Abstract
Four cyanobacterial strains isolated from arsenic (As) contaminated area in Minas Gerais, Brazil namely: Pseudanabaena spp. CCM-UFV063 and CCM-UFV065, Tolypothrix sp CCM-UFV067, and Desmonostoc sp. CCM-UFV070 were grown on BG-11 and BG-110 culture media respectively supplemented with di-sodium hydrogen arsenate heptahydrate (As5+) at concentrations of 0, 1, 10, 100, 1000 and 5000 mg L−1. The growth was measured by means of optical density daily for seven (7) days. Contents of chlorophyll a, carbohydrates, protein, and amino acids were also evaluated. Fluorescence Spectroscopy and ICP-OES were used to quantify As in cells. The cellular growth, based on OD750nm, was significantly lower (P < 0.05) in 5000 mg L−1 As. Chlorophyll a, carbohydrates and protein contents were significantly (P < 0.05) lowest in 5000 mg L−1 As. Amino Acid content of studied cyanobacteria varied and were higher in higher As concentrations except for Pseudanabaena sp. CCM-UFV065. Similarly, As cells accumulation was dependent on concentration and showed the order of 5000 mg L−1 > 1000 mg L−1 > 100 mg L−1 > 10 mg L−1 > 1 mg L−1 All studied cyanobacteria showed ability to absorb As(V) especially Pseudanabaena sp. CCM-UFV065 and could all be potential isolates for bioremediation of As-contaminated waters.
Article Highlights
-
Cyanobacteria spp growth was reduced at 5000 mg L−1 arsenate amendment.
-
Chlorophyll a, carbohydrates and protein contents of cyanobacteria spp were low at 5000 mg L−1 arsenate amendment.
-
Cyanobacteria spp accumulation of arsenate was dose dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water pollution by arsenic (As) due to both natural and anthropogenic activities is one of the most common environmental issues worldwide [1,2,3,4,5,6]. It is estimated that 35 million to 77 million people have been chronically exposed to As through drinking water in Bangladesh alone [5, 7, 8]. Human exposure to As well over the recommended limits of 10 µg L−1 can lead to an array of diseases including bladder, skin and lung cancers; diabetes; cardiovascular disease; developmental disorders; neurological disorders and metabolic disorders [9,10,11,12].
Cyanobacteria are essential primary producers in the ecosystem and can accumulate large amounts of arsenic from their surroundings making them suitable ecological indicators for As bioavailability and its remediation [13,14,15,16,17,18]. Potentially, they could remediate arsenic-contaminated waters in wetlands through adsorption and biotransformation of inorganic arsenic [17, 19].
In recent years, special attention has been paid to cyanobacterial growth analyses in As contaminated environments [17, 20, 21]. However, the majority of physiological and metabolic studies conducted with cyanobacterial under As stress have been done with model strains such as Nostoc, Anabaena sp. PCC7120 and Synechocystis sp. PCC6803 [16, 22,23,24]. Few studies have been carried out with indigenous strains [15, 17, 20], which can present high diversity and adaptability. Cyanobacterial strains isolated from these sites can be used in decontamination studies, once they are capable of accumulating and transforming As [3, 17].
Pentavalent arsenate (As (V)) is the most common form in Aquatic systems and it is thermodynamically stable under oxic conditions [1]. It is a phosphate analogue and therefore, enters the cells through phosphate transporters, mediating its toxicity by replacing phosphate in essential biochemical reactions, such as oxidative phosphorylation and glycolysis [12, 16, 18, 23, 25]. Therefore, changes in biochemical parameters of arsenic exposed organisms could help in understanding the resistant nature of particular taxon to As (25). Since cyanobacteria adaptation to stress is coupled with profound changes in proteome repertoire, proteomic studies can unravel the possible relationships between protein abundance and stress acclimation [23]. Arsenic adversely affects the metabolic processes and leads to (i) oxidative stress through reactive oxygen species (ROS) generation thereby damaging proteins, lipids and nucleic acids, (ii) inhibition of RubisCO, chlorophyll biosynthesis, photosynthetic pigments, photosynthesis, and (iii) inhibition of adenosine triphosphate (ATP) by uncoupling phosphorylation [6, 23].
Paucity of studies on the effect of As on cyanobacteria growth and metabolism necessitated this study which assessed the impact of increasing concentrations and accumulation of As (arsenate) on the growth and metabolism of four cyanobacteria characterized as Tolypothrix sp (CCM-UFV 067; heterocytous), subgenus Pseudanabaena (CCM-UFV 063; homocytous), Desmonostoc sp (CCM-UFV 070; heterocytous) and Pseudanabaena sp (sub genus Ilyonema) (CCM-UFV 065; homocytous) [26].
2 Materials and methods
2.1 Cyanobacteria strains and culture conditions
A total of four cyanobacterial strains were used in this study. All strains are available at the Collection of Cyanobacteria and Microalgae (CCM–UFV) [Universidade Federal de Viçosa (Viçosa, Minas Gerais state, Brazil)]. The strains were previously characterized [26] and are identified as Pseudanabaena spp. CCM-UFV063 and CCM-UFV065, Tolypothrix sp. CCM-UFV067, and Desmonostoc sp. CCM-UFV070. Stock cultures of these strains were placed into 125 mL Erlenmeyer flasks containing 60 mL of fresh sterile culture media. The strains CCM-UFV063 and CCM-UFV065 (filamentous homocytous) were grown in BG-11 while the strains CCM-UFV067 and CCM-UFV070 (filamentous heterocytous) were cultured in BG-110 (BG-11 without N source) [27]. The growth conditions, for maintenance and experiments, were as follow: rotation at 110 rpm, at room temperature (24 °C), under light intensity of 70 ± 5 µmoles·m−2 s−1 (16:8 h of light: dark cycle).
2.2 As treatments and growth curves
For inoculum production, 1 mL aliquots of the above-mentioned strains were taken from stock cultures and inoculated in Erlenmeyer flasks (500 mL) containing 200 mL of culture medium. The flasks were kept under the photoautotrophic conditions described above for ~ 10 days.
