Abstract
Botulinum neurotoxins (BoNTs) serotypes A and B are the two most common of the four BoNTs that cause the high mortality botulism disease in individuals consuming contaminated foods. The gold standard assay for BoNT detection is the live mouse bioassay, which has several major disadvantages, including tedious procedures and animal sacrifice requirements. In this study, we developed an immuno-based assay using magnetic streptavidin nanoparticles (mSNP) functionalized with specific synthetic biotinylated, 6xHis-tagged peptide substrates (Peptides A, PA and B, PB) designed for BoNT/A and BoNT/B proteolytic reactions, respectively. In the presence of active toxins that possess endopeptidase activity, upon cleavage, the released fragments with His-tag were dotted on a blotting membrane, ultimately producing color signals after incubation with anti-His antibody, alkaline phosphatase (AP)-conjugated antibody, and then AP substrates. The results showed that the efficiency of peptide-mSNP complex formation reached up to 81%, and the dot blot immunoassay allowed peptide detection from 10 ng of His-tagged peptides. Preliminary testing with the extracellular extracts from the isolated Clostridium botulinum strains indicated that the botulinum toxin in the 2020 botulism outbreak in Vietnam belonged to serotype A, the most potent BoNT. The established assay could be applied to construct a portable biosensor for BoNT detection and a high throughput device to screen potential BoNT inhibitors for drug development.
Article highlights
-
The biotinylated peptide substrates could be conjugated onto streptavidin nanoparticles with 81% efficiency. The estimated limit of detection was 50 pg of the targets.
-
The peptide A-nanoparticle complexes were cleaved by the extracellular extracts from the strain isolated in the 2020 botulism outbreak in Vietnam, confirming the toxinotype for the first time, despite the dual AB genotype.
-
The nanomaterials functionalized with specific peptide substrates are potentially transformed into an automated device for BoNT detection, and more importantly, anti-toxin compound screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Food-borne illness has been estimated to affect almost 600 million people annually, resulting in nearly half a million deaths, equivalent to 33 million healthy life years lost. Among the 31 most common hazards found in unsafe food compiled by the WHO in 2015 [38], Clostridium botulinum (C. botulinum) producing the botulinum neurotoxin (BoNT), one of the deadliest toxins known, and causing botulism is the second most life-threatening agent. Yet, in many countries, mainly in Africa and Asia, the lack of awareness and, more importantly, reliable diagnostic tools has been limiting botulism surveillance capacity, hampering both personal health and socioeconomic development [34]. Food-borne botulism was first reported in Vietnam in July 2020 in a food poisoning outbreak related to the consumption of vegan pate occurring in multiple provinces [22], causing at least five deaths, dozens in critical conditions, and nearly a hundred hospitalized. Since then, a number of outbreaks linked to C. botulinum have been annually reported nationwide. Generally, the botulinum detection methods used in suspected botulism cases are live mouse bioassay (MBA), microbiological culture techniques to identify the presence of the bacteria, nucleic acid amplification tests for unique encoding genes, and immunological and enzyme assays (i.e., toxinotype identification) [7, 15, 35]. Notably, neither the routine culture method nor the standard mouse bioassay is robust enough to detect the pathogen or its toxins, and there is no standardized kit available for testing.
Botulinum neurotoxins form an extraordinary family of proteins with distinctive sequences and structures, further complicating detection procedure development. From the original 7 serotypes from A through G classified based on antisera-neutralizing ability, more than 40 subtypes have been documented, with up to 70% difference in amino acid sequences [27]. It is important to note that, due to the clinical relevance, as pointed out by different studies, each toxin type has a distinct mode of action, thus potency [5, 28], toxin typing capability is a desirable feature while developing BoNT detection methods. With serotype information, proper treatment and accurate prognosis could be delivered to patients suffering from botulism. Among all of the detection methods listed above, the enzyme activity assay is the only method capable of determining the true toxin type in cases of silent or defective genes. Among the four serotypes causing human botulism, BoNT/A is considered the most common and potent serotype worldwide [31]. The strains isolated in the recent outbreaks in Vietnam were also shown to carry BoNT/A and BoNT/B encoding genes [21]. However, the toxinotype has not been investigated yet.
