Abstract
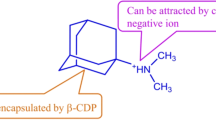
To entrap the water-insoluble medicine, the current innovation provides a cost-effective solution to the increasing need for hydrophobic gel. Improving the bioavailability of a medicine that is poorly soluble begins with entrapping the substance. By only switching out the monovalent ions for divalent ones, the researchers hope to increase the hydrophobicity of their material. In this experiment, barium ions were used instead of potassium ions in the iota carrageenan to make structural, chemical, and physicochemical changes easier during transformation. Hydrophobicity was determined qualitatively by observing the ability to trap small oil particles and repel water, moreover, the quantitative investigation was carried out using the weight loss method to determine the metal ion water isolation value and drug entrapment value within the core of barium linked carrageenan gel. The metal’s weight loss metal water affinity was determined to be 90% after 24 h, but it was only 67% with the synthesized gel coating the metal, this clearly shows that the barium gel had greater water protection activity. Furthermore, the barium-linked gel exhibited three times the entrapment capacity of the parent gel, and it successfully encased the water-insoluble medication with controlled release. The current study shows how the attached ion to the polymer changes its hydrophilic behavior into a hydrophobic one. This is a new and inexpensive way to do things. However, up until this point, the addition of the hydrophobic substrate and the functionalization of the polymer have been utilized. This leads to a plan that shows promise for improving the ability of hydrophobic hydrocolloid gels to hold drugs that do not dissolve in water.
Article Highlights
-
Role of ions in carrageenan Gel formation.
-
Ion replacement of iota Carrageenan.
-
Barium ion linked carrageenan characterization and its hydrophobicity investigation.
-
Entrapment of water insoluble drug for the enhancement of bioavailability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The polymer underwent various modifications, including functionalization and grafting, because of its extreme sensitivity to functional group substitution [1]. Hydrogels and hydrocolloids are types of polymers which shows sensitivity to water, and forms network with the water [2] Hydrocolloids are defined as three-dimensional polymers that are composed primarily of water. The inadequate energy dissipation mechanism within the gel network causes conventional hydrogel to be brittle, resulting in a low resistance to crack propagation. [3] When it comes to biomedical applications and drug delivery, hydrogel and hydrocolloids gel is a popular choice. [4] Hydrogel, mostly utilized in the food industry for the food related preparation, targeted drug delivery and tissue engineering [5,6,7] The carrageenan comes under this class with essential food and drug additive activity and has been well proven for targeted delivery, food items and others. [8] The carrageenan is widely used in the food industry as a hydrocolloid or hydrogel, because of its stable gel forming capability. Carrageenan originates from sea source and finds in the various forms. Like kappa, iota, gamma, etc., which is mostly classified based on the sulfated group present. The iota carrageenan (Fig. 1) represents the carrageenan family with inherent biological properties. [9,10,11]
The hydrophilic nature of carrageenan limits its use due to its poor mechanical strength, which includes brittleness and low strength. [12] Nevertheless, the utility of hydrocolloids is constrained when water-insoluble pharmaceuticals and their entrapment are considered. [13] The water insoluble drug and its bioavailability is the bottle neck for drug discovery scientist, various formulations have been tried so far with the hydrophobic drug carrier system. [14, 15], The overcome this limitation, the hydrophobic gel was used to extend the use to the non-polar drug was tried to enhance the mechanical strength, which repels water and does not mix with the water-based system and possess many oils entrapment like properties. Hydrophobic gels, also known as oleogels, are gels that have a high affinity for non-polar solvents, such as oils or lipids, which repel water and do not mix with the water-based system and possess many oils entrapment and water insoluble drug like properties. [16,17,18]. The carrageenan hydrocolloids were tried to convert in the hydrophobic in the various ways for the enhancement of the uses. [19,20,21]. Previous approaches relied on the addition of fat-soluble substances such as copolymers, amino acids, and lipids; however, these have their own set of cost and compatibility concerns when combined with drugs. To circumvent these problems, we are attempting to alter carrageenan-based hydrocolloids by substituting ions to achieve an enhanced hydrophobic character, which will allow us to entrap the non-polar and water-insoluble drug. The current study asserts that it can be utilized as an eco-friendly and economical technique by merely exchanging ions to create the hydrophobic gel, without the need for supplementary materials and the consequent loss of its inherent biological characteristics. [22, 23]