Aiming to determine the effects of different arsenate [As (V)] concentrations (hereafter As) over the cyanobacterial growth and metabolism eight-days growth curves (n = 3) were carried out. The As solution was prepared by dissolving analytical reagent-grade di-sodium hydrogen arsenate heptahydrate (Na2HAsO4.7H2O PA: > 98%, Sigma scientific) in Milli-Q water to give concentrations of 0, 1, 10, 100, 1000 and 5000 mg L−1 [28]. A final volume of 5 mL of each As solution was introduced into culture media (Final volume of 65 mL) after two days of growth (exponential growth phase of each cyanobacterial strain), while the control had 5 mL of each As solution introduced into culture media without cyanobacteria. Growth was evaluated by optical density (OD750 nm) which were measured at intervals of 24 h, according to [29].
2.3 Metabolic analyses
For the metabolic analyzes, freeze-dried biomasses sampled at days 2 of growth (before As addition) and days 4, 6 and 8 (after As addition) were used. The metabolic quantifications were only carried out in the treatments 0, 1000 and 5000 mg L−1 As, following the results observed in the growth curves. The chlorophyll a concentration was determined spectrophotometrically immediately after the methanol extraction, according to [30]. Quantification of total amino acids was performed as described by [31]; the carbohydrate content was determined according to [32]; and the content of total soluble proteins was determined as described by) [33].
Amino acid (aa) contents of the different cyanobacteria without arsenate amendment (control) and those grown in 1000 mg/L (CCM-UFV 067; 063 and 070) and 5000 mg/L) (CCM-UFV 067. 063, 070 and 065) As (V) amended media were quantified using the method of [31]. At 4, 6 and 8 d of cell growth, the supernatants (50 µL) from sequential ethanolic extraction were used for AA quantification. Na-Citrate Buffer (50 µL) was added to the ethanolic extract or leucine dilutions (standard curve) in 1.5 ml screw capped Eppendorfs as well as 100 µL Ninhydrin solution. The tubes were covered and heated at 95 °C for 20 min, quickly spun at 400 rpm and transferred to a µ-plate. Absorbance was read at 570 nm using spectrophotometer for plates. Standard curve for µ plate were prepared at 0, 2, 5, 10, 20, and 50 µl/mM leucine in 70% EtOH concentration.
Soluble carbohydrate contents of the different cyanobacteria without arsenate amendment (control) and those grown in 1000 mg/L (CCM-UFV 067; 063 and 070) and 5000 mg/L As (V) (CCM-UFV 067. 063, 070 and 065) were quantified using the method of [32]. The use of these two concentrations were based on the observation of significant changes in OD, and chlorophyll a at these concentrations. At 4, 6 and 8 d of cell growth, 5 ml of frozen culture from ultra-storage freezer (Thermo Scientific) were brought out and kept in ice box and 1 ml of H2SO4 (2N) were added to the culture in appendorf tubes and were heated at 80 °C for 30 min. Cultures were centrifuged at 13,400 rpm and 100 µL of samples as well as glucose dilutions were transferred to new tubes. Phenol (60 µL at 5% v/v) were added to the tubes, followed by 300 µL of H2SO4 (98%) and tubes were heated at 90 °C for 5 min, finally, absorption was read at 490 nm using spectrophotometer for plates. Glucose standard solutions (1 µg/µl) were prepared from a glucose stock solution (10 µg/µl in 2N H2SO4) [4 °C] and standard curve for µ plate were prepared at 0, 5, 10, 15 30 and 50 µg/µl glucose concentration.
Protein contents of the different cyanobacteria strains without arsenate amendment (control) and those grown in 1000 mg/L (CCM-UFV 067; 063 and 070) and 5000 mg/L (CCM-UFV 067. 063, 070 and 065) As (V) amended media were quantified using the method described by [33]. At 4, 6 and 8 d of cell growth, the remaining pellets from 5 ml of frozen culture used for sequential ethanolic extraction (chlorophyll a quantification) were used for protein quantification. NaOH (500 µL at 0.1 M) were added to the pellet with 1% (w/v) sodium dodecyl sulfate (SDS) and heated at 95 °C for 30 min. Samples were cooled in ice for 5 min and 125 µL of HCL (0.4 M) was added and these were centrifuged at 13,000 rpm for 10 min. The extracts (200 µL) and bovine serum albumen (BSA; 200 µL) were transferred to new 2 mL tubes where 1000 µL of solution D (60 mL of NaOH 0.1 M, 600 µL of 2% CuSO4 and 600 µL of 4% potassium sodium tartrate) were added and kept at room temperature for 20 min. Then, 200 µL of Folin and Ciocalteu’s phenol reagent (diluted 1:1 (v/v) with water) were added and the reactions were kept in the dark for 40 min after which absorbance was read at 750 nm using spectrophotometer for plates. BSA standard solutions (1 µg/µl) were prepared from a stock BSA solution (10 µg/µl in 0.1 M NaOH) [− 20 °C] and standard curve for µ plate were prepared at 0, 0.01, 0.02, 0.04, 0.08, 0.12, 0.16, 0.2, 0.24 and 0.28 µg/µl BSA concentration.
2.4 As quantification in cyanobacterial cells
As content in the cells of CCM-UFV 067; 063; 070 and 065 controls and 1, 10, 100, 1000 and 500 mg·L−1 amendments were determined. Cells were harvested at 1, 3 and 5 day after As amendment by centrifugation at 1500 g for 8 min and the supernatant removed. The cell pellets were then lyophilized using a lyophilizer (Scanvac; Bioma) for approximately 24–48 h and were placed in a desiccator before total As analysis. Total As in cells (0.01 g) were quantified for each treatment in I ml of conc. HNO3 using perfluoroalkoxy (PFA) vessels (CEM) and were digested in a microwave (Model MARs, CEM) at 55 °C for 10 min, 75 °C for 10 min and 95 °C for 30 min with 5 min ramp time between each stage [34, 35]. After the digestion program, the vessels were cooled to room temperature; opened and 10 ml of deionized water was added. Vessels were then filtered into 15 ml polypropylene (PP) tubes using JP42 (11 cm; slow filtration) paper. Samples were stored in 15 ml polypropylene (PP) tubes until quantification of total As using Hydride Generation Fluorescence Spectrometry (HG-AFS) (PSA Analytical Model 10.033 Excalibur) for lower As amendments (1, 10 and 100 mg/l) and Inductively Coupled Plasma Optical Emission Spectrometry ICP-OES (Optima 7300 DV) for higher As amendments (1000 and 5000 mg/l) after appropriate dilutions.
