Abstract
Management of wastewater from diverse sources are important because effluents are discharged into the aquatic environment after treatment. In this study, the water quality of the Veldwachters River and associated ecotoxicity risks of the wastewater treatment plant’s (WWTP) effluent were evaluated. Physicochemical characterisation over four seasons and ecological risk assessment of WWTP’s effluent using aquatic organisms comprising of the producer Raphidocelis subcapitata, consumer Daphnia magna and decomposer Tetrahymena thermophila as experimental models were investigated. The crustaceans were subjected to 48 h exposure for mortality while both algae and the protozoan were exposed for growth inhibitions at 72 h and 24 h respectively. Physicochemical characteristics were within regulatory limits. Growth inhibition was observed in autumn and winter for R. subcapitata and in summer and spring for T. thermophila. Crustaceans D. magna (consumer) was classified as Class III (acute toxicity) for the effluent in all seasons. Meanwhile, the most sensitive organism in the applied battery of biotests was the protozoan T. thermophila (decomposer), demonstrating a toxic unit (TU) > 100, while R. subcapitata and D. magna demonstrated 1 TU < 10. These results showed that the effluent have potential toxicological effects on aquatic organisms and provided insights into the required intervention strategies for pollution reduction.
Graphical abstract

Article highlights
-
Water quality and toxicity assessment of WWTP effluent using aquatic organisms.
-
There is a significant difference between the growth inhibition of D. magna and T. thermophila (p < 0.05).
-
Redox potential was positively correlated with the toxicity values in R. subcapitata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water is among the major essentials provided by nature, to support life, and is regarded as the most precious resource [32]. However, over the past few decades, a lot of water systems around the world have undergone tremendous amount of change [29]. Population growth, industrialisation, urbanisation and consumption patterns are amongst the changes contributing to the worlds rising demand for freshwater resources [39]. With demand of water exceeding supply, the world is far from being water secure and inadequate water supplies pose great challenges to the economic development of many nations [45]. Currently, there is a shortage of freshwater in developing countries, including countries that were considered as water rich [19].
South Africa (SA) is a semi dry country and its freshwater resources are scarce and extremely limited [7]. With an average rainfall of 450 mm, SA is the 30th driest country in the world. This is far less than global average of 860 mm per year [9]. As a result, SA is categorised as a water stressed country, with an annual freshwater availability of less than 1700 m3 per capita. The comparison of SA’s available water per capita, with neighbouring and other countries, highlights the challenge that the country is currently facing. The recent Western Cape drought ‘Day Zero’ (2017–2018) is another example that shows that SA’s water scarcity issues are severe [29]. The drought brought to light SA's water system's flaws and potential problems with water scarcity that could also arise in other provinces [12, 29]. Additionally, water security does not only depend on the availability of water, but also on the quality of the water and globally, about 80% of wastewater is discharged into the environment untreated or partially treated, leading to adverse environmental effects [15]. Wastewater is generated through various sources such as homes, industries, farms, and various other ways. As a result, the complexity of wastewaters comprising of both natural and synthetic organic substances and high volumes of industrial chemicals are possible contaminants that cause toxic effects in wastewater [3]. These constitute potential dangers to the natural water system and hence becomes hazardous to life and the environment [30].
The rise in water scarcity and wastewater production has led to the reuse of wastewater which is a promising source of water supply and a crucial component of sustainable water resource management [5]. Therefore, a response to water scarcity and deteriorating water quality has been to develop wastewater treatment technologies. Wastewater treatment plants (WWTPs) play a crucial role in preventing human health and the environment from being negatively affected by removing pollutants in the wastewater [6]. However, inadequate wastewater and sewage treatment operations and maintenance of infrastructure are regarded as major causes of the deteriorating water quality in the many regions and South Africa is no exception [16]. Conventional wastewater technologies which refer to the traditional methods such as physical, chemical and biological processes used for treating wastewater have proved to be inadequate to handle the rapidly increasing load caused by population growth [26]. WWTP effluents contribute a diverse variety of contaminants such as polychlorinated biphenyls (PCBs), antibiotics and antiviral drugs, as well as pesticides to freshwater ecosystems downstream because not all toxins from sewage waters are removed [35, 40].
The different treatment steps at the WWTP, the composition of the wastewater that enters the WWTP, the type of treatment processes conducted, seasonal variations in temperature and other external factors such as agricultural and urban runoff along the river play a great role in the quality and toxic levels of the surface water. As a result, possible adverse effects on aquatic organisms and the environment change according to seasons [24]. Therefore, seasonal variation studies are important to monitor pollutant levels across a range of time scales to determine the best treatment processes [23].
Our dependence on treated water is increasing thus, the importance of maintaining and upgrading WWTP technologies becomes a need as it serves as the final barrier between polluted water and a healthy and functioning ecosystem [41]. Water quality monitoring and sampling becomes the best way to assess contamination of water and to further ascertain compliance of a WWTP’s efficiency to the relevant regulatory standards [2]. When assessing the water quality parameters, it is also important to complement the physicochemical characterisation with biological indicators of test organisms that can be verified to the current list of accepted direct toxicity assessments (DTA) for better characterisation of freshwater ecosystems and effectiveness of WWTPs effluent quality [5]. In this way, ecotoxicological assessments predict potentially harmful biological effects of pollutants, they can be used to determine community tolerance tendencies and offer information on the global bioavailability and toxicity of various chemical stressors. [40]. The use of a battery of bioassays with organisms each representing a different trophic level (producers, consumers and decomposers), is recommended [26, 40].
Previous studies have shown that ecotoxicological assessments can be carried out using various model species that have been developed for years and generally used to assess water safety [21]. As a result, the commonly used organisms include algae, luminescent bacteria, protozoa, daphnia, and fishes [21, 38]. For instance, a study [46] assessed the acute toxicity of polychlorinated diphenylethers (PCDEs) using Scenedesmus obliquus, Daphnia magna, and Danio rerio which all represented different trophic levels. Furthermore, these species are known standard test organisms used for aquatic risk assessments and have been widely selected as models for ecotoxicology studies for assessing chemical pollutants [46]. Meanwhile, Mendonca et al and Reque et al [25, 37] stated that, when conducting ecotoxicological studies, due to variations in the relative sensitivities of the organisms, it is essential to study effects at various trophic levels. Additionally, sensitivities of single species vary from one toxicant to another. and it is therefore difficult to apply a model of action of a set of chemicals found for one species to another species [37].