The carrageenan remains with the K+ ion in its natural form and possess some of the essential character because of the ions like the gel strength and gelling process because for the gel formation dissociation of the carrageenan is important and the dissociation is totally dependent on the ion present, so we tried here to replace the K+ ion with Ba2+ to exert different property, however the role of ions plays important role in gelling property is well explored, and exits as hydrocolloids without enhancing hydrophobic character. [24,25,26]
2 Material and methods
Marine hydrocolloids (Cochin) provided Watergel GU 8684 (K-linked iota carrageenan) for research and the degree of polymerization was calculated as 2, through the NMR, Mass spectroscopic analysis of the benzoyl linked iota -carrageenan, where the one aromatic link was found with 2 polysaccharide units[27] Aldrich (Merck) sold reagent-grade sodium chloride, barium chloride, HCl (6 M), and analytical-grade solvents like ethanol, ethyl acetate, dichloromethane (DCM), dimethylformamide (DMF), Sudan Red, and acetone. Used instruments included Adarsh Model Number 9001–2815 magnetic stirrers, Zhart India (2018/108) ultra-sonicators an electronic compact scale (SF-400C) weighing machine, a Labman digital rotational viscometer (LMDV-60), a Hanna Instruments EC 215 conductivity metre, and a Labtronics Lt114 digital photoelectric colorimeter. All glassware and instruments were used properly and calibrated.
-
i)
Synthesis and characterization of compound 2 and its gel formation.
Step 1) Replacement of ion: Compound 1 was screened to get solubilized and found that the alcohol and water (3:4) combination in 10 mL to make the completely soluble solution, while with water it was forming gel. [28] Compound 1 (1 equiv.) was solubilized in the above-mentioned solvent system, till the complete solution forms, later the 1 mL of hydrochloric acid (6 M) was added to start the dissociation, and after 30 min, BaCl2 (2 equiv.) was added to the reaction mixture until a clear solution was obtained. (Fig. 2).
The reaction was kept in the ice water for the settlement of the precipitate completely, followed by the filtration of the solid particle, dried through rotatory evaporator, and stored in an airtight container as a powder of compound 2, and evaluated through the IR, and Mass spectroscopy. [NMR, Mass Spectra is in supporting information].
-
a)
NMR Spectroscopy: NMR spectroscopy was recorded for the confirmation of carrageenan skeleton with the proton in the aliphatic region with slight de-shielding. The sample was prepared by dissolving in deuterated DMSO (di methyl sulfoxide), Samples were examined to check the iota carrageenan’s 1H peaks.
-
b)
IR: The Spectrum (Model-2 and Serial number- 89258)) was used for FTIR equipment. The data analysis programme used was NIOS2. The sample was prepared with KBr as palates and the sample was analysed through the intense and characteristic peak and fingerprint region.
-
c)
Mass spectroscopy: The Shimadzu mass spectrometer QP 2010 Ultra Mass Spectrometry (MS) machine with electron capture detector (ECD) flame ionisation detector (FID) was used for the fragment-based analysis. The molecular fragment peaks were used to confirm the attachment of Ba.2+ ion [28]
Step 2) Gel formation: Furthermore, the powdered form of compound 2 was transformed into a gel by utilizing distilled water at a temperature of 80 degrees Celsius. This was accomplished with the assistance of a probe sonicator for a period of up to thirty minutes, until the mixture was completely dissolved. The gel was then formed by immediately keeping the solution in the ice condition.
-
ii)
Computational studies:
The software Chem 3D 16, which was utilized to perform the calculation of the bond length between carrageenan and the K+ and Ba2+ ions. Ionic interaction distance was calculated after the MM2 method through the normalization of the molecular energy and bond. This was followed by the calculation of the ionic interaction distance, which was correlated with the bond dissociation energy.
-
iii)
Viscosity:
A Labman digital Rotational Viscometer (model no. LMDV 60) was used to determine the viscosity of the prepared sample gel states. The working conditions for the instrument were as follows: the speed was set to 30 revolutions per minute, the spindle was set to SPL 2, and the frequency range was from 50 to 60 Hz. The viscosity analysis of the gel that contained Ba-linked carrageenan was carried out at room temperature to observe any potential changes in the physical constitution of the substance.
-
iv)
Conductivity Test.
To determine the conductivity of the gel phase in a normal and enhanced temperature to see the dissociated ion and its role in the gel formation The conductivity metre that had been calibrated with distilled water solution was utilised.