2.5 Statistical analysis
SPSS V23 (ONE WAY ANOVA) was used to analyze the difference in the means of the OD, DM, chlorophyll a, CHO, protein, AA and As in cells in the controls of CCM-UFV 067; 063; 070 and 065 and the different As amendments. Significance was measured at p < 0.05.
3 Results
3.1 Cyanobacteria growth and physiology during incubation with As
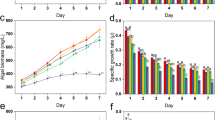
The observed OD during cyanobacteria growth in the different As amendments is shown in Fig. 1. The OD of Tolypothrix sp CCM-UFV 067 control and the different As amendments increased with incubation time although no significant difference (p > 0.05) between the OD of the control and As amendments (1, 10, 100 and 1000 mg L−1) was observed. However, OD decreased significantly in 5000 mg L−1 amendment as compared to the control and other As amendments (Fig. 1). OD increased with time in both the control of Pseudanabaena sp CCM-UFV 063 and As amendments. However, it was reduced significantly (p < 0.05) in the highest As amendment (5000 mg L−1) as compared to the control and other As amendments (Fig. 1). Increase in OD with time was also observed in both the control and As treatments with Desmonostoc sp CCM-UFV 070. However, OD of 100 mg L−1 As amendment was higher (p < 0.05) than all other treatment at 5 d (Fig. 1). Significant decreases (p < 0.05) in OD was observed in the 5000 mg L−1As amendment at 5, 6 and 7 day of incubation studies as compared to the control and other As amendments. OD of the control of Pseudanabaena sp (sub genus Ilyonema) CCM-UFV 065 and As amendment increased with incubation time although OD at 5000 mg L−1 As amendment was significantly (p < 0.05) lower than the control and other As amendments (Fig. 1). Cyanobacterial cells OD was significantly lower (p < 0.05) in 5000 mg L−1 As amendment as compared to controls without As amendment (Fig. 1).
Chlorophyll a content of all studied Cyanobacteria (control) during growth in As amendments (1000 and 5000 mg L−1 As) is shown in Fig. 2. This was same in Tolypothrix sp CCM-UFV 067 control all through incubation period except a decrease on the last day of incubation. However, it increased significantly (p < 0.05) with time in the 1000 mg L−1 As amendments. Conversely, a significant decrease (p < 0.05) in the 5000 mg L−1 As amendment was observed (Fig. 2). Initial decrease (p < 0.05) in chlorophyll a content of Pseudanabaena sp CCM-UFV 063 control at 4 d was followed by a significant increase (p < 0.05) at 6 and 8 d of incubation. As amendment of 1000 mg L−1 had chlorophyll a content only significantly lower (p < 0.05) than the control at 6 and 8 d however, this was lower (p < 0.05) in 5000 mg L−1 As amendment as compared to the control and 1000 mg L−1 As treatment (Fig. 2). Chlorophyll a content of Desmonostoc sp CCM-UFV 070 (control) increased with time (Fig. 2). An initial decrease observed at 4 d after As amendment in both As amendments (1000 and 5000 mg L−1) was followed by an increase at 6 d. However, this increase was significantly lower (p < 0.05) as compared to the control with 5000 mg L−1 As amendment having the lowest (P < 0.05) chlorophyll a content. Chlorophyll a content of Pseudanabaena sp (sub genus Ilyonema) CCM-UFV 065 increased with time in both the control and 1000 mg L−1 As amendment with a drop at last day of incubation (Fig. 2). However, this was significantly higher in the control as compared to that of 1000 mg L−1 As amendment. The highest As amendment (5000 mg L−1) had a significant drop in chlorophyll a content at 4 and 6 d after As amendment with an increase observed at 8 d relative to other days (Fig. 2). There was a decrease (p < 0.05) in chlorophyll a content of all studied cyanobacterial cells at 1000 and 5000 mg L−1 As amendment as compared to the control except with Tolypothrix sp CCM-UFV 067 where chlorophyll a content was significantly higher in 1000 mg L−1 As amendment as compared to the control at 4. 6 and 8 d (Fig. 2). Chlorophyll a content of cyanobacterial cells (controls) were greenish in color as compared to yellowish brown color observed in the highest As amendment after 8 d incubation period (Fig. 2).
3.2 Metabolic analyses: soluble carbohydrates
Soluble carbohydrates were determined at 4, 6 and 8 d in the control (CCM-UFV 067. 063, 070 and 065), 1000 mg L−1 (CCM-UFV 067; 063 and 070) and 5000 mg L−1 (CCM-UFV 067. 063, 070 and 065) As amendments (Tables 1 and 2). Soluble carbohydrate in Tolypothrix sp CCM-UFV 067 control was significantly higher (p < 0.05) than the two As amendments at 4 d. However, no significant difference was observed between the control and 1000 mg L−1 amendment at 6 d but 5000 mg L−1 amendment was significantly lower than both at this time. At 8 d, no significant difference was observed in the control and As amendments. Soluble carbohydrate in Tolypothrix sp CCM-UFV control did not change with time however, there were significant increases in soluble carbohydrates in both As amendments at 8 d as compared to 4 and 6 d.
Soluble carbohydrate in Pseudanabaena (CCM-UFV 063) control was higher (p < 0.05) than As amendments at 4 d (Table 1). Although 5000 mg L−1 As amendment had significantly lower soluble carbohydrate than that of the control and 1000 mg L−1 As amendment at 6 and 8 d this was significantly higher at 6 and 8 d in 1000 mg L−1As amendment as compared to the control. Soluble CHO generally increased with time in the control and As amendment at 1000 mg L−1.
Soluble CHO in Desmonostoc sp (CCM-UFV 070) control was significantly higher (p < 0.05) as compared to both As amendments at 4, 6 and 8 d (Table 2). Generally, CHO decreased with time in the control and As amendments although it increased at 6 d relative to 4 and 8 d in 5000 mg L−1 As amendment (Table 2).