These holistic approach is important in water scarce countries where water reuse for agricultural and recreational purposes is widely practiced. Therefore, this research intends to provide an assessment of the potential influence the WWTP effluent has on the Veldwachters River and provide more insight on the benefits of aquatic toxicity tests in WWTP effluents. The results obtained from this study could further provide useful information for improving operations in WWTPs, contribute to the selection of suitable treatment technologies and assist in establishing aquatic toxicity criteria.
2 Materials and methods
2.1 Selection criteria of the sampling points
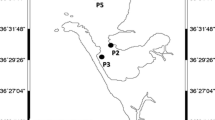
A site inspection was conducted, to critically observe the study area and select sites that would meet the objectives of the study. The selected five sampling points were points in the WWTP, outside the WWTP and in the vicinity of the WWTP. The selection of sampling points upstream and downstream of the river were considered based on accessibility and water availability for most part of the sampling period. A global positioning system (GPS) was used to obtain geographic coordinates of the sample sites. The WWTP is situated in a small metropole 50 km East of Cape Town, South Africa as shown in Fig. 1. The WWTP is fully functional and receives wastewater from both municipal and industrial sources. The study area is characterised by small and big commercial farms which contributed a high percentage to the South African agricultural capacity.
The WWTP has a treatment capacity of 35 megalitres per day, Average Dry Weather Flow (ADWF), which consists of the Membrane bioreactor (MBR) for liquid and solid separation instead of conventional clarifiers, with a treatment capacity of 27 megalitres per day (ML/d) ADWF and the modified old Activated Sludge Process (ASP) with a treatment capacity of 8 ML/d ADWF. The WWTP treats 74–85% domestic and 15–26% industrial sewage from its local area via a combination of gravitational sewers and 9 pump stations. The MBR technology is beneficial in terms of the small footprint of the bioreactor and the production of effluent that can be reused in future. Furthermore, the incorporation of the membrane technology will significantly improve the state of the river downstream. The final effluent is discharged into the Veldwachters River. The River is a tributary of the Eerste River, flows through agricultural areas and supplies farmers in the area with water for irrigational purposes.
2.2 Sampling procedure
Samples were collected using glass bottles with a storage capacity of 2.5 L and the sampling procedure was according to the standard methods [22]. Field sampling commenced in September 2019 and ended in June 2021. The plan was to sample for 12 consecutive months to cover all four seasons and provide information of possible seasonal variation within one year. However, due to Covid 19 and lockdown restrictions the sampling regime extended to 13 inconsecutive months. The samples were collected in September, October November, and December 2019; January, February, August September, October, November, and December 2020; March and June 2021. After sampling, the bottles were placed in cooler boxes containing ice, transported for approximately 50 km to the laboratory, and stored in the refrigerator in darkness at 4 °C until further analysis. A total of 26 influent and effluent samples; and 37 river water samples were collected and analysed.
2.3 Determination of physicochemical parameters
The following physicochemical parameters, pH, Dissolved Oxygen (DO), Total Dissolved Solids (TDS), Oxidation–Reduction Potential (ORP) and temperature were measured with a portable multi parameter reader (SensoDirect 150 Multiparameter—Lovibond, Germany) after calibration as per manufacturer manual. Chemical oxygen demand (COD) was measured using a photometer and its ready-to-use kits (Lovibond MD 100, Germany) and the biochemical oxygen demand, (BOD) was measured using the BOD system (Lovibond, BD 600, Germany) in the laboratory.
2.4 Ecotoxicity tests
Raphidocelis subcapitata, (a primary producer), Daphnia magna (a primary consumer), and Tetrahymena thermophila (a decomposer), were used as test species to further assess the quality of the WWTP effluent. The Toxkits were supplied by MicroBioTests Inc., Belgium as Algaltoxkit, Daphtoxkit F™, FTM and Protoxkit F™. The experiments complied with all applicable international standards and guidelines (ISO 6341 and OECD 201, 202, and 244). The test organisms are widely used for investigation of WWTP effluent toxicity due to their rapid, low-cost, and easy to run test kits. Detailed information of the aquatic toxicity experiments used is illustrated in Table A1. No reference test was done in all experiments, however, the experiments included cells that were not exposed to test samples (negative control). Serial dilution of the effluent samples was done using the respective organisms’ standard media, the OECD 202 medium artificial freshwater (AFW) composed of 294 mg/L CaCl2·2H2O; 67.75 mg/L NaHCO3; 123.25 mg/L MgSO4·7H2O; and 5.75 mg/L KCl. A dilution series of the WWTP effluent sample at 100% (undiluted, C1), 50% (C2), 25% (C3), 12.5% (C4), and 6.25% (C5) and control were prepared, using similar methods to Carvalho et al. [5]. While it would have been ideal to include a positive or negative control to validate the results, this was not possible due to limitations of resources and equipment. Therefore, the results were instead validated through multiple independent experiments, to ensure consistency and reliability. This was done to obtain valid LC50 or EC50 endpoint and the experimental data for all tests were analysed using ToxRAT Professional 3.2® for the determination of growth inhibition, mortality, statistical significance, and critical concentrations. Detailed information regarding the EC50 calculations can be found in Table A2 and Figures A1–A2.