-
v)
Microscopic examination.
The microscopic study of compound 1 and compound 2 based gel was conducted with the addition of oil droplet, the observation of engulfing of oil (Olive oil- Tatco food company) was done by using binocular microscope (MV-LED) and high-quality images were captured by the capture pro software.
-
vi)
Water repellent activity through weight loss method.
To quicken the oxidation process, two iron balls of equal weight were dissolved in two distinct beakers filled with 0.1 M HNO3. In one beaker, the iron balls were submerged without any coating, while in another, a gel based on compounds 1 and 2 was applied to the iron balls. This process was carried out for 24 h, during which time the weight loss of both plates was measured four times at intervals of six hours. The weight loss was computed by removing the ball at each interval until the 24 h. [29]
-
vii)
Visualization study of metal abrasion.
-
viii)
Two different iron balls were dipped in 3 ml of hydrogen peroxide and kept for 5 h to give the balls an oxidative surface. After that, we added 0.5 ml of strong HCl into each test tubes. After 5 h, both the test tube and the visual observation showed that the hydrochloric acid sped up the oxidation and rusting process. [30].
-
ix)
Drug Entrapment efficiency inside the core of compound 1 and 2 based gel.
During the gel formation process, an equal quantity of both compound 1 and compound 2 gel was impregnated with celecoxib, with each compound containing 100 mg of substance. To determine whether the hydrophobic drug (celecoxib) was entrapped by the gel, an equal amount of gel was diluted to convert into solution phase and then passed through the UV spectrophotometer at 255 nm to calculate the absorption of the solution (matrix and celecoxib), Later the concentration of the drug was calculated through the absorption calibration curve (Y = 0.0266X) of the celecoxib. The calibration curve was form by the known concentration of drug (10, 20 and 30 mg/ml) against the absorption from the UV–Visible spectrophotometer. The concentration of drug calculated by using the calibration curve, which indicates the amount of the drug inside the core. The amount which was obtain considered as entrapped drug and compared in both the cases (K+ and Ba2+ carrageenan).
-
x)
Drug release profile.
Drug release investigations on celecoxib-based carrageenan simple were carried out in vitro utilizing the Franz diffusion cell apparatus. A correctly weighted amount of 500 mg of barium carrageenan was combined with 10 mg of celecoxib to make the gel, with a 90% entrapment rate and a total drug entrapment of 9 mg. The gels were put into separate membranes and dialyzed against dissolving media. The amount of celecoxib released into the dialyzing liquid was measured with a UV spectrophotometer at 255 nm wavelength over a 30-min period. The drug release pattern was analyzed through the curve with the best fit R2 value.
3 Result and discussion
3.1 Confirmation of Ba2+ linkage to carrageenan
The synthesized compound 2, where characterized through the Mass spectra first, the fragment peaks of 359.21 and 421.10 (Supporting information) confirm the presence of fragment from glycosidic bond cleavage, confirming the fragments of Ba-O-S with the mass of 359 in the mass spectra. Further NMR spectroscopy (Supporting information) with the characteristic carrageenan range 3–8 ppm with the hump of 20 protons, confirms the presence of carrageenan skeleton. The IR showed a slight alteration in the fingerprint region with the addition of an intense peak at 1017 cm.−1 of the BaSO4 and the absence of bending H2O, which was present with all ions, and the absence of the water deformation peak, somewhat gives indication of its hydrophobic nature. (Fig. 3) [31]
The confirmation of the Ba2+ linkage with the carrageenan backbone was one of the characteristic peaks displayed in Table 1, as stated in the previous sentence.
Considering that the class of carrageenan belongs to the best known for its hydrogel, we attempted to create a gel from compound 2 by the above-mentioned method with high temperature, however, the Compound 1 form gel in the normal; water condition. The investigation of the dissociation energy, which directly proportional to bond length between the ions, were calculated through the MM2 calculation, and found the distance of bond is less in the case of Ba2+ (2.6 Ao) as compared to the K+(2.7 Ao) as depicted in the Fig. 4. [32] The energy calculation of the bond length (dissociation energy) shows the low dissociation energy of ion in case of compound 1, so it forms gel in the normal water, however, the compound 2 requires more energy to dissociates and converted to gel form.