CHO content of Pseudanabaena sp (sub genus Ilyonema) CCM-UFV 065 was significantly higher in the control as compared to 5000 mg L−1As amendment at 4, 6 and 8 d. However, CHO was significantly higher at 4 d as compared to 8 d in the control but reverse was the case in 5000 mg L−1As amendment as there was significant increase at 8 d as compared to 4 and 6 d (Table 2).
3.3 Metabolic analyzes: protein
Protein content were determined at 4, 6 and 8 d in the control (CCM-UFV 067. 063, 070 and 065), 1000 mg L−1 (CCM-UFV 067; 063 and 070) and 5000 mg/L) (CCM-UFV 067. 063, 070 and 065) As amendments (Tables 1 and 2). Tolypothrix sp CCM-UFV 067 control and 1000 mg L−1 As amendment protein content were significantly higher (p < 0.05) than that 5000 mg L−1As amendments at 4 d. At 6 and 8 d however, the control had significantly higher protein content as compared to both As amendments. Protein content was significantly lower in 5000 mg L−1 relative to the control and 1000 mg L−1 As amendment. The control had higher protein content at 6 d as compared to 4 and 8 d but this was highest at 4 d as compared to 6 and 8 d in 1000 mg L−1 As amendment. No significant difference in protein content was observed in the 5000 mg L−1 As amendment with time.
Protein content of Pseudanabaena sp (CCM-UFV 063) control was significantly higher (p < 0.05) than both As amendment at 4 and 8 d however, no significant difference in protein content were observed between the control and 1000 mg L−1 As amendment at 6 d. Sampling days had no impact on the protein content of the control though this was higher at 6 d as compared to 4 and 8 d in 1000 mg L−1 As amendment. Protein content was significantly lower in 5000 mg L−1 relative to the control and 1000 mg L−1 As amendment.
At 4 d, protein content of Desmonostoc sp (CCM-UFV 070) control and 1000 mg L−1 was significantly higher as compared to 5000 mg L−1 As amendments (Table 2). However, the control protein content was significantly higher than both As amendments at 6 and 8 d. Protein content was highest in the control at 6 d, and higher in 6 and 8 d as compared to 4 d in 5000 mg L−1 As amendment. Protein content was significantly lower in 5000 mg L−1 relative to the control and 1000 mg L−1 As amendment.
The control of sub genus Ilyonema (CCM-UFV 065) had consistently higher protein content as compared to 5000 mg L−1 As amendment, and had significantly higher protein content at 4 and 6 d relative to 8 d while in 5000 mg L−1 As amendment this was higher at 4 d as compared to 6 and 8 d (Table 2).
3.4 Cyanobacteria metabolism: amino acid
Amino acid (AA) content was determined at 4, 6 and 8 d in the control (CCM-UFV 067, 063, 070 and 065), 1000 mg L−1 (CCM-UFV 067; 063 and 070) and 5000 mg L−1 (CCM-UFV 067. 063, 070 and 065) As amendments (Tables 1 and 2). Varying concentrations of AA were observed during the experiment. At 4 and 8 d, amino acid content was highest in the 1000 mg L−1 As amendment with Tolypothrix sp CCM-UFV 067. However, this was least in 5000 mg L−1 As amendment at 4 d. Conversely, at 6 d, 1000 mg L−1 amendment was the least while the control and 5000 mg L−1 amendment were the same nonetheless (Table 1). Amino acid content of both the control and As amendments were highest at 4 d (Table 1).
Amino acid content of Pseudanabaena sp (CCM-UFV 063) at 1000 mg L−1 As amendment was significantly lower than that of the control and 5000 mg L−1 As amendment at 4 d. At 6 d however, this was highest in 5000 mg L−1 As amendment followed by the control, and was least in 1000 mg L−1 As amendment. Conversely, at 8 d this was least in 5000 mg L−1 As amendment as compared to the control and 1000 mg L−1 As amendment. Amino acid content of the control and As amendments was highest at 8 d (Table 1).
At 4 d, AA content of Desmonostoc sp (CCM-UFV 070) control and As amendments were not significantly different (Table 2). However, at 6 and 8 d the two As amendments had significantly higher (p < 0.05) AA content as compared to the control (Table 2). Although no significant difference in AA content was observed with the control overtime this was significantly higher at 8 d in the two As amendments.
In sub genus Ilyonema (CCM-UFV 065), control had consistently higher AA content as compared to 5000 mg L−1 As amendment. The control had significantly higher AA content at 4 d while no significant difference in AA was seen in 5000 mg L−1 As amendment with time (Table 2).
3.5 As quantification in cyanobacterial cells
As content in the cells of CCM-UFV 067; 063; 070 and 065 controls and at 1, 10, 100, 1000 and 500 mg L−1 amendments were determined at 4, 6 and 8 d of growth. Generally, As cell accumulation was concentration dependent with Tolypothrix sp CCM-UFV 067 As accumulation following the pattern of 5000 > 1000 > 100 > 10 > 1 mg L−1 at 4, 6 and 8 d (p < 0.05) (Table 3). As seemed to have accumulated more with time (at 8 d as compared to 4 d) in the lower concentrations but less with time in the higher As concentrations (1000 and 5000 mg/L) (Table 3). Similarly, As content of Pseudanabaena (CCM-UFV 063) was dependent on concentration. Cells amended with 5000 mg L−1 As had higher (p < 0.05) As concentration at 4 d and 8 d as compared to 1000 mg L−1 As amendment while this was least (p < 0.05) in 1 mg L−1 As amendment at both time points. Cells with high As concentrations (1000 and 5000 mg L−1) had highest (p < 0.05) As accumulation at 6 d and lowest at 4 d.
As accumulation in Desmonostoc sp (CCM-UFV 070) cells was based on concentration. At 4, 6 and 8 d cells As accumulation followed the pattern of 5000 > 1000 > 100 > 10 > 1 mg L−1 (p < 0.05) (Table 4). However, cells amended with high As concentrations (1000 and 5000 mg/L) had highest (p < 0.05) As accumulation at 8 d (Table 4). In Ilyonema (CCM-UFV 065), cells amended with higher As concentrations had consistently higher As content as compared to cells amended with lower As concentrations. Cells amended with 5000 mg L−1 As had highest (p < 0.05) As accumulation at 8 d and least at 6 d.