In each serotype, the toxin is a 150-kDa zinc-binding metalloprotease (holotoxin) that consists of two domains covalently linked by a disulfide bridge. The first domain is the 50-kDa light chain (LC), the zinc-containing active site for the endopeptidase activity. The second domain is the 100-kDa heavy chain (HC), which consists of a 50-kDa C-terminal receptor-binding domain (HC) and a 50-kDa translocation domain (HN). Only cleavage assays with the specific N-ethylmaleimide-sensitive-factor attachment receptor (SNARE) proteins as endopeptidase substrates can determine the true toxin type (in cases of silent, defective, or mosaic genes). Endopeptidase assays monitor the toxin’s catalytic activity, which is the major determinant of BoNT toxicity. Basically, endopeptidase assays display the serotype-specific proteolytic cleavage of SNARE proteins in conjunction with technically different readouts, e.g., mass spectrometry (Endopep-MS assay) [4, 18], immunological detection of cleavage products (Endopep-ELISA) [13, 17], protein-based fluorescence resonance energy transfer (FRET) sensors [3], proteolytic-PCR [20]. Each BoNT serotype cleaves the substrate at a distinct site, based on which detection methods can be designed to recognize and identify the exact serotype. Endopeptidase assays are fast and relatively simple, with proven applications in screening BoNT inhibitors and toxin detection in food. The major disadvantage of mass spectrometry and FRET-based methods is that they utilized sophisticated instruments.
For the first time, this study developed a simple method using the dot immunoblotting technique to detect BoNT/A activity and indicated the presence of active BoNT/A in the strain isolated in the 2020 botulism outbreak in Vietnam.
2 Materials and methods
2.1 Bacterial culture and toxin preparation
C. botulinum strains isolated from the 2020 botulism outbreaks in Vietnam and stored at the National Institute for Food Control were inoculated in cooked meat medium (Oxoid, Thermo Fisher Scientific, USA) or Tryptone-Peptone-Glucose-Yeast Extract (TPGY) broth (Difco, Becton Dickinson, USA) in an anaerobic environment at 37 °C for 5 days. The presence of viable cells and spores was examined by Gram staining. The C. botulinum culture was centrifuged at 4000 rpm for 15 min to remove bacterial cells, and then the supernatant was filtered with a 0.22-μm filter membrane (Sartorius, Germany). Aliquots were prepared and stored at − 70 °C for further experiments. Prior to the MBA, the samples were treated with trypsin and diluted with PBS buffer at different dilution factors. Control samples were heated at 100 °C for 10 min. The mice were injected with 0.5 ml of samples and observed for symptoms of botulism.
2.2 Materials
Peptide substrates A and B (PA: HHHHHHTRIDEANQ|RATKMK-Biotin, 20 amino acids, 2.7 kDa, and PB, Biotin-LSELDDRADALQAGASQ|FETSAAKLKRKHHHHHH, 34 amino acids, 4.1 kDa, |: cleavage site), biotinylated at one end and tagged with 6xHis at the other end, was delivered from Yao-hong Bioechnology (Taiwan). Glutaraldehyde was purchased from Sigma (USA), nitrocellulose membrane (0.45 µm), NBT (p-nitroblue tetrazolium chloridez)/BCIP (5-bromo-4-chloro-3-indolyl phosphate), and alkaline phosphatase (AP)-conjugated antibody were products of Thermo Scientific (USA), Streptavidin magnetic particles were manufactured by Roche (USA), and anti-6xHis-tag monoclonal antibody was from Clontech (USA).
2.3 Dot blotting
The precut nitrocellulose membrane was soaked in 4% BSA for 5 min and then transferred into 4% glutaraldehyde for 10 min with gentle shaking (modified from [23]). After air drying for 20 min, 1 μl of each sample was dotted onto the membrane and let dry before the blocking step with 4% BSA for 1 h at room temperature. The membrane was incubated for 1 h each with primary and subsequently secondary antibodies (1:5000 and 1:3000 dilutions from 0.5 mg/ml stocks, respectively) with three washes in 1× PBS buffer containing 0.1% Tween 80 (pH 7.4) in between, 5 min each. Color development was performed with NBT/BCIP solution and stopped with 1X PBS buffer containing 20 mM EDTA when the dots were visible.
