial activity of nanomaterials such as graphene-based materials are physical direct interaction of extremely sharp edges of nanomaterials with cell wall membrane [70], reactive oxygen species (ROS) generation even in dark condition [71, 72], wrapping the cells within the aggregated nanomaterials for bacteria [73] and other motile cells [74], oxidative stress [28], interruption in the glycolysis process of the cells [75], DNA damaging [76], metal ion release [77] and contribution in generation and explosion of nanobubbles [78]. Antibacterial properties of synthesized rGO-Au Au mainly through the direct contact and wrapping mechanism. The direct contact and bacteria wrapping mechanism was observed in Fig. 9. However, the carbon tape substrate makes rGO sheets challenging to observe. Therefore, the substrate was changed to a glass substrate, and the SEM images reveal that rGO sheets wrapped the bacteria (Fig. 9c and 9d). Due to the high conductivity of rGO sheets and AuNPs, they appear very bright under the SEM microscope compared to the bacterial cell. SEM images display that AuNPs in rGO-Au attached to E. coli cell walls (Fig. 9b). Apparently, the morphology of E. coli was also damaged after incubation with the rGO-Au nanocomposite. Direct contact of bacteria with the sharp edges of rGO surfaces rupture the bacteria membrane, eventually resulting in bacterial death [28]. The rGO sheets also wrap the bacterial cells, preventing nutrients intake to the cells and leading to growth inhibition [73, 79]. Furthermore, the presence of AuNPs on the rGO-Au nanocomposite enhances antibacterial properties by interacting with bacterial cell walls and cytoplasm. Bacteria cell walls contain phospholipid bilayers, creating negatively charged cell surfaces surface [80]. This characteristic makes AuNPs easily bind with the cell wall through electrostatic interaction and potentially penetrating the inner cell. It also possible that rGO-Au damaging the DNA due to AuNPs exhibit an affinity for binding with thiol groups in DNA molecules and enzymes in the cytoplasm, causing disruptions in bacterial metabolic processes that eventually lead to bacterial death [26]. Hence, this study demonstrated the synergistic effect between rGO and AuNPs could effectively inhibit bacterial growth at remarkably low concentrations.
4 Conclusion
In conclusion, the microwave-assisted synthesis of rGO-Au using ascorbic acid proves to be a promising method for rapid and efficient production of nanocomposites with ultrasmall size of AuNPs. Microwave heating might offer a unique reaction mechanism, preventing agglomeration during the nucleation of AuNPs through molecular vibration and collisions induced by electromagnetic waves. The optimal microwave irradiation time was found to be 4 min, ensuring a high reduction degree of rGO-Au. Characterization of the synthesized nanocomposite clarifies the successful reduction of GO to rGO, showcasing a uniform distribution of ultrasmall AuNPs with the average size 2.2 nm on the rGO surface. This synthesized nanocomposite displays remarkable bacteriostatic activity at very low concentration despite the limited quantity of AuNPs. Compared to disk diffusion method, the OD method more suitable to observed antibacterial properties in graphene-based materials. The inhibition of bacterial growth is highly influenced by both the size and quantity of AuNPs present in graphene-based materials. These findings pave the way for further exploration in nanomaterials for biomedical applications such as developing new antibiotics and antimicrobial coatings. Although the microwave heating provides advantages in nanomaterial synthesis, the scalability of this method presents challenges. Further research is necessary to optimize AuNPs quantities in graphene-based materials while maintaining their ultrasmall size. It could make rGO-Au nanocomposite as a remarkable antibacterial agent in very low concentration.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Liang J, Liu J, Guo L, Wang W, Wang C, Gao W, et al. CO2 hydrogenation over Fe–Co bimetallic catalysts with tunable selectivity through a graphene fencing approach. Nat Commun. 2024;15:512. https://doi.org/10.1038/s41467-024-44763-9.
Akhavan O, Saadati M, Jannesari M. Graphene jet nanomotors in remote controllable self-propulsion swimmers in pure water. Nano Lett. 2016;16:5619–30. https://doi.org/10.1021/acs.nanolett.6b02175.
Safian MT, Umar K, Mohamad Ibrahim MN. Synthesis and scalability of graphene and its derivatives: a journey towards sustainable and commercial material. J Clean Prod. 2021;318:128603. https://doi.org/10.1016/j.jclepro.2021.128603.
Mayes M, Farahmand F, Grossnickle M, Lohmann M, Aldosary M, Li J, et al. Mapping the intrinsic photocurrent streamlines through micromagnetic heterostructure devices. Proc Natl Acad Sci. 2023;120:e2221815120. https://doi.org/10.1073/pnas.2221815120.