4 Discussion
The growth, physiology and metabolic analyses of four cyanobacterial strains isolated from As-contaminated environment [26] were investigated in As supplemented culture media. Growth analyses were based on OD while chlorophyll a was used to assess the physiology of the cyanobacteria. Cyanobacteria metabolism was assessed based on CHO, protein and AA and also As cell accumulation. Generally, results revealed that low As concentrations (1, 10, 100 mg/L) had no impact on OD, this was not surprising as these cyanobacteria were isolated from As contaminated area and therefore, may have As resistance and detoxification mechanisms [26, 36]. Reduction in OD in 5000 mg L−1 As amendment relative to other treatments necessitated further assessments of chlorophyl a, CHO, protein, AA (1000 and 500 mg L−1) and As cell accumulation.
All four cyanobacteria sp. studied had OD that was significantly lower (p < 0.05) in 5000 mg L−1As amendment as compared to the control and other As amendments (Fig. 1). This could be as a result of As cellular accumulation and subsequent toxicity thereby, reducing the cyanobacteria cell growth (18). Initial As(V) concentration has been reported as one of the factors that control the amount of As(V) taken up by cyanobacterial cells, and increase in arsenate concentration in the culture leads to an increased arsenate concentration in the biomass [13, 19, 36]. Franco et al. [17] reported decrease in Synechococcus sp cell number with increasing As concentration. Anabaena sp PCC7120 OD was inhibited by 43% at 40 mM As after 24 h of treatment (23). Similarly, reduction in cell numbers and growth rate of three cyanobacteria cultures with increasing As(V) concentration was reported by [36]. Again, (18) observed decrease in the growth of Nostoc muscorum when it was exposed to 50, 100 and 150 mM of arsenate. Although it has been reported that As toxicity is concentration dependent, resistance and detoxification mechanisms of the organisms are also important [36].
As toxicity at high concentrations (5000 mg/L) could be as result of reactive oxygen species (ROS) generation, presence of As(III) in the cell as well as, disruption of phosphate metabolism [6, 18]. Induction of several genes coding for protein involved in redox scavenging was observed during arsenic exposure to Synechocystis sp. PCC 6803 [24]. Since As(V) is taken up by cyanobacteria cells due to its similarity to phosphate and quickly reduced to As(III) therefore, toxicity could be due to the presence of As(III) in the cell. When As(III) ability to be excreted or sequestered is overwhelmed it binds to intracellular thiols, thereby inhibiting cell division [19]. The disruption of phosphate metabolism by incorporation of As(V) into phosphorylated compounds vital to the cycling of ATP, also contribute to As toxicity [6, 18, 19, 37].
The non-existence of difference in OD in 1000 mg L−1As amendment relative to the control and lower As concentrations could be because of resistance and detoxification mechanisms of the cyanobacteria especially since they were isolated from As contaminated sediments [26]. Again, this could be attributed to the Phosphate content and concentration (0.04 g/L) of the BG 11 media used. The high phosphate concentration in the media may not have induced phosphate transport system (which is the main arsenate transport system) usually induced under phosphate deprivation, therefore decreasing arsenate transport at 1000 mg L−1 As amendment relative to 5000 mg L−1 As treatment [12]. Phosphate concentration interferes with arsenate transport and has a great influence on arsenic resistance in cyanobacteria [5, 12, 24]. Sanchez-Riego et al. [24] showed repression of phosphate transport genes by both arsenate and arsenate in Synechocystis sp. PCC 6803 preventing acquisition of arsenate after both treatments. It has also been reported that more than 3000 fold difference in arsenate over phosphate concentration invivo is needed to observe a negative effect in growth [22]. Reduction in phosphate concentration in the media has been shown to be essential in detecting growth inhibition by the addition of arsenate during the growth of cyanobacteria [12, 24]. Xioa et al. [6] reported a much higher As(V) concentration in low phosphate (0.0003 g/L) than in high phosphate (0.007 g/L) media during the growth of Microcystis wesenbergii, this was attributed to inhibition of As(V) by phosphate. However, As cell accumulation was higher in 1000 mg L−1 relative to the lower As concentrations therefore, it could be that OD was not affected at this concentration as a result tolerance and resistance.
Chlorophyll a was significantly lower (p < 0.05) in 1000 and 5000 mg L−1 As amendments as compared to the control of Pseudanabaena sp, Desmonostoc sp and Pseudanabaena sp (sub genus Ilyonema) but was lowest in 5000 mg L−1 amendments in all the four studied cyanobacteria (Fig. 2). Several authors have reported decline in Chl a with increasing As concentrations [18, 20, 21, 23, 36, 38]. Huang et al. 2014 showed reduction in cell numbers, growth rate and chlorophyll a of three cyanobacteria sp (Oscillatoria tenuisa, Anabena affinis and Microcystis aeruginosa) with increasing As(V) concentration. This may be as a result of high internal As(V) concentration thereby inhibiting/reducing ɖ-aminolevulinic acid dehydrogenase and protochlorophyllide reductase which actively participate in pigment synthesis [18, 23]. High As concentrations can eliminate the order of chloroplasts arrangement and affect photosynthetic processes [36]. Pandey et al. [23] reported a 36.1% decline in chlorophyll a on day 1 of arsenic amendment using Anabaena sp. PCC7120. Similarly, Microcystis aeruginosa showed 31.5% reduction in chlorophyll a as compared to the control at As(V) concentration of 50 mg·L-1 [36]. Again, [18] reported 15, 34 and 44% reduction in Chlorophyll a content following 50 mM, 100 mM and 150 mM arsenate treatment. Lower Chlorophyll a content in high As concentration could also be as a result of enhanced activity of chlorophyllase (a chlorophyll degrading enzyme), replacement of Mg atom from the tetra-pyrrole ring of chlorophyll and the substitution of central atom with that of As thereby, inhibiting its biosynthetic pathway and the efficiency of photosynthetic light harvesting complexes [15, 18, 23, 38]. Generally, there was an increase in chlorophyll a content with time in the controls of Desmonostoc sp and Pseudanabaena sp (sub genera Ilyonema) and Tolypothrix sp at 1000 mg L−1As amendment (Figure 2). This agrees with the findings of [23] who reported increase in chlorophyll a content of Anabena sp PCC7120 with time. Conversely, Tolypothrix sp had