3.2 Optimization of the gel formation from compound 2
Maintaining a high temperature in the water solution system enabled the provision of energy in the solution. In the case of the compound 2 the ionic attraction was more, so requires more energy to dissociates, so the energy for the gel formation was optimized from the range of 30 to 75 °C, but the gel form was not observed even after the instant and long process colling, The temperature above 80 °C and, instant colling was found to get the gel with extra stiff ness as it is showing in the Fig. 5, where it was not flowing, even in the direction of gravitational force.
It was observed that there was an increase in conductivity around 80 o C, as depicted in Fig. 6 of the graph that can be found below. This was since the gel formation took place at a higher temperature (80 °C), which led us to believe that dissociation was the cause of the gelling phenomenon. The solution in which compound 2 was stored was analyzed with a conductometer from the temperature of room temperature (25 °C) to the temperature of 100 °C. It was concluded that the dissociation of the compound 2 requires energy to get the gel unlike other ion linked like Ca, K ion.
Therefore, it is evident that the maximum dissociation occurred at that point, and the subsequent collision that occurred from that point resulted in the formation of a gel by means of ionized carrageenan and a robust network of divalent Ba2+. This interaction, which outbids the water molecules, could be the culprit behind the stiffness. After investigating that was optimized, it was determined that the optimal combination for gel formation was 100 mg of carrageenan in 10 mL of water at 80 °C with rapid colling. The Gel was further evaluated for its enhanced predicted hydrophobicity through qualitative and quantitative analysis.
3.3 Evaluation of compound 2 based gel
3.3.1 Qualitative method
In the qualitative approach, rather than using numerical value, visual inspection was used to measure the hydrophobicity of different substances.
3.4 Observation of hydrophobicity inside the water
The gels of compound 1 and compound 2 were added to a solution of water and kept for 1 h. It was noticed that the gel of compound 2 moved from top to bottom without dissolving (Fig. 7left side), whereas the gel of compound 1 was completely dissolved (Fig. 7right side), and the stiffness of compound 2 remained constant for many months even outside the container, demonstrating the water protection effect after the gel formation. The visual manifestation and its observation strongly reveal the hydophobic character in a qualitative sense.
Immediately following the visual evaluation of the gel, an attempt was made to explore the oil-loving and oil-taking capabilities of the gel by means of the dyes.
3.5 Microscopic Analysis of oil uptake study
The further investigation for the oil-loving nature was attempted to be carried out through the microscopic analysis of the engulfment process. Initially, the analysis of the compound 1 based gel was carried out by the addition of the oil, which later transforms into larger globules without attaching to the compound 1 asdepicted in Fig. 8. This visual presentation clearly demonstrates that the oil phobic nature of the compound 1 based gel.
The oil engulfment study of compound 2 was carried out; however, the compound 2 was not visible in a clear manner. Therefore, the sudan red dye was utilised in order to identify the process. The part of Fig. 8 was with gel (blue colour), and then a drop of oil (yellow colour) was added to see the effect on the gel. After ten minutes, the yellow colour spreaded throughout the entire surface (Fig. 9), which was indicating that the gel possessed a lipophilic quality. On the other hand, in the hydrogel, the oil was separated as droplets.
In addition to hydrophobicity, the lipophilic character was further employed for the purpose of obtaining some industrial applications. The analysis of the gel was based on the most important application, as indicated by the experimental study that is presented below.
3.6 Visualization study of metal abrasion
The metal abrasion study was conducted by placing the metal ball inside the solution, which contains the oxidizing agent to accelerate the abrasion process for 5 hr, the abrasion was observe at that test tube, where the compound 1 coated gel was applied to the metal ball, however, the test tube where the metal coated with compound 2 was kept, which remain intact after 5 hr (Figure 10). It was observed that the coating of hydrophobic gel influences the abrasion process, by providing water reppelent property. This finding also shows that compound 2 based gel, in contrast to compound 1 gel, has an isolated coating and capability to repel water, which corelated with the lipophilicity and hydrophobicity
3.6.1 Quantitative method
Instead of relying on visual inspection, the quantitative method uses numerical value as the method for measuring hydrophobicity levels.