2.4 Bradford assay
Reaction mixtures with a serial dilution of 10 µg/ml BSA to generate BSA solutions from 0 to 9 µg/ml were mixed with equal volumes of Bradford reagent and incubated for 5 min at room temperature for complete reaction. The absorbance was measured by a UV–Vis spectrophotometer at a wavelength of 595 nm. Each concentration was performed in triplicate, and the average measurements were used to construct a standard curve based on A595 nm values. From this standard curve, the samples were subjected to this assay, and the recorded absorbance was used to calculate the protein concentration.
2.5 Peptide conjugation onto magnetic streptavidin nanoparticles
Before the conjugation reaction, 10 μl of the magnetic streptavidin nanoparticles (mSNP) were washed in 1X PBS buffer (pH 7.4) three times. The reactions containing 200 µl of 1× PBS buffer (pH 7.4) and 50 µl of Peptide A at 2 mg/ml were incubated at room temperature with intermittent mixing to form peptide-conjugated magnetic streptavidin nanoparticles (P-mSNP) (adapted from [29]). Upon completion, 150 µl of the supernatant was removed. The P-mSNP complexes were washed three times in 200 µl of 1× PBS buffer, pH 7.4. The supernatant and wash fractions were used for residual peptide content using Bradford and dot immuno-assays. The conjugation efficiency was calculated using the following formula [36]:
where H% is P-mSNP complex formation efficiency, and N and Ne are the total input peptide amount (µg) and the unbound peptide amount (µg) measured in the supernatant, respectively.
2.6 Endopeptidase reaction
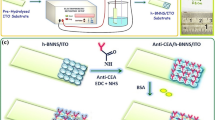
Extracellular proteins in the culture medium that contained BoNT were used in the reaction with a fixed amount of 3 μl in a total volume of 50 μl containing P-mSNP in HEPES buffer (50 mM HEPES (pH 7.4), 5 mM DTT, 0.2 mg/ml BSA). The reaction mixture was incubated at 37 °C for 3 h, and the results were analyzed by Bradford and dot blot immuno-assays (Fig. 1).
3 Results and discussion
3.1 Immunoblotting condition optimization for the detection of short peptides
Peptide A was smaller than 3 kDa and could easily dissociate from the membrane in the wash steps. According to Tomisawa et al. [36], using the vacuum pump after dotting samples helped retain small peptides on the membrane, yet our peptide substrates did not show any signal. To solve this issue, we used glutaraldehyde as a fixing reagent after saturating the blotting membrane with BSA. This chemical would form covalent bonds with the free amine groups of the peptide, facilitating its absorption onto the membrane. We tested different combinations of BSA and glutaraldehyde treatments and found out that the optimized conditions, including pre-saturation with 4% BSA in 5 min followed by 4% glutaraldehyde fixation in 10 min, allowed the detection of 0.01 µg PA (Fig. 2).
The buffer did not show any color signal as expected for a negative control, while a His-tagged protein yielded a clear purple dot (positions labeled – and +, respectively). This sensitivity was comparable to a 15-aa peptide recognized by its specifically raised antibody, calculated at 0.02 µg [10]. In our peptide substrate design, the His-tagged fragments (Pf), which would be cleaved off PA into the supernatant in the presence of the active toxin, was 14 amino acids long. Thus, this assay condition was applied to evaluate the efficiency of the peptide conjugation reaction and BoNT endopeptidase activity discussed below.
3.2 Conjugation of the peptide substrates onto magnetic streptavidin beads
The efficiency of the conjugation reaction was calculated using the formula in the Experimental section with both Bradford assay and dot blot analysis. After subtraction of the unbound peptide intensity in the supernatant (Fig. 3A—dots 3–4, B—columns 2–3) and the dissociated peptide intensity in the wash fraction (Fig. 3A, dot 5, and Fig. 3B, column 4), it was estimated that the conjugation efficiency consistently reached 81%.