significantly higher (p < 0.05) chlorophyll a content in 1000 mg L−1 As amendment as compared to the control and 5000 mg L−1 As amendment at 4, 6 and 8 d (Fig 2). This could be because As could be utilized under non inhibitory concentrations as additional energy source in metabolic processes that in turn improves nutritional status when grown in culture [39]. Gong et al. [3] similarly reported enhanced chlorophyll a in the presence of As(V) during Microcystis aeruginosa FACHB 905 growth. Chlorophyll a was also stimulated during the growth of a diazotrophic cyanobacteria (Nostoc ellipsosporum) at 50 and 200 µM sodium arsenate salt [39]. Again, chlorophyll a content of Gloeothece sp. GO9 was stimulated at 2.5 mg L−1 zinc concentration [40]. Plate pictures of cyanobacterial cells (Fig. 2) showed that control cells were greenish in color as compared to yellowish brown color observed in the highest As amendment (5000 mg L−1). As(V) has been reported to negatively affect cyanobacteria pigment levels [36]. Further, [23] observed that As treatment within 48–72 h transformed bluish green Anabaena sp. PCC7120 into yellow brown and dense pigmentation at one side of the cell. Debnath and Bhadury [20] also showed loss of pigment fluorescence in their study as a result of As(III) toxicity to four (4) diazotrophic cyanobacteria isolated from rice fields of As affected Bengal Deltan Plain (BDP). Low OD and chlorophyll a content at highest studied concentration could be attributed to As cellular accumulation and toxicity thereby, reducing cell growth, biosynthetic pathway and the efficiency of photosynthetic light harvesting complexes. Protein degradation, reduced photosynthetic pigment content as well as decrease in exopolysaccharide could be the cause of reduced protein and carbohydrate content at highest studied concentration.
Generally, carbohydrate and protein content of studied cyanobacteria were concentration dependent and were lowest in the highest As concentration (5000 mg L−1) studied (Tables 1 and 2). The low protein content in 5000 mg L−1 As amendments could be as result of negative impact of As on protein synthetic pathways, enhanced protease activity and ROS generation while that of carbohydrate could be associated with reduced photosynthetic pigment content as well as decrease in exopolysaccharides [21, 39]. Patel et al. [21] similarly reported reduced protein and carbohydrate contents of Nostoc muscorum and Anabaena sp PCC7120 when they were exposed to increasing concentrations of As(V) and As (III). Sanchez-Riego et al. [24] showed that the main response in Synechocystis sp PCC 6805 to arsenate (1 mM) and arsenate (50 mM) treatment included repression of growth-related genes or energy generation systems (photosynthesis, ATP synthetase and respiration) as well as, protein degradation. Again, As at high concentrations exerts oxidative stress by generating reactive oxygen species (ROS) that could damage proteins, lipids, and nucleic acids [6]. Sharma and Rathore [41] similarly reported reduced growth, protein, carbohydrate and amino acid content of Nostoc muscorum, Anabaena doliolum and Aulosira fertillissima when exposed to As, albeit the concentrations studied (1–15 mg/L) where much lower than that of this study. Varied amino acid content were exhibited by CCM-UFV 067, CCM-UFV 063 and CCM-UFV 070 which were a times higher in 1000 and 5000 mg L−1 As amendments relative to the control (Tables 1 and 2). This maybe a reflection of high antioxidants responses as cells try to adjust and tolerant high As dose [38]. However, AA content of sub genus Ilyonema (CCM-UFV 065) was dependent on As concentration and was higher in the control relative to 5000 mg L−1 As amendment, this may be related to diminished growth and cell death caused by high As concentration [38]. Interestingly, CCM-UFV 065 accumulated more As in cells relative to other studied cyanobacteria (Table 4).
As accumulation in cells were concentration dependent. Cells with higher As concentrations in media accumulated more As in the cells relative to cells with low As concentrations (Tables 3 and 4). The BG 11 media used in this study allowed high As accumulation although higher arsenic concentrations have been reported to accumulate in cells with low nutrient content relative to high nutrient content [6]. Again, lowest percentage As concentration in media (data not shown) were observed in higher As concentrations (1000 and 5000 mg/L) in this study. As cell accumulation could mean detoxification capacity by cells normally achieved by adsorption of As to cell surface, reduction, and thiol mediated sequestration in the vacuoles [38, 42]. The low As concentration in media with high As concentration (data not shown) is similar to the work of [20] where a total arsenite decrease of 72–84% within 7 days of cyanobacterial incubation was reported. Several Authors have reported dose dependent accumulation of As in cells [6, 18, 20, 21, 36, 38]. Patel et al. [18] reported increasing As in cells as the concentration of arsenic increased in medium. Similarly, concentration-dependent increase in As accumulation in Nostoc muscorum and Anabaena sp. PCC7120 during growth in As(V) and As(III) was illustrated by [21]. Upadhyay et al. [38] showed a significant dose dependent accumulation pattern in Nannochloropsis sp grown in different concentrations of As(III). Increasing arsenic concentration in the medium led to elevation of arsenic concentration in the cells of Nostoc sp. PCC 7120 [43], and in cells of Nostoc muscorum grown under arsenate [18]. Further, arsenicals’ accumulation in M. wesenbergii increased with increasing iAs (V) concentrations [6]. Again, in agreement with our study, [20] showed increase in intracellular As concentrations with increase in As(III) dose. High accumulation of As may also be due to different functional groups (hydroxyl, carboxyl and sulphate) in the cell wall that confer negative charge on its surface thus, toxic elements get sorbed electrostatically to the cell surface and enhance accumulation capacity [38]. In contrast to observations in this study, [36] gave evidence of decreases in the accumulation of As(V) by three cultures of cyanobacteria as AS(V) concentration increased. The Authors stated that cyanobacterial cells could not biosorb and bioaccumulate more As(V) as they were exposed to higher initial concentration (50 mg/L). In this study, As accumulation by cyanobacteria correlated with reduction in OD, Chlorophyll a, CHO and proteins. However, increase in AA was also observed a times at high As concentrations (1000 and 5000 mg L−1 As amendments), with the exception of CCM-UFV 065 which had less AA at 5000 mg L−1As amendment.