3.7 Water repellent activity through weight loss method.
To achieve water repellency, it is common practice to modify the surface qualities of materials by applying hydrophobic coatings or treatments. This is done to get the desired properties. In addition, the amount of weight loss that occurs in materials because of deterioration or breakdown is assessed, and this, in turn, serves as an indicator of the presence of hydrophobic coating. The two metal balls (initial weigh 0.798 g) were put through an accelerated abrasion process, and the weight of the balls was determined throughout the course of a period of six hours, which lasted until twenty-four hours. The findings of the weight loss study are presented in Table 2, where the ball 1, in column 1, contains the findings of the coated compound 1 based gel, while column 2, ball-2 presents the observed weight reduction due to coating with compound 2 based gel.
During the observation that lasted for twenty-four hours, the calculation of weight loss revealed that ball 1 had a reduction of ninety percent in weight, while ball 2, which had been coated with compound 2 based gel, had a reduction of sixty-seven percent in weight only. In the highly oxidizing condition, the compound 2 based coated ball was able to save 22 percent of the material. This not only demonstrates the effectiveness of compound 2 for the separation of the metal, but it also demonstrates the hydrophobic nature of the gel of compound 2 based gel. A good indication of the isolation behavior is provided by the comparison graph of the compound 1 and compound 2 based coated material with time, which is illustrated in Fig. 11.
3.8 Drug entrapment efficiency inside the core of compound 1 and 2 based gel
Entrapment efficiency is one of the most important physiochemical characteristics for knowing the concentration of drug entrapped in any formulation, so it is the best way to explain the affinity of polymer with respect to the hydrophilic and hydrophobic drug. The entrapment of the water insoluble the core of the gel of compounds 1 and 2 were done by calculating the absorption PG of compound 1 in the gel phase and it was found 0.221 and in the case of compound 2 it was 0.665, The entrapment of the water insoluble drug was found three times more in the case of compound 2 and it clearly indicates the hydrophobic core (Fig. 12) and its importance to enhance the loading of poorly soluble drug, which ultimately will enhances the bioavailability of the drug.
The qualitative descriptor and quantitative value, such as the decrease in weight loss of 27% and the drug entrapment value that is three times higher in the case of coated material, strongly suggest that the compound 2 gel behaves in a manner that is isolated from the water. The gel that was prepared through ion replacement is more favorable than the gel of the parent compound because of its better hydrophobic nature and the increased trapping of the medication that is insoluble in water. The improved trapping of the insoluble drug in the core of the compound 2 based gel allowed us to examine the drug's release pattern, which is an important feature of the matrix associated drug.
3.9 Drug release profile of the drug from compound 2 based gel
The In vitro drug release investigations were conducted utilizing Franz diffusion cells on gel of compound 2. Due to the 90% drug entrapment capability previously observed, the release profile test was conducted using the gel with 18 mg entrapped. The amount of drug released in dialyzing medium was followed UV spectrophotometrically at 255 nm within a time interval of 30 min (Fig. 13). The R2 for best fit was 0.9774 and the regression linear line relation was with the equation y=0.261x−1.76. Despite the drug’s insolubility, the release pattern indicates an early onsite, allowing for controlled release without dosage dumping and trapping.
4 Conclusion
The increasing requirement for the development of drug delivery system for the water insoluble drug to enhance the bioavailability of the drug. The hydrophobic core-based gel and its stable networking addressed this current issue. The goal of the study was to enhance the hydrophobic qualities by replacing the polymer’s ions K+ to Barium (Ba2+) in a novel and environ friendly way. This research provides new and cost-effective method through the attached ion to change the polymer's behavior from hydrophilic to hydrophobic. Nevertheless, hydrophobic substrate and polymer functionalization have been employed thus far. Hydrophobic hydrocolloid gels can load drugs that are insoluble in water, and this strategy shows promise for improving their entrapment property. The effectiveness of the entrapment of the water insoluble drug to a material's ability to resist the water exposure makes this research valuable for the drug discovery scientist. Visual and microscopic imaging verifies the oil loving nature of the gel through the stability of the hydrophobicity in the aquas medium was also taken into consideration. Furthermore, experiments on the metal coated with gel confirm the effective ness and enhanced hydrophobic behavior than the hydrocolloid. The numerical descriptor like the three times more entrapment and 23% of protection concludes the effectiveness of gel as water repellent material. Finally, when barium ions are added in place of potassium ions, the gel "carrageenan" becomes hydrophobic and improves the drug loading and drug release capability.
Data availability
Data is provided within supplementary information files.