A Dot blot immunoassay (1: unconjugated peptide; 2: conjugation buffer; 3, 4: 1 and 2 µl of the supernatant; 5: wash fraction), B Densitogram of the corresponding dots (1: unconjugated peptide; 2, 3: 1 and 2 µl of the supernatant; 4: wash fraction), C peptide release reaction (1: conjugation buffer; 2: PA 0.2 µg; 3: reaction buffer before incubation; 4: after dissociation)
Approximately 17% of the peptide remained unbound, and 2% could be dissociated from the PA-mSNP complex, as indicated by the faint dot in Fig. 3A (dot 5) and the corresponding dot density in Fig. 3B (column 4). The affinity between biotinylated molecules and streptavidin has been calculated at 10−15 M, and the reaction is considerably irreversible unless harsh chemicals are used [39]. To confirm P-mSNP crosslinking, the complex was treated with 0.35 M glycine, pH 3.3, and as predicted, the peptide was completely released into the supernatant (Fig. 3C, dot 4).
The strong binding affinity between biotin and streptavidin has provided a valuable advantage in bioanalytical studies of protein–ligand interactions [2]. As a result, this interaction has been explored using various methods, including colorimetric techniques and, most commonly, FTIR. Figure 4 displayed the FTIR spectra of the PA-mSNP sample, juxtaposed with the spectra of m-SNP and PA sample for comparison.
The m-SNP sample comprised a magnetic bead linked with streptavidin, a protein composed of 183 residues. Consequently, its FTIR spectrum showcased distinctive vibrational peaks from both the magnetic oxide material and streptavidin. The peak observed at 580 cm−1 was typically associated with the stretc.hing vibration of Fe–O metal oxide [24]. Peaks at 972 cm−1 and 1032 cm−1 corresponded to the asymmetric vibrations of the Si–O–Si linkages formed during the activation of the magnetic particle surfaces through silane treatment prior to the deposition of biocompatible molecules [1]. Peaks at 1169 cm−1, 1646 cm−1, and 2925 cm−1 affirmed the presence of characteristic amid I and II vibrations in streptavidin [11, 24]. Following the reaction between m-SNP and PA, a reduction in peak height at 1169 cm−1 and 2925 cm−1 was observed, possibly indicating the covalent binding of streptavidin to biotin [11, 24, 26]. Moreover, additional distinctive bonds within the protein were detected in both the sample containing PA and the sample containing PA-mSNP. These bonds, observed at 1530 cm−1 and 1636 cm−1, signified the O=C–N in peptide bonds, while those at 3230 cm−1 and 3454 cm−1 were indicative of O–H bonds within peptide linkages [12, 26], which substantiated the robust interaction between biotin and streptavidin, facilitating the connection between the particles and the biotinylated peptide substrates.
3.3 Detection of BoNT/A endopeptidase activity in the culture medium
In the presence of active botulinum neurotoxins that possess endopeptidase activity, the designed peptide substrates would release the corresponding peptide fragments (HfA and HfB) into the supernatant while the biotinylated fragments remained on the mSNPs. The results demonstrated with PA and BoNT/A showed that the unheated extracellular extract containing the active toxin yielded a strong signal (Fig. 5A, dot 6), whereas the heated extract, supposedly inactivating the endopeptidase activity, failed to produce any color (Fig. 5A, position 5). All of the buffers and the extract per se did not show a clear color signal (Fig. 5A, positions 1, 3, 4), indicating successful peptide cleavage according to our design with the extracellular extract obtained from the strain isolated from the vegan pate outbreak in 2020 in Vietnam. Consistent with the presence of the released peptides, the Bradford assay showed a markedly higher protein concentration, from 0.05 mg/ml in the supernatant before the endopeptidase cleavage reaction to 0.152 mg/ml afterward. A similarly constructed nanoparticle-peptide complex with BoNT/B substrate (PB-mSNP) was used in the established dot immunoassay (Fig. 5C). Interestingly, despite carrying both BoNT/A and BoNT/B encoding genes, only serotype A activity was detected (Fig. 5C, dots 5–6, corresponding to the toxin extracts from the Vietnamese strain and the active BoNT/B strain), in agreement with the report by La et al. of the fact that the bont/b gene from the Vietnamese strain harbors a mutation that generates a silent truncated neurotoxin form [21]. It is important to note that various subtypes of BoNT/A and BoNT/B have been reported to possess different catalytic capacities (i.e., substrate recognition and cutting efficiency) depending on the tested substrates [19, 33, 40, 14]. The peptides used in this study, PA and PB, modified from SNAPtide and Bnew sequences [9, 32], were shown to be more suitable for detecting cleavage reactions by recombinant BoNT/A1 and BoNT/B1 light chains compared to other subtypes, respectively [14, 19]. Inefficient substrate cleavage could result in false negatives for the weaker BoNT subtypes. While using larger amounts of enzyme for cutting (e.g., decreasing the sample dilution factor) might solve the problem, the established dot immunoassay would definitely benefit from the development of substrates with similar binding and cleavage efficiency to different BoNT subtypes.