5 Conclusion
Findings from this study showed that all indigenous cyanobacteria isolates were able to grow at both low and high As concentrations which showed their resistant to As contamination probably due to exposure to As. Although As at the highest (5000 mg/L) studied concentration showed reduction in growth (OD), physiology (Chlorophyll a) and metabolism (CHO and protein) in indigenous cyanobacteria, the increase AA a times would have been detoxification mechanisms applied to deal with high As concentration in cells. All studied cyanobacteria showed ability to absorb As(V) especially Pseudanabaena sp (sub genus Ilyonema) CCM-UFV 065 and these could be potential isolates for bioremediation of As-contaminated waters.
Data availability
My data is available and can be viewed via this link. https://drive.google.com/drive/folders/1eRF05l-FRlrOCRxHcUruYr2D5ej8j3UI?q=parent:1eRF05l-FRlrOCRxHcUruYr2D5ej8j3UI.
References
Morelli E, Mascherpa MC, Scarano G. Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure. Biometals. 2005;18:587–93. https://doi.org/10.1007/s10534-005-2998-1.
Rubio R, Ruiz-Chancho MJ, Lopez-Sanchez JF. Sample pre-treatment and extraction methods that are crucial to arsenic speciation in algae and aquatic plants. Trends Anal Chem. 2010;29:53–69. https://doi.org/10.1016/j.trac.2009.10.002.
Gong Y, Ao H, Liu B, Wen S, Wang Z, Hu D, Zhang X, Song L, Liu J. Effects of inorganic arsenic on growth and microcystin production of a Microcystis strain isolated from an algal bloom in Dianchi Lake, China. Chin Sci Bull. 2011;56:2337–42. https://doi.org/10.1007/s11434-011-4576-y.
Rezende PS, Costa LM, Windmoller CC. Arsenic mobility in sediments from Paracatu River Basin, MG, Brazil. Arch Environ Contam Toxicol. 2015;2015(68):588–602. https://doi.org/10.1007/s00244-015-0134-y.
Wang Y, Wang S, Xu P, Lin C, Liu M, Wang Y, Wang C, Zhang C, Ge Y. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev Environ Sci Biotechnol. 2015;2015(14):427–51. https://doi.org/10.1007/s11157-015-9371-9.
Zhe X, Xiaochuang Li, Shouliang H. Arsenic accumulation and biotransformation affected by nutrients (N and P) in Common blooming-forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria). Water. 2022;14:245. https://doi.org/10.3390/w14020245.
Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012;17:155–62. https://doi.org/10.1016/j.tplants.2011.12.003.
Rahman M, Mamun AA, Karim MR, Islam K, Amin HA, Hossain S, Hossain MI, Saud ZA, Noman ASM, Miyataka H, Himeno S, Hossain K. Association of total arsenic in drinking water, hair and nails with serum vascular endothelial growth factor in arsenic-endemic individuals in Bangladesh. Chemosphere. 2015;120:336–42. https://doi.org/10.1016/j.chemosphere.2014.08.003.
Páez-Espino D, Tamames J, DeLorenzo V, Cánovas D. Microbial responses to environmental arsenic. Biometals. 2009;22:117–30. https://doi.org/10.1007/s10534-008-9195-y.
Huang JH, Kretzchmar R. Sequential extraction method for speciation of arsenate and arsenite in mineral soils. Anal Chem. 2010;82:5534–40. https://doi.org/10.1021/ac100415b.
Pisani T, Munzi S, Paoli L, Backor M, Loppi S. Physiological effects of arsenic in the lichen Xanthoria parietina (L) Th. Fr. Chemosphere. 2011;82:963–99. https://doi.org/10.1016/j.chemosphere.2010.10.079.
Jose Huertas M, Lopez-Maury L, Giner-Lamia J, Sanchez-Riego AM, Florencio J. Metals in cyanobacteria; Analysis of the copper, nickel, cobalt and arsenic homeostasis mechanisms. Life. 2014;4:865–86. https://doi.org/10.3390/life4040865.
Murray LA, Raab A, Marr IL, Feldmann J. Biotransformation of arsenate to arsenosugars by Chlorella vulgaris. Appl Organomet Chem. 2003;17:669–74. https://doi.org/10.1002/aoc.498.
Salgado SG, Nieto MAQ, Simon MMB. Determination of soluble toxic arsenic species in alga samples by microwave-assisted extraction and high performance liquid chromatography-hydride generation-inductively coupled plasma-atomic emission spectrometry. J Chromatogr A. 2006;1129:54–60. https://doi.org/10.1016/j.chroma.2006.06.083.
Bhattacharya P, Pal R. Response of cyanobacteria to arsenic toxicity. J Appl Phycol. 2011;23:293–9. https://doi.org/10.1007/s10811-010-9617-4.
Miyashita S, Fujiwara S, Tsuzuki M. Cyanobacteria produce arsenosugars. Environ Chem. 2012;9:474–84. https://doi.org/10.1071/EN12061.
Franco FM, Ferreire FAG, Vasconcelos IF, Batista BL, Pujoni DGF, Magalhaes SMS, Barbosa F Jr, Barbosa FAR. Arsenic biotransformation by cyanobacteria from mining areas: evidences from culture experiments. Environ Sci Pollut Res. 2015;22:18607–15. https://doi.org/10.1007/S11356-015-5425-3.
Patel A, Tiwari S, Prasad SM. Toxicity assessment of arsenate and arsenite on growth, chlorophyll a, fluorescence and antioxidant machinery in Nostoc muscorun. Ecotoxicol Environ Saf. 2018;157:369–79. https://doi.org/10.1016/j.ecoenv.2018.03.056.
Levy JL, Stauber JL, Adams MS, Maher WA, Kirby JK, Jolley DF. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ Toxicol Chem. 2005;24:2630–9. https://doi.org/10.1897/04-580r.1.