2.4.1 72 h algal growth inhibition test with Rhaphidocelis Subcapitata
The algae growth inhibition test method was used to determine the toxicity of samples on freshwater R. subcapitata. The algal beads were de-immobilized according to the Algaltoxkit F™ instructions as per the OECD guideline 201. An algal density of 1 × 106 cell/mL was prepared from the concentrated algal inoculum by measurement of the optical density of the inoculum on a spectrophotometer (Jenway 6300) at a wavelength of 670 nm. Each flask inoculated with 1 × 104 cells/mL as the test start concentration. The various dilution series were transferred in three replicates into five long cells. The long cells were positioned randomly in the holding tray and were incubated at 23 °C with a sideway illumination of 10,000 Lux for 72 h. Measurements of the algal growth inhibition and OD at 670 nm of the algal suspensions in the long cells was performed at 24 h intervals for 72 h. The data was used to determine yield and growth inhibition of R. subcapitata after exposure to the water samples. According to the ISO Standard 8692 the test is deemed valid when the average growth rate in the control is at least 1.4 per day which corresponds to an increase in cell density by a factor 67 in 72 h. While the OECD 201 indicates that the control growth rate must be at least 0.92 per day (a multiplication factor of only 16).
2.4.2 48 h Acute immobilization test on crustacean Daphnia Magna
The crustaceans D. magna was exposed for 48 h to the different water samples collected for toxicity testing and hatching of the ephippia was achieved as per the supplier’s Daphtoxkit F™ instructions. The ephippia were rinsed using tap water, transferred into the hatching petri dish in 50 ml pre-aerated standard freshwater, and finally incubated at 20 ± 2 °C under continuous light intensity of 6000 lx for 72 h. Thereafter, the young daphnids (< 24 h old) were pre-fed spirulina 2 h prior to the start of experiments to prevent “starvation to death”. Each test consisted of exposing 5 replicates of 5 neonates to each of the 5 diluted concentrations. Approximately 20 neonates were first transferred into the multiwell rinsing plate and subsequently into the definite test multiwell plate. The rinsing step prevented the dilution of the test solutions during the transfer of the neonates from the stocks into the toxicant solutions. Thereafter, the neonates were covered in a transparent plastic lid and were incubated in darkness at 20 °C for 24–48 h. Following the 24 h and 48 h period, the neonates were visually observed and counted, neonates that were unable to swim after gentle agitation of the liquid for 15 s were immobile. The experimental results of D. magna were accepted when the observed mortality and immobilization rate were ≤ 10% in the controls [27].
2.4.3 24 h growth inhibition test on the ciliate protozoan Tetrahymena Thermophila
The ciliate protozoan microbiotest kit provided material to perform a growth inhibition of the ciliate protozoan T. thermophila within 24 h following the Protoxkit F™ instructions of OECD guideline 202. In this period normal growing cultures complete at least 5 generation cycles. The test is performed in disposable polystyrol spectrophotometric cuvettes of 1 cm in diameter. The only tools required were a photometer (filter 440 nm) and an incubator (30 °C). Ciliate inoculum and food suspension were used to prepare the dilution series, which were added to the control tubes and the test tubes.
-
(a)
To calculate the dilution factor that was needed to arrive at a ‘theoretical’ optical density (OD) value of 0.04
F = OD value/0.040
V = 0.5 × (F − 1)
For instance, if the original OD is 0.090, the 1.5 ml ciliate suspension should be diluted by a factor 0.090/0.040 = 2.25 times [28].
Where, F = the dilution factor, OD value = the optical density value, V = the dilution volume.
All the cells were put into a holding tray and the tray was put in an incubator (in darkness) at 30 °C for 24 h. After the 24 h incubation, the measuring equipment was recalibrated with a test cell containing 2 ml distilled water and all cells were gently shaken to determine the OD at 440 nm. The test was considered valid when the OD of the controls after 24 h incubation showed a decrease of the initial reading (T0) value by at least 60%, the OD at final reading (T24) should be 40% or less of the OD at T0 [28].
2.5 Ecotoxicity test evaluation
The results were expressed as either effective concentration (EC) or lethal concentration (LC). The toxicity endpoint (EC50) was determined as the relative concentration of the that resulted in 50% of immobilization in the tested sample after exposure time in different volumes. The L(E)C50 values were determined using nonlinear regression analysis and the 4-parameter logistic model which involves extrapolation beyond the range of the data, this was determined at 24, 48 and 72 by using the Toxicity Response Analysis and Testing (ToxRat) Professional 3.2 software. Toxicities and L(E)C50 values are inversely related and so, toxicity units (TUs) are used to describe concentration-based toxicity measurements. Acute toxicity unit (Tua) is used to express acute toxicity of concentration-based toxicities. To determine the toxicity effect of the test, toxicity values EC50 were converted to TU based on the equation (TU = [1/L(E)C50] × 100) as reported by [36] and were classified according to Persoone et al. [34] hazard classification system for wastewaters discharges into aquatic environments. The hazard classification system suggests that samples are classified as non-toxic when TU < 0.4,slightly toxic when 0.4 < TU < 1; toxic when 1 < TU < 10, very toxic when 10 < TU < 100 and extremely toxic when TU > 100 [34]. To classify TU for samples that had no EC50 values, the hazard classification method based on assumption was used [18]. Assuming that samples for which EC50 values could not be calculated have TU > 1, therefore, TU is assumed to be 1 [18].
To demonstrate the quantitative significance (weight) of the toxicity in each hazard class, a weight score was generated according to Persoone et al. [34] as shown in Table 1. Class weight scores were evaluated by the allocation of a test score for the effect results of each test of the battery and were calculated using Eq. 1.
-
(b)
To determine weight class score
$${\text{Class}}\,{\text{weight}}\,{\text{score}} = \sum all\,test\,scores/n$$(1)where n = number of tests performed.
-
(c)
To calculate the class score in percentage
$$\% \,{\text{Class weight score}} = {\text{Class score/maximum class weight score}} \times 100$$(2)
2.6 Statistical analysis
The data obtained from the river water, influent and effluent samples were analysed using the IBM SPSS Statistics version 28 package for statistical analysis. Pearson’s correlation analysis at 5% significance level was used to show the relationships between observed physicochemical parameters values obtained for the sampling duration. The statistical design for the ecotoxicity experiments was based on the ToxRat Professional 3.2 Software to evaluate the effect concentrations and confirm fulfilment of the validity criteria, hypothesis testing (NOEC) and regression (ECx). The endpoints such as EC50 and LC50 values were calculated with the corresponding 95% confidence limits. Statistical data was analysed for normality distribution with the Shapiro–Wilk’s W test while Levene’s test was confirmed with the variance homogeneity. Thereafter, the one-way ANOVA followed by the Bonferroni post-hoc test was performed, and the level of significance was p < 0.05.