A dot blot immunoassay for BoNT/A detection (upper panel: 1—reaction buffer, 3—no substrate control, 5—heated toxin extract; lower panel: 2—0.2 µg PA, 4—P-mSNP storage buffer, 6—unheated toxin extract). B Protein concentration (mg/ml) quantified by Bradford assay of the supernatants before (1) and after (2) cleavage. C Dot blot immunoassay for BoNT/A and BoNT/B detection (1—reaction buffer, 2—0.2 µg PB, 3—toxin extract, 4—BoNT/B, 5—PB-mSNP + toxin extract, 6—PB-mSNP + BoNT/B)
The major advantage of employing the dot blot immunoassay to detect BoNT activity was the use of a polyclonal secondary antibody that recognized distinct epitopes in the primary anti-His antibody, hence greatly amplifying the signal [30]. It has been calculated that approximately 115 antibodies could be generated against insulin [33]. Similarly, for each Pf bound to the primary antibody, multiple secondary antibodies would bind, increasing the number of alkaline phosphatase molecules that catalyzed the final color development reaction. According to a study by Ye et al., the limit of detection of such an immunoblotting assay could be as low as 50 pg of the targets [40] in a volume of 2 µl, equivalent to 25 ng/ml, close to the threshold of 7 ng/ml recommended [25]. Nevertheless, it should be noted that the performance of all immuno-based assays might vary significantly depending on the antibodies used. Previously, when comparing four different anti-His antibodies, Debeljak and colleagues confirmed a single antibody being able to recognize their target proteins [6]. To ensure consistent performance using different antibodies, we recommend titrating the antibodies with the dotted peptides to achieve equivalent or better limits of detection (e.g., 0.01 µg of PA in this study). It was recently reported that a nanosensor using gold nanoparticles assembled based on Langmuir–Blodgett films was capable of detecting 1 pg/ml of BoNT/A [16]. However, this limit of detection was achieved with purified toxin without testing with complicated food matrices, and this assay design did not provide information regarding BoNT activity, which could be of clinical relevance. It is important to note that, prior to the endopeptidase activity assay, an additional enrichment step is often recommended to separate BoNT from the sample matrix that could interfere with the substrate cleavage reaction. The strategy of using the beads conjugated with specific antibodies to capture and stabilize the enzyme during enrichment could produce results similar to or better than those of the MBA regarding sensitivity [8, 37]. A potential limitation of this study was the use of the extracts from C. botulinum culture instead of pure BoNT/A and BoNT/B, which were unavailable in our country due to biosafety concerns. Hence, the next direction is to include those enzymes in future method development for more accurate quantification of BoNT activity.
For further development, we introduced an automated sensing model designed to detect the presence of the BoNT toxins, presented in Fig. 6. This model was composed of two main components: (A) an automated 3D positioning reagent injection system tailored for the dot immunoblotting assay, and (B) a portable apparatus working with the mechanism of optical density. Specifically, the automated 3D positioning reagent injection system could automatically load and precisely dispense the required reagents for the immunoassay through three linear injectors. The solutions containing the reagents would be delivered onto the cover slip's surface with their spatial positions finely adjusted using programmable 3D positioning actuators. Following the incubation period, a vacuum pump system automatically withdraws the solutions (Fig. 6A). This automatic positioning injection was applied in this study. In future work, portable equipment will be designed for the quantification of His-tagged peptide fragments (illustrated in Fig. 6B), which is based on optical density measurement. Essentially, an array of LEDs situated opposite to the dot blot surface emits parallel light. The intensity of the transmitted light varies according to the density of the captured dots, which is subsequently processed before being displayed on the LCD screen.