Dasari TP, Danielle S, Asok M, Paul KD. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano Micro Lett. 2018;10:1–34. https://doi.org/10.1007/s40820-018-0206-4.
Mohan VB, Lau K, Hui D, Bhattacharyya D. Graphene-based materials and their composites: a review on production, applications and product limitations. Compos Part B Eng. 2018;142:200–20. https://doi.org/10.1016/j.compositesb.2018.01.013.
Smith AT, LaChance AM, Zeng S, Liu B, Sun L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater Sci. 2019;1:31–47. https://doi.org/10.1016/j.nanoms.2019.02.004.
You S, Luzan SM, Szabó T, Talyzin AV. Effect of synthesis method on solvation and exfoliation of graphite oxide. Carbon. 2013;52:171–80. https://doi.org/10.1016/j.carbon.2012.09.018.
Akhavan O, Kalaee M, Alavi ZS, Ghiasi SMA, Esfandiar A. Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon. 2012;50:3015–25. https://doi.org/10.1016/j.carbon.2012.02.087.
Guex LG, Sacchi B, Peuvot KF, Andersson RL, Pourrahimi AM, Ström V, et al. Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale. 2017;9:9562–71. https://doi.org/10.1039/C7NR02943H.
Akhavan O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon. 2010;48:509–19. https://doi.org/10.1016/j.carbon.2009.09.069.
Goncalves G, Marques PAAP, Granadeiro CM, Nogueira HIS, Singh MK, Grácio J. Surface modification of graphene nanosheets with gold nanoparticles: the role of oxygen moieties at graphene surface on gold nucleation and growth. Chem Mater. 2009;21:4796–802. https://doi.org/10.1021/cm901052s.
Hernández-Sánchez D, Villabona-Leal G, Saucedo-Orozco I, Bracamonte V, Pérez E, Bittencourt C, et al. Highly stable graphene oxide-gold nanoparticle platforms for biosensing applications. Phys Chem Chem Phys. 2018;20:1685–92. https://doi.org/10.1039/c7cp04817c.
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, et al. Synthesis of au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci Rep. 2014;4:1–7. https://doi.org/10.1038/srep07515.
Khalil I, Julkapli NM, Yehye WA, Basirun WJ, Bhargava SK. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced Raman scattering biosensor. Materials (Basel). 2016;9:406. https://doi.org/10.3390/ma9060406.
Thangamuthu M, Hsieh KY, Kumar PV, Chen G-Y. Graphene- and graphene oxide-based nanocomposite platforms for electrochemical biosensing applications. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122975.
Assali A, Akhavan O, Adeli M, Razzazan S, Dinarvand R, Zanganeh S, et al. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomed Nanotechnol Biol Med. 2018;14:1891–903. https://doi.org/10.1016/j.nano.2018.05.016.
Zhang Z, Chen H, Xing C, Guo M, Xu F, Wang X, et al. Sodium citrate: A universal reducing agent for reduction/decoration of graphene oxide with au nanoparticles. Nano Res. 2011;4:599–611. https://doi.org/10.1007/s12274-011-0116-y.
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS. Hydrazine-reduction of graphite- and graphene oxide. Carbon. 2011;49:3019–23. https://doi.org/10.1016/j.carbon.2011.02.071.
Zhuo Q, Ma Y, Gao J, Zhang P, Xia Y, Tian Y, et al. Facile synthesis of graphene/metal nanoparticle composites via self-catalysis reduction at room temperature. Inorg Chem. 2013;52:3141–7. https://doi.org/10.1021/ic302608g.
Xie X, Zhou Y, Huang K. Advances in microwave-assisted production of reduced graphene oxide. Front Chem. 2019;7:1–11. https://doi.org/10.3389/fchem.2019.00355.
De Silva KKH, Huang H-H, Yoshimura M. Progress of reduction of graphene oxide by ascorbic acid. Appl Surf Sci. 2018;447:338–46. https://doi.org/10.1016/j.apsusc.2018.03.243.
D’souza SL, Pati RK, Kailasa SK. Ascorbic acid functionalized gold nanoparticles as a probe for colorimetric and visual read-out determination of dichlorvos in environmental samples. Anal Methods. 2014;6:9007–14. https://doi.org/10.1039/C4AY01004C.
Kang H-H, Oh S-G. Synthesis of L-ascorbic acid derivative including 3-aminopropane phosphoric acid as a novel whitening agent. Bull Korean Chem Soc. 2003;24:1169–71. https://doi.org/10.5012/bkcs.2003.24.8.1169.