3 Results and discussion
3.1 Physicochemical characteristics of surface water and treated sewage wastewater
Guidelines for water quality are intended to assist in the development and implementation of risk management strategies that safeguard sources of drinking water by limiting potentially dangerous water components [44]. These guidelines are based on health-based targets, and the criteria used to determine these standards are constantly being reviewed. As a result, each country’s drinking water standards may differ in nature and form, and no single approach is universally applicable [44]. The results obtained for this study were compared with the accepted limits values applicable to discharge of wastewater into a watercourse as shown in Table 2
The overall measured temperature, pH, DO, EC, TDS, ORP, BOD and COD were in the range of 14.2–29.5 °C; 4.7–9.75; 1.7–9.5 mg/L; 559–1241 µm/cm; 376–840 ppm; -350–273 mV; 0.9–175.6 mg/L respectively. Temperature is one of the important guides to water quality as it affects chemical reactions, aquatic life, and suitability of water. In this study, there were no significant changes in temperature between the points and the effluent’s temperature complied with the set limits for discharged effluent into a waterbody by DWAF (≤ 35 °C) and WHO (40 °C). pH values tended towards neutral and alkaline for most of the seasons, which is an indication of disinfection in the water. This could be due to bicarbonate, carbonate, and hydroxide caused by CO2 and microbial decomposition of organic matter [4] and similar observations were found in studies by Kalwale and Savale [17], Olabode et al. [30]. The effluent values for pH were not all within the limits for discharged effluents into receiving waterbodies both from DWAF; USEPA and WHO.
DO concentrations in unpolluted water typically range between 8 and 10 mg/L, concentrations below 5 mg/L affect aquatic life, while concentrations below 2 mg/L cause fish kills [10]. Agoro et al. [1], states that the acceptable standard of DO for aquatic organisms is 4–5 mg/L. The effluent values show that about 46% of the values for DO were within that range and are beneficial for aquatic life. However, 54% of the effluent shows low DO values, which according to Edokpayi et al. [13], may pose an adverse effect on aquatic organisms in receiving environments. EC is primarily due to the dissolved ions obtained from decomposed plant matter and is also an important indicator of salinity found in surface water [1].
The EC values for the influent were too high when compared with the values of the effluent, which shows that the WWTP is efficient in the reduction of EC. Although some effluent values were higher than the DWAF (≤ 750 µS/cm) standards, they were still within the WHO (1000 µS/cm) and USEPA (2000 µS/cm) standards. TDS is directly proportional to EC; hence it is observed that the trends of these results corroborate with the values recorded for EC. Differences in values recorded upstream and downstream were observed which suggested that there could be external pollution sources such as agricultural and urban runoff, upstream the river. These could have contributed to high nutrient loads which could have led to eutrophication in the river. A similar observation by Agoro et al. [1], in a study on physicochemical properties of wastewater further states that high concentrations of TDS are toxic to aquatic organisms and thus lead to dehydration and thermal shock, which affects the organisms osmoregulatory strength.
ORP readings in water are considered as an important indicator of pollution levels. Currently, there are no regulatory standards for ORP in South Africa, therefore, ORP in this study was compared with the WHO standards which were well within the WHO standards of 700 mV. Thus, suggesting that the effluent poses little to no negative effect on the river. The DWAF guideline values for BOD is 3–6 mg/L while the WHO is 50 mg/L, however this threshold was exceeded by most of the seasons sampled. This is detrimental as continuous discharge of the WWTP effluent will impact the receiving waterbody to some degree and may negatively impact the aquatic organism, especially those downstream. COD is known as an alternative test to BOD to determine the concentration of organic matter in wastewater samples. According to Aniyikaiye et al. [2], Baharvand and Daneshvar [4] COD values are typically greater than BOD values, due to organic components in the water that are resistant to microbial oxidation.
The recorded values suggested that the WWTP effluent complied with the set limits ≤ 75 mg/L by DWAF and ≤ 100 by WHO for COD for all sampling seasons. Nevertheless, the COD values for March (226 mg/L) were above the required standards, which could be attributed to seasonal variation. Moreover, this high COD value is an indication that due to these seasonal variations, the effluent contains materials that are resistant to microbial degradations and can detrimentally affect the receiving river.