References
Purohit P, Bhatt A, Mittal RK, Abdellattif MH, Farghaly TA. Polymer grafting and its chemical reactions. Front Bioeng Biotechnol. 2023;11(10):1044927. https://doi.org/10.3389/fbioe.2022.1044927.
Zhang H, Zhang F, Yuan R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels based on natural polymers Beijing: Academic; 2020 .pp. 357–410 Elsevier. https://doi.org/10.1016/B978-0-12-816421-1.00015-X
Jayakody MM, Kaushani KG, Vanniarachchy MP, Wijesekara I. Hydrocolloid and water soluble polymers used in the food industry and their functional properties: a review. Polym Bull. 2023;80(4):3585–610. https://doi.org/10.1007/s00289-022-04264-5.
Mirzaei A, Esmkhani M, Zallaghi M, Nezafat Z, Javanshir S. Biomedical and environmental applications of carrageenan-based hydrogels: a review. J Environ Polym Degrad. 2023;31(5):1679–705. https://doi.org/10.1007/s10924-022-02726-5.
Koko MY, Hassanin HA, Qi B, Han L, Lu K, Rokayya S, Harimana Y, Zhang S, Li Y. Hydrocolloids as promising additives for food formulation consolidation: a short review. Food Rev Int. 2023;39(3):1433–9. https://doi.org/10.1080/87559129.2021.1934004.
Yang Z, McClements DJ, Li C, Sang S, Chen L, Long J, Qiu C, Jin Z. Targeted delivery of hydrogels in human gastrointestinal tract: A review. Food Hydrocoll. 2023;1(134): 108013. https://doi.org/10.1016/j.foodhyd.2022.108013.
MohanKumar BS, Priyanka G, Rajalakshmi S, Sankar R, Sabreen T, Ravindran J. Hydrogels: potential aid in tissue engineering—a review. Polym Bull. 2022;79(9):7009–39. https://doi.org/10.1007/s00289-021-03864-x.
Dattilo M, Patitucci F, Prete S, Parisi OI, Puoci F. Polysaccharide-based hydrogels and their application as drug delivery systems in cancer treatment: a review. J Funct Biomater. 2023;14(2):55. https://doi.org/10.3390/jfb14020055.
Ishaq A, Nadeem M, Ahmad R, Ahmed Z, Khalid N. Recent advances in applications of marine hydrocolloids for improving bread quality. Food Hydrocoll. 2023;23: 109424. https://doi.org/10.1016/j.foodhyd.2023.109424.
Udo T, Mummaleti G, Mohan A, Singh RK, Kong F. Current and emerging applications of carrageenan in the food industry. Int Food Res J. 2023;11: 113369. https://doi.org/10.1016/j.foodres.2023.113369.
Akanksha B, Nidhi N, Priyank P. The impact of carrageenan on pharmascience. Curr Tradit Med. 2024. https://doi.org/10.2174/0122150838266638231117180516.
Qamar SA, Junaid M, Riasat A, Jahangeer M, Bilal M, Mu BZ. Carrageenan-based hybrids with biopolymers and nano-structured materials for biomimetic applications. Starch-Stärke. 2024;76(1–2):2200018. https://doi.org/10.1002/star.202200018.
Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Del. 2007;4(4):403–16. https://doi.org/10.1517/17425247.4.4.403.
Raizada A, Bandari A, Kumar B. Polymers in drug delivery: a review. Int J Pharm Res Dev. 2010;2(8):9–20.
Rashid M, Malik MY, Singh SK, Chaturvedi S, Gayen JR, Wahajuddin M. Bioavailability enhancement of poorly soluble drugs: the holy grail in pharma industry. Curr Pharm Des. 2019;25(9):987–1020. https://doi.org/10.2174/1381612825666190130110653.
Flöter E, Wettlaufer T, Conty V, Scharfe M. Oleogels—their applicability and methods of characterization. Mol. 2021;26(6):1673. https://doi.org/10.3390/molecules26061673.
Dassanayake LS, Kodali DR, Ueno S. Formation of oleogels based on edible lipid materials. COCIS. 2011;16(5):432–9. https://doi.org/10.1016/j.cocis.2011.05.005.
Kuzina MA, Kartsev DD, Stratonovich AV, Levkin PA. Organogels versus hydrogels: advantages, challenges, and applications. Adv Funct Mater. 2023;33(27):2301421. https://doi.org/10.1002/adfm.202301421.