Debnath M, Bhadury P. Adaptive responses and arsenic transformation potential of diazotrophic cyanobacteria isolated from rice fields of arsenic affected Bengal Delta Plain. J Appl Phycol. 2016;28:2777–92. https://doi.org/10.1007/s10811-016-0820-9.
Patel A, Tiwari S, Prasad SM. Effect of time interval on arsenic toxicity to paddy field cyanobacteria as evident by nitrogen metabolism, biochemical constituent, and exopolysaccharide content. Biol Trace Elem Res. 2020. https://doi.org/10.1007/s12011-020-02289-3.
López-Maury L, Sánchez-Riego AM, Reyes JC, Florencio FJ. The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. Strain PCC 6803. J bacterial. 2009;191(11):3534–43. https://doi.org/10.1128/JB.01798-08.
Pandey S, Rai R, Rai LC. Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J Proteomics. 2012;75:921–37. https://doi.org/10.1016/j.jprot.2011.10.011.
Sanchez-Riego AM, Lopez-Maury L, Florencio FJ. Genomic responses to arsenic in the cyanobacterium Synechocystis sp. PCC6803. PLoS ONE. 2014;9(5):e96826. https://doi.org/10.1371/journal.pone,0096826.
Rahman MA, Hassler C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat Toxicol. 2014;146:212–9. https://doi.org/10.1016/j.aquatox.2013.11.009.
Obuekwe IS, Vaz MG, Genuario DB, Castro NV, Almeida AV, Veloso RW, Pinto GN, Alvarenga LV, Mello JV, Nunes-Nesi A, Araujo WL. Arsenic-contaminated sediment from mining areas as source of morphological and phylogenetic distinct cyanobacterial lineages. Algal Res. 2019;42:101589. https://doi.org/10.1016/j.algal.2019.101589.
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. https://doi.org/10.1099/00221287-111-1-1.
Association of Analytical Chemists (AOAC). Standard official methods of analysis of the association of analytical chemists. In: Williams SW, ed. Washington, DC; 1984. p. 121.
Alvarenga LV, de Almeida AVM, de Castro NV, Oder JC, Esteves-Ferreira A, Nunes-Nesi A, Araújo WL, Vas Marcelo GMV. Physiological responses to light intensity and photoperiod of the halotolerant cyanobacterium Desmonostoc salinum CCM-UFV059. Bioresour Technol Rep. 2020;11:100443. https://doi.org/10.1016/j.biteb.2020.100443.
Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg. 1989;975:384–94. https://doi.org/10.1016/S0005-2728(89)80347-0.
Yemm EW, Cocking EC, Ricketts RE. The determination of amino-acids with ninhydrin. Analyst. 1955;80:209–13. https://doi.org/10.1039/an9558000209.
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura SI, Lee YC. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339:67–72. https://doi.org/10.1016/j.ab.2004.12.001.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. https://doi.org/10.1016/0003-2697(76)90527-3.
Yin XX, Zhang YY, Yang J, Zhu YG. Rapid biotransformation of arsenic by a model protozoan Tetrahymena thermophila. Environ Pollut. 2011;159:837–40. https://doi.org/10.1016/j.envpol.2010.12.033.
Zhang S, Rensing C, Zhu YG. Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments. Environ Sci Technol. 2014;48:994–1000. https://doi.org/10.1021/es403836g.
Huang Winn-Jung Wu, Chih-Chao C-C. Bioaccumulation and toxicity of arsenic in cyanobacteria cultures separated from eutrophic reservoir. Environ Monit Assess. 2014;186:805–14. https://doi.org/10.1007/s10661-013-3418-6.
Nagy CI, Vass I, Rakhely G, Vass IZ, Toth A, Duzs A, Peca L, Kruk J, Kos PB. Coregulated genes link sulphide:quinone oxidoreductase and arsenic metabolism in Synechocystis sp. Strain PCC6803. J Bacteriol. 2014;196:3430–40. https://doi.org/10.1128/JB.01864-14.
Upadhyay AK, Mandotra SK, Kumar N, Singh NK, Singh L, Rai UN. Augmentation of arsenic enhances lipid yield and defense responses in alga Nannochloropsis sp. Bioresource Technol. 2016;221:430–7. https://doi.org/10.1016/j.biortech.2016.09.061.
Dutta S, Bhadury P. Effect of arsenic on exopolysaccharide production in a diazotrophic cyanobacterium. J Appl Phycol. 2020. https://doi.org/10.1007/s10811-020-02206-0.
Okmen K, Bozanta E, Ugur A, Ceyhan N. Zinc effect on chlorophyll a, total carbohydrate, total protein contents and biomass of cyanobacterial species. J Appl Biol Sci. 2011;5(2):67–73.
Sharma P, Rathore DK. Toxicity of arsenic on nitrogen-fixing cyanobacteria. J Algal Biomass Utln. 2018;9(1):68–71.
Wang Z, Luo Z, Yan C, Che F, Yan Y. Arsenic uptake and depuration kinetics in Microcystis aeruginosa under different phosphate regimes. J Hazard Mater. 2014;276:393–9. https://doi.org/10.1016/j.jhazmat.2014.05.049.
Xue XM, Yan Y, Xiong C, Raber G, Francesconi K, Pan T, Ye J, Zhu YG. Arsenic biotransformation by a cyanobacterium Nostoc sp. PCC 7120. Environ Pollut. 2017;228:111–7. https://doi.org/10.1016/j.envpol.2017.05.005.
Acknowledgements
The authors are grateful to the Brazilian Research Council (CNPq) and Third World Academy of Sciences (TWAS), as well as Departmentos de Solos and Biologia Vegetal, Universidade Federal de Viçosa, Minas Gerais, Brazil for provision of laboratory space and equipment.
Funding
No external funding was used for this research.
Author information
Authors and Affiliations
Contributions
IS, GMV, RV did the laboratory work and data collection. JVM and CJO supervised and reviewed the manuscript. IS and GMV wrote and prepared the manuscript
Corresponding author
Ethics declarations
Competing interests
The corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obuekwe, I.S., Vaz, M.G.M.V., Veloso, R. et al. Impact of arsenate on the growth and metabolism of cyanobacterial strains isolated from gold-mining areas. Discov Appl Sci 6, 292 (2024). https://doi.org/10.1007/s42452-024-05966-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05966-3