3.2 R. subcapitata 72 h growth inhibition test
As primary food producers in aquatic ecosystems, any disturbance on the microalgae can influence the entire balance of such ecosystems [21]. Furthermore, due to their sensitivity, algae can be easily cultivated which makes them an important kind of test organism for acute toxicity evaluation [21]. The ToxRat Professional 3.2 software was used to assess R. subcapitata in terms of the number of cells and growth rate for a period of 72 h for four seasons (Table 3). There was an increase in the number of cells observed in summer (S1), autumn (S2) and spring (S4) (6.25–100%) from 24 to 72 h exposure. In autumn (S2), however, the increased number of cells were observed at a low concentration (6.25%) and at the control after 24 h to 72 h. Furthermore, from 12.5 to 100% the number of cells increased at 24 h, decreased at 48 h, and increased at 72 h exposure, respectively. A non-linear regression without weighting was performed to estimate the effective concentration for growth rate. The growth rate of R. subcapitata decreased from 24 to 72 h exposure and fluctuated with each % dilution per season. Meanwhile, the percentage inhibition at the different concentrations revealed no significant pattern of effect. The percentage inhibition was found to be highest in autumn (S2) (27.5%) at 50% dilution, and winter (S3) (15.2%) at 100% whole effluent following the 72 h exposure. The growth inhibition of this study never reached 100% which was like an observation in some samples collected from four municipal WWTPs by Szklarek et al. [40]. In the summer (S1) and autumn (S2) seasons, at 100% whole effluent, the growth of R. subcapitata decreased to 4% and -15.2% respectively. Moreover, in summer (S1) and spring (S4) the growth of R. subcapitata was inhibited in some samples to the highest concentration of the effluent, which proves that dilution plays a role in the toxicity. The different percentage dilutions of the effluent were toxic and inhibited the growth of R. subcapitata, while others favoured algal growth. Increased nutrient availability; decreased competition in the more diluted samples or higher concentrations of toxic compounds found in the more diluted samples could be the reason for higher inhibition in more diluted samples. While the results showed that R. subcapitata is sensitive to various percentage dilutions of the effluent, they also showed that the percentage inhibition of the growth rate were dependent on the test item concentration and time with decreasing inhibition growth rate values. The results are similar to the observation by Pereao et al., [33]. The statistical analyses for summer (S1) showed Shapiro–Wilk’s (W) = 0.971,and probability p(W) = 0.813 which was observed to be greater than the selected significance level of 0.010. Thus, the treatment data did not significantly deviate from normal distribution. While Levene’s Test indicated variance homogeneity (p > 0.010). Therefore, normal distribution and variance homogeneity check (p > 0.01) were passed. However, for autumn (S2) the normal distribution is poor (p < = 0,01) while variance homogeneity check was passed (p > 0,01). Meanwhile, the winter (S3) and spring (S4) seasons passed both the homogeneity check (p > 0.01) and the variance homogeneity.
3.3 D. magna 48 h acute immobility test
The acute freshwater D. magna is an excellent test model organism in aquatic toxicology and its toxicity was assessed by exposing it to different dilutions (6.25–100%) of WWTP effluent over a period of 48 h as shown in Fig. 2. In the present test none (0%) of the daphnids died, thus, confirming the validity of the test.
The total percentage mortality of D. magna exposed to these concentrations was compared to the control for all seasons. No neonate mortality was observed in the control and the lowest concentrations of the effluent samples in all seasons except for the summer (S1) season (Fig. 2). D. magna mortality rate increased 8x- 2 × dilution of the original samples in the spring (S4) season resulting in 5–10% mortality. The results indicated that the higher WWTP effluent concentration (100%) led to no mortality rate of D. magna following the 48-h exposure, which is an indication that dilution may play a part in the toxicity of D. magna. The results also showed that at lower dilution concentrations of 6.25–50% there was 5–10% mortality of the test organisms, indicating that the low percentage concentrations of the WWTP effluent may induce toxicity to D. magna. The release of the WWTP effluent will affect freshwater organisms like D. magna by causing direct acute toxicity. A study by Wagner et al. [43] showed no mortalities on D. magna following the 48 h acute exposure on WWTP final effluent. While da Costa et al. [8] used Ceriodaphnia silvestrii and Daphnia similis to verify the possible adverse effects of secondary disinfected effluent. The statistical analysis was done using the step-down Cochran-Armitage test which was later replaced by the Bonferroni Fisher test because the analysis of contrasts did not reveal a linear trend (p > 0.05).
3.4 T. thermophila 24 h growth inhibition test
The results of the acute freshwater tests for T. thermophila, is presented in Fig. 3. The results for summer (S1) and spring (S4) showed significant growth inhibition level values which show a high inhibition response of T. thermophila to the WWTP effluent. In autumn (S2) and winter (S3) there is a dose-dependent inhibition of proliferation on T. thermophila, which suggests stimulation of bacteria and is similar to what was observed in the study by [33]. The maximum percentage of growth inhibition for all season (S1, S2, S3 and S4) was 128.3%; − 10.3%; 3.3% and 143%, respectively. The effluent showed significant percentage inhibition in the different dilution treatments of T. thermophila in contrast to the control.
To assess environmental risks and impacts, test species should be eukaryotic, their biology and general responses should be known, laboratory handling should be easy, and a short generation time is advantageous for studies of long-term effects. The ubiquitous ciliated protozoa fulfils these requirements [14]. Furthermore, their abundance is an indicator of healthy aquatic environments, and they represent a significant trophic level where bioaccumulation or bio-concentration are important processes. The T. thermophila ciliate tests not only play an important ecological role in self-purification and matter cycling of natural aquatic ecosystems but they serve as a powerful tool for the prediction of possible hazards to wastewater treatment processes. They are ideal early warnings indicators of aquatic ecosystem deterioration and have proven to be excellent tools of assessing pollution, which is why it was one of the test species used for this study. Following the one-way ANOVA test which showed a level of significance of p < 0.05, the Bonferroni post-hoc test revealed a significant difference between D. magna and T. thermophila (p < 0.05.