Qiu Y, Wang Z, Owens ACE, Kulaots I, Chen Y, Kane AB, et al. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale. 2014;6:11744–55. https://doi.org/10.1039/C4NR03275F.
Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials. 2016. https://doi.org/10.3390/nano6040071.
Iliut M, Leordean C, Canpean V, Teodorescu C-M, Astilean S. A new green, ascorbic acid-assisted method for versatile synthesis of Au–graphene hybrids as efficient surface-enhanced Raman scattering platforms. J Mater Chem C. 2013;1:4094–104. https://doi.org/10.1039/C3TC30177J.
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–80. https://doi.org/10.1021/nn202451x.
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim J-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomed. 2012;7:5901–14. https://doi.org/10.2147/IJN.S37397.
Tang J, Chen Q, Xu L, Zhang S, Feng L, Cheng L, et al. Graphene oxide-silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl Mater Interfaces. 2013;5:3867–74. https://doi.org/10.1021/am4005495.
Sandhya PK, Jose J, Sreekala MS, Padmanabhan M, Kalarikkal N, Thomas S. Reduced graphene oxide and ZnO decorated graphene for biomedical applications. Ceram Int. 2018;44:15092–8. https://doi.org/10.1016/j.ceramint.2018.05.143.
Kurmarayuni CM, Chandu B, Yangalasetty LP, Gali SJ, Khandapu BMK, Bollikolla HB. Studies on the antioxidant and antibacterial activities of in situ green synthesized graphene-gold nanocomposite. ChemistrySelect. 2021;6:11832–7. https://doi.org/10.1002/slct.202103236.
Kadiyala NK, Mandal BK, Ranjan S, Dasgupta N. Bioinspired gold nanoparticles decorated reduced graphene oxide nanocomposite using Syzygium cumini seed extract: evaluation of its biological applications. Mater Sci Eng C. 2018;93:191–205. https://doi.org/10.1016/j.msec.2018.07.075.
Hegab HM, ElMekawy A, Zou L, Mulcahy D, Saint CP, Ginic-Markovic M. The controversial antibacterial activity of graphene-based materials. Carbon. 2016;105:362–76. https://doi.org/10.1016/j.carbon.2016.04.046.
Barbolina I, Woods CR, Lozano N, Kostarelos K, Novoselov KS, Roberts IS. Purity of graphene oxide determines its antibacterial activity. 2D Mater. 2016;3:25025. https://doi.org/10.1088/2053-1583/3/2/025025.
Chen J, Yao B, Li C, Shi G. An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon. 2013;64:225–9. https://doi.org/10.1016/j.carbon.2013.07.055.
Cobos M, González B, Fernández MJ, Fernández MD. Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int J Biol Macromol. 2018;114:599–613. https://doi.org/10.1016/j.ijbiomac.2018.03.129.
De Silva KKH, Huang H-H, Joshi RK, Yoshimura M. Chemical reduction of graphene oxide using green reductants. Carbon. 2017;119:190–9. https://doi.org/10.1016/j.carbon.2017.04.025.
Johnson SR, Evans SD, Mahon SW, Ulman A. Alkanethiol molecules containing an aromatic moiety self-assembled onto gold clusters. Langmuir. 1997;13:51–7. https://doi.org/10.1021/la9607520.
Yonezawa T, Kunitake T. Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Colloids Surfaces A Physicochem Eng Asp. 1999;149:193–9. https://doi.org/10.1016/S0927-7757(98)00309-4.
Varnavski O, Ramakrishna G, Kim J, Lee D, Goodson T. Critical size for the observation of quantum confinement in optically excited gold clusters. J Am Chem Soc. 2010;132:16–7. https://doi.org/10.1021/ja907984r.
Tepe S. Angular Light Scattering of Gold Nanoparticles on Graphene Oxide. Gothenburg: Chalmers University of Technology; 2016.
Pan H, Low S, Weerasuriya N, Shon Y-S. Graphene oxide-promoted reshaping and coarsening of gold nanorods and nanoparticles. ACS Appl Mater Interfaces. 2015;7:3406–13. https://doi.org/10.1021/am508801e.
Zedan AF, Moussa S, Terner J, Atkinson G, El-Shall MS. Ultrasmall gold nanoparticles anchored to graphene and enhanced photothermal effects by laser irradiation of gold nanostructures in graphene oxide solutions. ACS Nano. 2013;7:627–36. https://doi.org/10.1021/nn304775h.