The above-mentioned design not only facilitates automation to enhance the uniformity of the dot blot assay process but also mitigates the risk of testing personnel coming into contact with hazardous samples. Additionally, this approach boosts the effectiveness of the testing process by mechanizing all phases, thereby reducing the time required for each step. Moreover, the solvents and reagent systems can be appropriately controlled at the correct temperature during testing, curtailing resource depletion during prolonged or multi-sample testing scenarios. Furthermore, this automated system can be modified to accommodate a range of different biochemical validation procedures.
4 Conclusion
The gold standard MBA for botulism detection and surveillance usually takes several days to obtain results and cannot be carried out in regular laboratory settings or in the field. This study established a simple dot blot immunoassay and demonstrated the designs of an automated screening instrument and a portable device for identifying botulinum neurotoxin serotype A. Our assay allowed toxinotype determination for the strain causing the largest botulism outbreak in Vietnam. Furthermore, this platform could be easily adapted for other serotypes and employed to test novel substrates and screen antitoxin compounds for botulism treatment.
Availability of data and materials
The authors declare that the data that supports the findings reported in this study are available within this article and its supplementary file. Additional file formats or raw data files could be provided by the corresponding author upon reasonable request.
References
Abdelaziz MM, Hefnawy A, Anter A, Abdellatif MM, Khalil MAF, Khalil IA. Silica-coated magnetic nanoparticles for vancomycin conjugation. ACS Omega. 2022;7:30161–70.
Austin RJ, Smidansky HM, Holstein CA, Chang DK, Epp A, Josephson NC, Martin DB. Proteomic analysis of the androgen receptor via MS-compatible purification of biotinylated protein on streptavidin resin. Proteomics. 2012;12(1):43–53. https://doi.org/10.1021/ac050485f.
Bagramyan K, Barash JR, Arnon SS, Kalkum M. Attomolar detection of botulinum toxin type A in complex biological matrices. PLoS ONE. 2008;3(4): e2041. https://doi.org/10.1371/journal.pone.0002041.
Boyer AE, Moura H, Woolfitt AR, Kalb SR, McWilliams LG, Pavlopoulos A, Schmidt JG, Ashley DL, Barr JR. From the mouse to the mass spectrometer: detection and differentiation of the endoproteinase activities of botulinum neurotoxins A–G by mass spectrometry. Anal Chem. 2005;77(13):3916–24. https://doi.org/10.1021/ac050485f.
Chen Y, Li H, Yang L, Wang L, Sun R, Shearer JES, Sun J. Rapid detection of clostridium botulinum in food using loop-mediated isothermal amplification (LAMP). Int J Environ Res Public Health. 2021;18(9):4401. https://doi.org/10.3390/ijerph18094401.
Debeljak N, Feldman L, Davis KL, Komel R, Sytkowski AJ. Variability in the immunodetection of His-tagged recombinant proteins. Anal Biochem. 2006;359(2):216–23. https://doi.org/10.1016/j.ab.2006.09.017.
Dorner MB, Schulz KM, Kull S, Dorner BG. Complexity of botulinum neurotoxins: challenges for detection technology. Curr Top Microbiol Immunol. 2013. https://doi.org/10.1007/978-3-642-33570-9_11.
Dunning FM, Ruge DR, Piazza TM, Stanker LH, Zeytin FN, Tucker WC. Detection of botulinum neurotoxin serotype A, B, and F proteolytic activity in complex matrices with picomolar to femtomolar sensitivity. Appl Environ Microbiol. 2012;78(21):7687–97. https://doi.org/10.1128/AEM.01664-12.
Feltrup TM, Singh BR. Development of a fluorescence internal quenching correction factor to correct botulinum neurotoxin type A endopeptidase kinetics using SNAPtide. Anal Chem. 2012;84(24):10549–53. https://doi.org/10.1021/ac302997n.
García G, Yuliana G, Sánchez AP, Marín Montoya M. Detection of PMTV using polyclonal antibodies raised against a capsid-specific peptide antigen. Rev Fac Nacl Agron Med. 2013;66(2):6999–7008.