3.5 Ecotoxicological test values for the WWTP effluent
The results for the probit analysis of the bioassays are shown in Table A2 and the toxicity classifications in Table A3. The effective concentration (EC50) values for R. subcapitata in autumn (S2), winter (S3) and spring (S4) could not be calculated, however, the EC50 for summer (S1) (122.2) was calculated and exhibited measurable EC50 values. Based on these EC50 values, the TU values were determined by using the equation TU = [1/L(E)C50] × 100, as reported by [36]. Thus, for summer (S1) the effluent can be classified as class II, slightly acute toxicity, with 0.4 < TU < 1. While for autumn (S2), winter (S3) and spring (S4), if TU > 1 the effluent is classified as class III, acute toxicity with 1 < TU < 10. This was different to the classifications made by Katsoyiannis and Samara, [18], where 7 out of 13 WWTP investigated were classified as slightly toxic. Moreover, their study also reported that different WWTP will show different results because toxicity of any plant is greatly influenced by the type of wastewater it receives. For D. magna, the 95% confidence limit was not detected in all seasons, and resulted in no LC10, LC20 and LC50 being measured by the ToxRat Professional 3.2 software following the 48-h exposure. The hazard classification method based on assumption [18] was used to classify the effluent on D. magna. Assuming that samples for which EC50 values could not be calculated have TU > 1, TU is assumed to be 1, as shown in Table A3. The results suggest that for all seasons, D. magna exposed to the effluent is classified as Class III (acute toxicity). Although EC50 was not determined in all seasons, these findings do not completely rule out the possibility of the effluents chronic toxicity. Kocbas and Oral [20], studied the toxicity of municipal wastewater treatment plant effluents, using D. magna as a test species. The results obtained were comparable to this study and showed TU values ranging from 3.0 to 4.2 suggesting that the WWTP is classified as Class III (acute toxicity). The diverse use of bioassays, using sublethal toxicity assessment methods or chronic tests as well as reconsidering toxicity end points may produce better results [21]. A chronic toxicity test with a longer exposure time, such as 21 days with the effluent, may also show different results. Thus, chronic toxicity studies need to be evaluated to better understand the actual toxicity effects of the effluent on the aquatic environment. The respective EC50 values, using Persoone et al. [34] classification methods, of summer (S1) effluent exposed to T. thermophila can be classified as Class III, acute toxicity, 1 < TU < 10. The autumn (S2) effluent is classified as Class V, very high acute toxicity, TU > 100, while the winter (S3) and spring (S4) effluent are both classified as Class I, no acute toxicity, TU < 0.4. Generally, the protozoan T. thermophila was more susceptible to the effluent as compared to other biotest, demonstrating a TU > 100, while other samples demonstrated 1 TU < 10. This could be attributed with the lack of cell walls that exposes T. thermophila to respond faster to environmental changes [11, 47]. These results correspond with a study by Udebuani et al. [42], indicating that T. thermophila proved to be more sensitive than other biotests used in their study.
The overall biotest results were further classified according to each season, as shown in Table A3. The sampling for summer (S1) was classified as acute toxicity (class III) as all three tests have a TU of 1 < TU < 10. The effect percentage is 55.7%, which shows that the WWTPs effluent contains toxic chemicals. The autumn (S2) season was classified as very high acute toxicity (class V) due to its high effect percentage of 89% and the TU results of T. thermophila (384.6). Although other biotests (D. magna and R. subcapitata) showed TU of 1, the toxic results may have appeared due to unbalance of nutrients or the presence of solids in the water in this season. In winter (S3) and spring (S4), the effluent was both classified as acute toxicity (class III) because all biotests had a TU of 1 < TU < 10. No other tests exhibited toxic effects and similarly to summer (S1), these results prove that there are toxic chemicals in the water, although the physicochemical properties indicated no pollution for most of its parameters. The results further demonstrated that the test organisms exhibited different behaviours in the same wastewater effluent sample. This could be associated with the level of pollutants found in the effluent and the sensitivity of the test organism used in the study. The order of sensitivity based on trophic levels is the decomposer (T. thermophila) > producer (R. subcapitata) > consumer (D. magna). In literature, similar observations were made in a study on effects of municipal wastewater treatment plant effluent quality on aquatic ecosystem organisms [33]. Therefore, the WWTP effluent has potential toxicological effect on aquatic organisms and physicochemical properties alone cannot assess the quality of effluents and how they affect receiving water bodies.
3.6 Correlation analysis of the bioassays and physicochemical parameters of the surface water and treated sewage wastewater
Pearson’s correlation coefficient (r) of the physicochemical properties and bioassays was investigated to understand the association and the strength of the linear relationship between the variables. Correlations were based on the original physicochemical parameters that were determined during field sampling. The results of these variables showed significant and insignificant relationships with each other as shown in Table 4: Correlation coefficient between physicochemical parameters and bioassays Table 4. There was a significant (p < 0.01) positive correlation between pH and DO while temperature with pH and DO indicate a negative correlation (r = − 0.450 and − 0.515 at p < 0.01), respectively. The observed correlation between pH; EC; TDS; ORP; COD and BOD was statistically insignificant (r = 0.003; 0.015; − 0.196; 0.011 and 0.003) respectively which showed that there was no relationship between these variables.
These results were different to those observed by Pereao et al. [33] where a strong positive correlation existed between pH; EC; ORP and TDS values and the research established strong positive correlation between EC, TDS, COD and BOD, while ORP showed a negative correlation with TDS and EC. According to Osode and Okoh [31] knowing how the variables correlate with each other helps with understanding the nature of the physicochemical parameters and their species speciation on the receiving water body and effluent. On the other hand, the potential relationship between the physicochemical parameters and bioassays was assessed based on the TU of each organism. ORP was positively correlated with the toxicity values found in R. subcapitata (r = 0.998**), while pH, EC and TDS showed a negative correlation (r = − 0.933, − 0.890, − 0.938), respectively. The correlation between temperature and the toxicity values of T. thermophila was (r = 0.920), while for pH and DO it was (r = − 0.641 and − 0.686). The partial positive and negative correlation between these variables suggested that a relationship does exist between the variables and these parameters influence the sensitivity of T. thermophila.
4 Conclusion
The findings of this study showed that the effluent met the recommended water quality standards for EC, ORP, COD, temperature, and TDS suggesting that the physicochemical properties of the WWTP can be acceptable for discharge into the Veldwachters River. The physicochemical properties were complemented with ecotoxicological tests which considered the toxic effects of the contaminants on aquatic organisms. The application of a battery of tests provided information on the biological activity of the WWTP effluent which physicochemical monitoring alone was unable to provide. The seasonal classifications of all bioassays based on the TU showed that the effluent in summer, autumn and winter were classified as acute toxicity class III, while spring was classified as very high acute toxicity class V. The results revealed that although the effluent contributes positively to the river recharge and health downstream, there is the potential to trigger eutrophication in the river system. The aquatic bioassays exhibited different levels of acute toxicities in the same WWTP effluent, the order of sensitivity based on trophic levels is the decomposer (T. thermophila) > producer (R. subcapitata) > consumer (D. magna). The findings further highlight the need for continuous monitoring and provides insights into the benefits and possible risks that must be mitigated by governments and WWTP authorities for reuse and optimal utilization of freshwater resources for sustainability. Additionally, the study showed that no single technique can represent a thorough strategy for protecting aquatic life. Therefore, the study recommends improved operational practices, such as monitoring and optimising treatment processes to reduce the potential toxicity of wastewater and enforcing aquatic toxicities when monitoring water quality. Further research is required to understand the effects of chronic tests on the WWTP effluent and methodologies of implementing them.