Pham TA, Choi BC, Lim KT, Jeong YT. A simple approach for immobilization of gold nanoparticles on graphene oxide sheets by covalent bonding. Appl Surf Sci. 2011;257:3350–7. https://doi.org/10.1016/j.apsusc.2010.11.023.
Xu C, Shi X, Ji A, Shi L, Zhou C, Cui Y. Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PLoS ONE. 2015;10:e0144842.
Kumar A, Kuang Y, Liang Z, Sun X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater Today Nano. 2020;11:100076. https://doi.org/10.1016/j.mtnano.2020.100076.
Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, et al. Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data. 2013;58:209–16. https://doi.org/10.1021/je300551c.
Xu Z, Gao H, Guoxin H. Solution-based synthesis and characterization of a silver nanoparticle–graphene hybrid film. Carbon. 2011;49:4731–8. https://doi.org/10.1016/j.carbon.2011.06.078.
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, et al. Raman spectrum of graphene and graphene layers. Phys Rev Lett. 2006;97:187401. https://doi.org/10.1103/PhysRevLett.97.187401.
Calizo I, Balandin AA, Bao W, Miao F, Lau CN. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007;7:2645–9. https://doi.org/10.1021/nl071033g.
Akhavan O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon. 2015;81:158–66. https://doi.org/10.1016/j.carbon.2014.09.044.
Malassis L, Dreyfus R, Murphy RJ, Hough LA, Donnio B, Murray CB. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016;6:33092–100. https://doi.org/10.1039/C6RA00194G.
Jasuja K, Linn J, Melton S, Berry V. Microwave-reduced uncapped metal nanoparticles on graphene: tuning catalytic, electrical, and Raman properties. J Phys Chem Lett. 2010;1:1853–60. https://doi.org/10.1021/jz100580x.
Peng S, McMahon J, Schatz G, Gray S, Sun Y. Reversing the size-dependence of surface plasmon resonances. Proc Natl Acad Sci USA. 2010;107:14530–4. https://doi.org/10.1073/pnas.1007524107.
Chen J, Cui X, Wang Q, Wang H, Zheng X, Liu C, et al. One-pot photochemical synthesis of ultrathin Au nanocrystals on co-reduced graphene oxide and its application. J Colloid Interface Sci. 2012;383:140–7. https://doi.org/10.1016/j.jcis.2012.06.007.
Hu H, Wang X, Xu C, Wang J, Wan L, Zhang M, et al. Microwave-assisted synthesis of graphene nanosheets-gold nanocomposites with enhancing electrochemical response. Fuller Nanotub Carbon Nanostructures. 2012;20:31–40. https://doi.org/10.1080/1536383X.2010.533307.
Vinodgopal K, Neppolian B, Lightcap IV, Grieser F, Ashokkumar M, Kamat PV. Sonolytic design of graphene−Au nanocomposites. Simultaneous and sequential reduction of graphene oxide and Au(III). J Phys Chem Lett. 2010;1:1987–93. https://doi.org/10.1021/jz1006093.
Tuz Johra F, Jung W-G. Low temperature synthesis of RGO-Au nanocomposite with apparently reduced time and its application as a chemical sensor. Appl Surf Sci. 2016;362:169–75. https://doi.org/10.1016/j.apsusc.2015.11.145.
Bilecka I, Niederberger M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale. 2010;2:1358–74. https://doi.org/10.1039/B9NR00377K.
Collins MJ Jr. Future trends in microwave synthesis. Future Med Chem. 2010;2:151–5. https://doi.org/10.4155/fmc.09.133.
La Hoz AD, Alcázar J, Carrillo J, Herrero MA, Muñoz JDM, Prieto P, et al. Reproducibility and scalability of microwave-assisted reactions. In: Chandra U, editor., et al., Microwave heating, Chapter 7. IntechOpen: Rijeka; 2011.
Ashry N, Bahgy H, Mohamed A, Alsubhi N, Alrefai G, Binothman N, et al. Evaluation of graphene oxide, chitosan, and their complex as antibacterial agents and anticancer apoptotic effect on HeLa cell line. Front Microbiol. 2022;13:922324.
Bhaisare M. MALDI MS analysis, disk diffusion and optical density measurements for the antimicrobial effect of zinc oxide nanorods integrated in graphene Q1 oxide nanostructures. Biomater Sci. 2015. https://doi.org/10.1039/c5bm00342c.