Ellis AL, Mills TB, Norton IT, Norton-Welch AB. The hydrophobic modification of kappa carrageenan microgel particles for the stabilisation of foams. J Colloid Interface Sci. 2019;7(538):165–73. https://doi.org/10.1016/j.jcis.2018.11.091.
Mavelil-Sam R, Ouseph EM, Morreale M, Scaffaro R, Thomas S. Recent developments and formulations for hydrophobic modification of carrageenan bionanocomposites. Polym Carbohydrate Polym. 2023;15(7):1650. https://doi.org/10.3390/polym15071650.
Sun W, Saldaña MD, Zhao Y, Wu L, Dong T, Jin Y, Zhang J. Hydrophobic lappaconitine loaded into iota-carrageenan by one step self-assembly. Carbohydrate Polym. 2016;137:231–8. https://doi.org/10.1016/j.carbpol.2015.10.060.
Garnier C, Michon C, Durand S, Cuvelier G, Doublier JL, Launay B. Iota-carrageenan/casein micelles interactions: evidence at different scales. Colloids Surf B. 2003;31(1–4):177–84. https://doi.org/10.1016/S0927-7765(03)00137-1.
Sedayu BB, Cran MJ, Bigger SW. A review of property enhancement techniques for carrageenan-based films and coatings. Carbohydrate Polym. 2019;216:287–302. https://doi.org/10.1016/j.carbpol.2019.04.021.
Bhattacharyya T, Palla CS, Dethe DH, Joshi YM. Rheological investigation of the network structure in mixed gels of Kappa and Iota Carrageenan. Food Hydrocoll. 2024;1(146): 109298. https://doi.org/10.1016/j.foodhyd.2023.109298.
Elfaruk MS, Wen C, Chi C, Li X, Janaswamy S. Effect of salt addition on iota-carrageenan solution properties. Food Hydrocoll. 2021;1(113): 106491. https://doi.org/10.1016/j.foodhyd.2020.106491.
Norton IT, Goodall DM, Morris ER, Rees DA. Role of cations in the conformation of iota and kappa carrageenan. J Chem Soc Faraday Trans. 1983;79(10):2475–88. https://doi.org/10.1039/F19837902475.
Bhatt A, Kailkhura S, Purohit P. Benzoylation of Iota Carrageenan: development of a stable, conductive, and hydrophobic drug carrier with reduced toxicity and improved gel-forming ability. Macromol Chem Phys. 2024. https://doi.org/10.1002/macp.202400017.
Shukla A, Kumar S, Bhatt A, Purohit P, Kailkhura S, Abdellattif MH. Iota carrageenan linked barium ion nanoparticle synthesis for the selective targeted imaging and inhibition of cancer cells. J Polym Eng. 2024. https://doi.org/10.1515/polyeng-2023-0278.
Rosu C, Lin H, Jiang L, Breedveld V, Hess DW. Sustainable and long-time ‘rejuvenation’of biomimetic water-repellent silica coating on polyester fabrics induced by rough mechanical abrasion. J Colloid Interface Sci. 2018;15(516):202–14. https://doi.org/10.1016/j.jcis.2018.01.055.
Qureshi D, Nayak SK, Maji S, Kim D, Banerjee I, Pal K. Carrageenan: a wonder polymer from marine algae for potential drug delivery applications. Curr Pharm Des. 2019;25(11):1172–86. https://doi.org/10.2174/1381612825666190425190754.
Seki T, Chiang KY, Yu CC, Yu X, Okuno M, Hunger J, Nagata Y, Bonn M. The bending mode of water: a powerful probe for hydrogen bond structure of aqueous systems. J Phys Chem Lett. 2020;11(19):8459–69. https://doi.org/10.1021/acs.jpclett.0c01259.
Luo YR, Kerr JA. Bond dissociation energies. CRC Handbook Chem Phys. 2012;89(89):65–98.
Acknowledgements
All authors express their gratitude to Graphic Era Hill University for providing the initial funding to start the project work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
A.S- Experiment performer S.K- Experiment performer A.B- Data analyser P.P- Supervised,
Corresponding author
Ethics declarations
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shukla, A., Kumar, S., Bhatt, A. et al. Conversion of iota carrageenan hydrocolloids to hydrophobic hydrocolloids, by the replacement of potassium to barium ion, for the entrapment of water insoluble drugs. Discov Appl Sci 6, 244 (2024). https://doi.org/10.1007/s42452-024-05925-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05925-y