Gong P, Peng Z, Wang Y, Qiao R, Mao X, Qian H, Zhang M, Li C, Shi S. Synthesis of streptavidin-conjugated magnetic nanoparticles for DNA detection. J Nanopart Res. 2013;15:1558.
Gonzalez M, Bagatolli LA, Echabe I, Arrondo JLR, Argarana CE, Cantor CR, Fidelio GD. Interaction of biotin with streptavidin. J Biol Chem. 1997;272(17):11288–94.
Hallis B, James BA, Shone CC. Development of novel assays for botulinum type A and B neurotoxins based on their endopeptidase activities. J Clin Microbiol. 1996;34(8):1934–8. https://doi.org/10.1128/jcm.34.8.1934-1938.1996.
Henkel JS, Jacobson M, Tepp W, Pier C, Johnson EA, Barbieri JT. Catalytic properties of botulinum neurotoxin subtypes A3 and A4. Biochemistry. 2009;48(11):2522–8. https://doi.org/10.1021/bi801686b.
Hobbs RJ, Thomas CA, Halliwell J, Gwenin CD. Rapid detection of botulinum neurotoxins-a review. Toxins. 2019;11(7):418. https://doi.org/10.3390/toxins11070418.
Huong NT, Nhiem LT. Facile detection of botulinum neurotoxin using LSPR nanosensor based on Langmuir–Blodgett films of gold nanoparticles. RSC Adv. 2023;13:31176–81.
Jones RG, Ochiai M, Liu Y, Ekong T, Sesardic D. Development of improved SNAP25 endopeptidase immuno-assays for botulinum type A and E toxins. J Immuno Methods. 2008;329(1–2):92–101. https://doi.org/10.3390/toxins11100588.
Kalb SR, Krilich JC, Dykes JK, Lúquez C, Maslanka SE, Barr JR. Detection of botulinum toxins A, B, E, and F in foods by Endopep-MS. J Agric Food Chem. 2015;63(4):1133–41. https://doi.org/10.1021/jf505482b.
Kalb SR, Baudys J, Kiernan K, Wang D, Becher F, Barr JR. Proposed BoNT/A and/B peptide substrates cannot detect multiple subtypes in the Endopep-MS assay. J Anal Toxicol. 2020;44(2):173–9. https://doi.org/10.1093/jat/bkz044.
Kolesnikov AV, Kozyr AV, Ryabko AK, Shemyakin IG. Ultrasensitive detection of protease activity of anthrax and botulinum toxins by a new PCR-based assay. Pathog Dis. 2016;74(1):ftv112. https://doi.org/10.1093/femspd/ftv112.
La TTH, Nguyen TL, Nguyen TT, Pham Y. Investigation of botulinum neurotoxin types from Clostridium botulinum causing a recent outbreak in Vietnam. VNU J Sci Nat Sci Tech. 2021;37(4):65–9.
Le QH, Vo NAT, Do TNK, Vo TTH, Day JN, Nguyen NS, Hua TT, Ho TCT, Nguyen TTN. Botulism outbreak after the consumption of vegetarian pâté in the south of Viet Nam [version 4; peer review: 3 approved with reservations]. Wellcome Open Res. 2021;5:257. https://doi.org/10.12688/wellcomeopenres.16372.4.
Littauer UZ, Giveon D, Thierauf M, Ginzburg I, Ponsting H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci USA. 1986;83(19):7162–6. https://doi.org/10.1073/pnas.83.19.7162.
Long J, Li X, Liu X, Jin X, Xie Z, Xu X, Lu C. Preparation of streptavidin-coated magnetic nanoparticles for specific immobilization of enzymes with high activity and enhanced stability. Ind Eng Chem Res. 2014;60(4):1542–52.
Luisa W, Kirkwood M, Larry H. Current methods for detecting the presence of botulinum neurotoxins in food and other biological samples, Bioterrorism. Rijeka: InTech; 2012. https://doi.org/10.5772/33638.
M.J., Swamy T., Heimburg D., Marsh. Fourier-transform infrared spectroscopic studies on avidin secondary structure and complexation with biotin and biotin-lipid assemblies. Biophys J. 1996;71(2):840–47. https://doi.org/10.1016/S0006-3495(96)79285-8.
Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P. Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindström M, Lista M, Lúquez C, Mazuet C, Pirazzini M, Popoff MR, Stringer MC. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins. 2017;9(1):38. https://doi.org/10.3390/toxins9010038.
Pellett S, Bradshaw M, Tepp WH, Pier CL, Whitemarsh R, Chen C, Barbieri JT, Johnson AE. The light chain defines the duration of action of botulinum toxin serotype a subtypes. MBio. 2018;9(2):e00089-e118. https://doi.org/10.1128/mBio.00089-18.
Pham Y, Nguyen ATV, Phan TN, Chu LL, Nguyen DQ, Nguyen MH, Nam NH, Luong NH. Specificity and processing rate enhancement of Mycobacterium tuberculosis diagnostic procedure using antibody–coupled magnetic nanoparticles. Int J Nanotechnol. 2013;12(5–7):335–46.
Pleiner T, Bates M, Görlich D. A toolbox of anti-mouse and anti-rabbit IgG secondary nanobodies. J Cell Biol. 2018;217(3):1143–54. https://doi.org/10.1083/jcb.201709115.
Popoff R. Botulinum toxins, diversity, mode of action, epidemiology of botulism in France. Rijeka: IntechOpen; 2018. https://doi.org/10.5772/intechopen.79056.
Rosen O, Feldberg L, Gura S, Zichel R. A new peptide substrate for enhanced botulinum neurotoxin type B detection by endopeptidase-liquid chromatography–tandem mass spectrometry/multiple reaction monitoring assay. Anal Biochem. 2015;473:7–10. https://doi.org/10.1016/j.ab.2014.09.016.
Schroer JA, Bender T, Feldmann T, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur J Immun. 1983;13:693–700. https://doi.org/10.1002/eji.1830130902.
The World Bank. The safe food imperative: accelerating progress in low- and middle-income countries. 2018. https://www.worldbank.org/en/topic/agriculture/publication/the-safe-food-imperative-accelerating-progress-in-low-and-middle-income-countries. Accessed 5 Aug 2023.
Thirunavukkarasu N, Johnson E, Pillai S, Hodge D, Stanker L, Sharma S. Botulinum neurotoxin detection methods for public health response and surveillance. Front Bioeng Biotechnol. 2018;8:80. https://doi.org/10.3389/fbioe.2018.00080.
Tomisawa S, Abe C, Kamiya M, Kikukawa T, Demura M, Kawano K, Aizawa T. A new approach to detect small peptides clearly and sensitively by western blotting using a vacuum-assisted detection method. Biophysics. 2013;9:79–83. https://doi.org/10.2142/biophysics.9.79.
Worbs S, Fiebig U, Zeleny UR, Schimmel H, Rummel A, Luginbühl W, Dorner BG. Qualitative and quantitative detection of botulinum neurotoxins from complex matrices: results of the first international proficiency test. Toxins. 2015;7(12):4935–66. https://doi.org/10.3390/toxins7124857.
World Health Organization. WHO estimates of the global burden of foodborne diseases. 2015. https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases. Accessed 5 Aug 2023.
Xiong Y, Tu Z, Huang X, Xie B, Xiong Y, Xu Y. Magnetic beads carrying poly (acrylic acid) brushes as “nanobody containers” for immunoaffinity purification of aflatoxin B 1 from corn samples. RSC Adv. 2015;5(94):77380–7. https://doi.org/10.3168/jds.2018-16109.
Ye F, Smith PB, Wu C, Chiu DT. Ultrasensitive detection of proteins on western blots with semiconducting polymer dots. Macromol Rapid Commun. 2013;34(9):785–90. https://doi.org/10.1002/marc.201200809.
Acknowledgements
This study was funded by Vietnam National University, Hanoi, with the grant code KLEPT.20.02.
Author information
Authors and Affiliations
Contributions
H-LN, YP and M-QL wrote the main manuscript text and prepared figures. YP, H-LN, H-LN, P-AL designed and conducted the experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, HL.T., Nguyen, HL., Le, PA. et al. A versatile immunoassay based on functionalized nanoparticles for botulinum neurotoxin detection and sensor development. Discov Appl Sci 6, 243 (2024). https://doi.org/10.1007/s42452-024-05900-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05900-7