Shalini A, Priya K, Kothai S, Pandian K, Anbalagan G, Jaisankar V. Synthesis and characterisation of graphene oxide decorated gold nano particles and their application towards antibacterial activity. Chem Pap. 2022;76:6861–7. https://doi.org/10.1007/s11696-022-02375-x.
Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, et al. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir. 2012;28:12364–72. https://doi.org/10.1021/la3023908.
Lin C, Tao K, Hua D, Ma Z, Zhou S. Size effect of gold nanoparticles in catalytic reduction of p-Nitrophenol with NaBH4. Molecules. 2013;18:12609–20. https://doi.org/10.3390/molecules181012609.
Li W-R, Sun T-L, Zhou S-L, Ma Y-K, Shi Q-S, Xie X-B, et al. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int Biodeterior Biodegrad. 2017;123:304–10. https://doi.org/10.1016/j.ibiod.2017.07.015.
Dat NM, Cong CQ, Phuc NM, Dat NT, Huong LM, Tai LT, et al. Facile phytosynthesis of gold nanoparticles-doped graphene oxide using Mangifera indica leaf extract: characterization, antibacterial activity, and catalytic reduction of organic dyes. Mater Today Sustain. 2022;19:100216. https://doi.org/10.1016/j.mtsust.2022.100216.
Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–6. https://doi.org/10.1021/nn101390x.
Dutta T, Sarkar R, Pakhira B, Ghosh S, Sarkar R, Barui A, et al. ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: comparison with graphene oxide (GO). RSC Adv. 2015;5:80192–5. https://doi.org/10.1039/C5RA14061G.
Lakshmi Prasanna V, Vijayaraghavan R. Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir. 2015;31:9155–62. https://doi.org/10.1021/acs.langmuir.5b02266.
Akhavan O, Ghaderi E, Esfandiar A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J Phys Chem B. 2011;115:6279–88. https://doi.org/10.1021/jp200686k.
Hashemi E, Akhavan O, Shamsara M, Rahighi R, Esfandiar A, Tayefeh AR. Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv. 2014;4:27213–23. https://doi.org/10.1039/C4RA01047G.
Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50:1853–60. https://doi.org/10.1016/j.carbon.2011.12.035.
Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med. 2011;51:1872–81. https://doi.org/10.1016/j.freeradbiomed.2011.08.025.
Wang Y-W, Cao A, Jiang Y, Zhang X, Liu J-H, Liu Y, et al. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces. 2014;6:2791–8. https://doi.org/10.1021/am4053317.
Jannesari M, Akhavan O, Madaah Hosseini HR, Bakhshi B. Oxygen-rich graphene/ZnO2-Ag nanoframeworks with pH-switchable Catalase/peroxidase activity as O2 nanobubble-self generator for bacterial inactivation. J Colloid Interface Sci. 2023;637:237–50. https://doi.org/10.1016/j.jcis.2023.01.079.
Zhang T, Tremblay P-L. Graphene: An antibacterial agent or a promoter of bacterial proliferation? Iscience. 2020;23:101787. https://doi.org/10.1016/j.isci.2020.101787.
Białas N, Sokolova V, van der Meer SB, Knuschke T, Ruks T, Klein K, et al. Bacteria (E. coli) take up ultrasmall gold nanoparticles (2 nm) as shown by different optical microscopic techniques (CLSM, SIM, STORM). Nano Sel. 2022;3:1407–20. https://doi.org/10.1002/nano.202200049.
Acknowledgements
The authors express gratitude to the Ministry of Research and Higher Education of the Republic of Indonesia for providing full funding for this research under the Master to Doctoral Education Program for Excellent Students (PMDSU) program with contract No. 2197/UN1/DITLIT/Dit-Lit/PT.01.03/2023; May 8, 2023.
Funding
This work was supported by the Ministry of Research and Higher Education of the Republic of Indonesia (Grant No. 2197/UN1/DITLIT/Dit-Lit/PT.01.03/2023; May 8, 2023).
Author information
Authors and Affiliations
Contributions
Pandji Zamzami Fathurrohman: Methodology, Data curation, Writing – original draft. Eko Sri Kunarti: Validation, Supervision. Nastiti Wijayanti: Conceptualization, Validation, Supervision. Sri Juari Santosa: Conceptualization, Project Administration, Writing – review & editing, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fathurrohman, P.Z., Kunarti, E.S., Wijayanti, N. et al. Straightforward microwave-assisted synthesis of ultrasmall gold nanoparticles acnhored on reduced graphene oxide for enhanced antibacterial application. Discov Appl Sci 6, 311 (2024). https://doi.org/10.1007/s42452-024-06002-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06002-0