Data availability
All data generated and analysed during this study are included in this published article.
References
Agoro MA, Okoh OO, Adefisoye MA, Okoh AI (2018) Physicochemical properties of wastewater in three typical South African sewage works. Pol J Environ Stud 27(2):491–499
Aniyikaiye TE, Oluseyi T, Odiyo JO, Edokpayi JN (2019) Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int J Environ Res Public Health 16(7):1235
Aydin ME, Aydin S, Tongur S, Kara G, Kolb M, Bahadir M (2015) Application of simple and low-cost toxicity tests for ecotoxicological assessment of industrial wastewaters. Environ Technol (UK) 36(22):2825–2834
Baharvand S, Daneshvar MRM (2019) Impact assessment of treating wastewater on the physiochemical variables of environment: a case of Kermanshah wastewater treatment plant in Iran. Environ Syst Res. https://doi.org/10.1186/s40068-019-0146-0
Carvalho AR, Isabel Pérez-Pereira A, Cavadas Couto CM, Tiritan ME, Cláudia, Ribeiro MR (2021) Assessment of effluents quality through ecotoxicological assays: evaluation of three wastewater treatment plants with different technologies. https://snirh.apambiente.pt/. 19 August 2022
Chen K-H, Wang H-C, Han J-L, Liu W-Z, Cheng H-Y, Liang B, Wang A-J (2020) The application of footprints for assessing the sustainability of wastewater treatment plants: a review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.124053
Cohen TM, Matthew M, Salit K, Dor R (2019) Global invasion in progress: modeling the past, current and potential global distribution of the common myna. Biol Invas 21:1295–1309. https://doi.org/10.1007/s10530-018-1900-3
da Costa JB, Rodgher S, Daniel LA, Espíndola ELG (2014) Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 23(9):1803–1813
Department of Water and Sanitation (2018) Strategic Overview of the Water Sector in South Africa. 81. http://nepadwatercoe.org/wp-content/uploads/Strategic-Overview-of-the-Water-Sector-in-South-Africa-2013.pdf.
DFID (1999) A Simple Methodology for Water Quality Monitoring. Department for International Development (DFID), pp 1–39
Doerder FP (2014) Abandoning sex: multiple origins of asexuality in the ciliate Tetrahymena. BMC Evol Biol 14(1):1–13
Donnenfeld Z, Hedden S, Crookes C (2018) A delicate balance: water scarcity in South Africa. https://www.africaportal.org/publications/delicate-balance-water-scarcity-south-africa/. 8 Sept 2022
Edokpayi JN, Odiyo JO, Durowoju OS (2017) Impact of wastewater on surface water quality in developing countries: a case study of South Africa. INTECH. https://doi.org/10.1039/C7RA00172J
Gerhardt A, Ud-Daula A, Schramm K-W (2010) Tetrahymena spp. (Protista, Ciliophora) as test species in rapid multi- level ecotoxicity tests. Acta Protozoologica 49(12):271–280
Harding G, Chivavava J, Lewis AE (2020) Challenges and shortcomings in current South African industrial wastewater quality characterisation. Water SA 46(2):267–277
Iloms E, Ololade OO, Ogola HJO, Selvarajan R (2020) Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. Int J Environ Res Public Health 17(3):1096
Kalwale AM, Savale PA (2012) Determination of physico-chemical parameters of Deoli Bhorus Dam water. Pelagia Research Library Adv Appl Sci Res 3(1):273–279
Katsoyiannis A, Samara C (2007) Ecotoxicological evaluation of the wastewater treatment process of the sewage treatment plant of Thessaloniki. Greece J Hazard Mater 141(3):614–621
Khanna A (2020) An overview of technology driven solutions to the water crisis in developing nations. Int J Soc Sci Econ Res. https://doi.org/10.46609/IJSSER.2020.v05i05.013
Koçbaş F, Oral R (2015) Daphnia magna as a test species for toxicity evaluation of municipal wastewater treatment plant effluents on freshwater cladoceran in turkey. Turk J Fish Aquat Sci 15(3):619–624
Luan X, Liu X, Fang C, Chu W, Xu Z (2020) Ecotoxicological effects of disinfected wastewater effluents: a short review of: in vivo toxicity bioassays on aquatic organisms. Environ Sci Water Res Technol 6(9):2275–2286
Madikizela LM, Chimuka L (2017) Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ Monitor Assess 189(7):348
Makuwa S, Tlou M, Fosso-Kankeu E, Green E (2022) The effects of dry versus wet season on the performance of a wastewater treatment plant in North West Province. South Africa Water SA 48(1):40–49
Maranho LA, Garrido-Pérez MC, Baena-Nogueras RM, Lara-Martín PA, Antón-Martín R, DelValls TA, Martín-Díaz ML (2014) Are WWTPs effluents responsible for acute toxicity? Seasonal variations of sediment quality at the Bay of Cádiz (SW, Spain). Ecotoxicology 24(2):368–380
Mendonca E, Picado A, Paixao SM, Silva L, Barbosa M, Cunha MA (2013) Ecotoxicological evaluation of wastewater in a municipal WWTP in Lisbon area (Portugal). Desalin Water Treat 51(19–21):4162–4170
Methneni N, Morales-González A, Jaziri A, Mansour HB, Fernandez-Serrano M (2021) Persistent organic and inorganic pollutants in the effluents from the textile dyeing industries: ecotoxicology appraisal via a battery of biotests. Environ Res 196:2022. https://doi.org/10.1016/j.envres.2021.1109564September
Microbiotests Daphtoxkit F (1996) Crustacean Toxicity Screening Test for Freshwater Standard Operating Procedure
Microbiotests Protoxkit F (2001) Freshwater Toxicity Test with a Ciliate Protozoan Standard Operating Procedure
Nyam YS, Kotir JH, Jordaan AJ, Ogundeji AA, Turton AR (2020) Drivers of change in sustainable water management and agricultural development in South Africa: a participatory approach. Sustain Water Resources Manag 6:62. https://doi.org/10.1007/s40899-020-00420-9
Olabode GS, Olorundare OF, Somerset VS (2020) Physicochemical properties of wastewater effluent from two selected wastewater treatment plants (Cape Town) for water quality improvement. Int J Environ Sci Technol
Osode AN, Okoh AI (2009) Impact of discharged wastewater final effluent on the physicochemical qualities of a receiving watershed in a suburban community of the eastern Cape Province. Clean: Soil, Air, Water 37(12):938–944
Pandey CL (2021) Managing urban water security: challenges and prospects in Nepal. Environ Dev Sustain 23:241–257. https://doi.org/10.1007/s10668-019-00577-0
Pereao O, Akharame MO, Opeolu B (2021) Effects of municipal wastewater treatment plant effluent quality on aquatic ecosystem organisms. J Environ Sci Health Part A 56(14):1480–1489
Persoone G, Marsalek B, Blinova I, Törökne A, Zarina D, Manusadzianas L, Nalecz-Jawecki G, Tofan L, Stepanova N, Tothova L, Kolar B (2003) A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ Toxicol 18(6):395–402
Phungela TT, Maphanga T, Chidi BS, Madonsela BS, Shale K, Studies O, Peninsula C, Town C, Africa S, Chidi B, Shale K (2022) The impact of wastewater treatment effluent on Crocodile River quality in Ehlanzeni District, Mpumalanga Province, South Africa. S Afr J Sci 118(7):64–71
Ramírez-Morales D, Masís-Mora M, Montiel-Mora JR, Cambronero-Heinrichs JC, Briceño-Guevara S, Rojas-Sánchez CE, Méndez-Rivera M, Arias-Mora V, Tormo-Budowski R, Brenes-Alfaro L, Rodríguez-Rodríguez CE (2020) Occurrence of pharmaceuticals, hazard assessment and ecotoxicological evaluation of wastewater treatment plants in Costa Rica. Sci Total Environ 746:141200. https://doi.org/10.1016/j.scitotenv.2020.141200
Reque R, Carneiro RD, Yamamoto FY, Ramsdorf WA, Martins LR, Guiloski IC, de Freitas AM (2021) Ecotoxicity of losartan potassium in aquatic organisms of different trophic levels. Environ Toxicol Pharmacol 87(12):103727
Rotini A, Manfra L, Spanu F, Pisapia M, Cicero AM, Migliore L (2017) Ecotoxicological method with marine bacteria Vibrio anguillarum to evaluate the acute toxicity of environmental contaminants. J Vis Exp 2017(123):1–5
Sun S, Zhou X, Liu H, Jiang Y, Zhou H, Zhang C, Fu G (2021) Unraveling the effect of inter-basin water transfer on reducing water scarcity and its inequality in China. Water Res 194(116931):2022. https://doi.org/10.1016/j.watres.2021.11693131August
Szklarek S, Kiedrzyńska E, Kiedrzyński M, Mankiewicz-Boczek J, Mitsch WJ, Zalewski M (2021) Comparing ecotoxicological and physicochemical indicators of municipal wastewater effluent and river water quality in a Baltic Sea catchment in Poland. Ecol Ind 126(April):1–12
Tortajada C (2020) Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. NPJ Clean Water. https://doi.org/10.1038/s41545-020-0069-3
Udebuani AC, Pereao O, Akharame MO, Fatoki OS, Opeolu BO (2021) Acute toxicity of piggery effluent and veterinary pharmaceutical cocktail on freshwater organisms. Environ Monit Assess 193(5):293
Wagner ND, Helm PA, Simpson AJ, Simpson MJ (2019) Metabolomic responses to pre-chlorinated and final effluent wastewater with the addition of a sub-lethal persistent contaminant in Daphnia magna. Environ Sci Pollut Res 26(9):9014–9026
WHO (2011) Guidelines for drinking-water quality. http://www.who.int 17 August 2022
Xia W, Chen X, Song C, Pérez-Carrera A (2022) Driving factors of virtual water in international grain trade: A study for belt and road countries. Agric Water Manag 262(107441):2022. https://doi.org/10.1016/j.agwat.2021.1074414September
Yang W, Huang X, Wu Q, Shi J, Zhang X, Ouyang L, Crump D, Zhang X, Zhang R (2022) Acute toxicity of polychlorinated diphenyl ethers (PCDEs) in three model aquatic organisms (Scenedesmus obliquus, Daphnia magna, and Danio rerio) of different trophic levels. Sci Total Environ 805:150366. https://doi.org/10.1016/j.scitotenv.2021.150366
Zahid MT, Shakoori FR, Zulifqar S, Jahan N, Shakoori AR (2014) A new ciliate species, Tetrahymena farahensis, isolated from the industrial wastewater and its phylogenetic relationship with other members of the genus Tetrahymena. Pak J Zool 46(5):1433–1445
Acknowledgements
The study was supported by Prof Opeolu’s South African National Research Foundation’s Competitive Support for Rated Researchers Grant (Grant Number: 129279), the Environmental Chemistry and Toxicology Research Group, Cape Peninsula University of Technology and the management and staff of the WWTP for access and sampling permission.
Funding
This study was funded by Prof Opeolu’s South African National Research Foundation’s Competitive Support for Rated Researchers Grant (Grant Number: 129279).
Author information
Authors and Affiliations
Contributions
SM: Material preparation, data collection and analysis, investigation, and writing the manuscript-original draft. OP: Material preparation, investigation, data collection and analysis, software and writing the manuscript-review & editing. BO: Supervision, study conception and design, project administration; funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mlonyeni, S., Pereao, O. & Opeolu, B. Application of biological assays to evaluate the aquatic toxicity of a WWTP effluent in Western Cape, South Africa. SN Appl. Sci. 5, 276 (2023). https://doi.org/10.1007/s42452-023-05490-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05490-w