Abstract
Single-Phase (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) perovskites were synthesized by the sol–gel method followed by sintering at 700 °C for 5 h. Samples with x = 0.0–0.4 are found to crystallize into rhombohedral structure (R-3c space group) while the ones with x = 0.6–1.0 crystallize into orthorhombic structure (Pbnm space group). The average particle size of the samples was in the range of 21–44 nm. All samples exhibit a ferromagnetic to paramagnetic second-order magnetic phase transition at Curie temperature, which is found to decrease linearly with increasing the Pr concentration. The magnetic coercivity was found to be small (~ 10 Oe) for all the samples, at T < TC. The experimental effective paramagnetic moment (µeff) is found to increase with increasing x from 3.99 µB (x = 0.0) to 5.05 µB (x = 1.0). The magnitude of the maximum magnetic entropy change (−ΔSM)max. and the relative cooling power (RCP) for the samples having orthorhombic structure increases as x increases reaching a maximum value of 4.67 J/kg.K and 574 J/kg at ΔµoH = 9 T for x = 1.0. While the ones with the rhombohedral structure have the maximum values of (−ΔSM)max. and RCP of 4.63 J/kg.K and 472 J/kg at ΔµoH = 9 T are for x = 0.2. The large values of (−ΔSM)max. and RCP (at room temperature (RT)) and the wider temperature range of −ΔSM for the sample with x = 0.6 suggests that this compound may be considered as magnetic refrigerant material at RT, and the other compounds can be candidates in the vicinity of RT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the magnetocaloric effect (MCE) has attracted wide attention for its possible practical applications in magnetic refrigerators. This technology has several advantages over conventional gas compression. First, the cooling efficiency in magnetic refrigerators is higher and can reach up to 30–60% of the Carnot cycle. Second, easily to build using solid substances as working materials. Third, they are environmentally friendly since there is no need to use global-warming gases, which cause ozone depletion [1, 2]. This technology is used in a lot of applications such as large-scale air conditioners, heat pumps, supermarket refrigeration units, waste separation, chemical processing, gas liquefication, liquor distilling, sugar refining, grain drying, etc. [1].
The MCE can be defined as the change in the temperature of the refrigerant in response to a change in the external magnetic field [3].The magnetocaloric effect depends on the fact that when an external magnetic field is applied the spin entropy of a magnetic material decreases resulting in an increase in the lattice entropy and hence an increase in the temperature of the sample. On the contrary, removing the magnetic field adiabatically causes the magnetic spins to randomize, which leads to an increase in the magnetic entropy and a decrease in the lattice entropy and henceforth lowering the temperature of the sample [4]. A giant magnetocaloric effect was discovered in Gd–Si–Ge alloys, and a large magnetic entropy change was reported in other alloys such as MnFeP0.45As0.55, Fe49Rh51, La(Fe, Si)13, and Mn1–xFexAs [4]. Gadolinium (Gd) is considered to be a potential material for magnetic refrigeration near room temperature. Gd has a ferromagnetic transition temperature, which occurs near room temperature (at 294 K) and a magnetic entropy change estimated at 5 J kg−1 K−1 for μ0H = 2 T [5] but its use may be limited due to its high cost.
Magnetic refrigerant materials can be classified according to their phase transition as first and second order magnetic phase transition [6]. In literature, it has been reported that the first and second order phase transition in manganites exhibit a large magnetocaloric effect. A first-order magnetic transition has high values of the magnitude of the change of entropy ΔSM with a large hysteresis that leads to low operation relative cooling power. On the contrary, despite that the second order magnetic transition induces a smaller ΔSM but the distribution is over a broader temperature range, which results in a larger relative cooling power (RCP). Hence, composite materials that undergo second order magnetic transition are more suitable for magnetic refrigeration [7].
Perovskites type manganites with general formulae RE1–xAxMnO3 (where R is a trivalent rare earth cation and A is a divalent alkaline earth cation) have received important attention from researchers due to their structural, electronic, and magnetic wide properties, which are important in many technological applications [5, 8]. These materials exhibit rich physical properties such as unique colossal magnetoresistance (CMR) behaviour, magnetocaloric effect, electrical, optical, and dielectric properties [5]. These properties are significant for many applications such as magnetic recording, magnetic sensors, and magnetic refrigerating technology [5, 9]. The properties can be tuned by different parameters such as the substitution of the A-site and/or the B-site with suitable elements, which causes changes in structural parameters, Mn4+/Mn3+ ratio, average ionic radius of A-site of the perovskite, cationic disorder, and oxygen non-stoichiometry [5, 10]. In addition, researchers found that the synthesis techniques will strongly affect some of these materials’ chemical and physical properties such as the morphology, the chemical composition, the grain size distribution, and their correlation with magnetic and magnetocaloric properties [10].
Perovskites manganites (RE1–xAxMnO3) are considered promising candidates for magnetic refrigerating due to 1) ease to tune their transition temperature by RE-site or Mn-site substitution 2) high chemical stability 3) high resistivity 4) lower cost 5) easier to fabricate [4, 11]. Large MCE (larger than MCE of Gd) has been measured in compounds such as La0.75Ca0.25MnO3 and La1–xAgxMnO3 and a large MCE of 1.53 Jmol−1 K−1 has been reported at low temperatures in Tb1–xHoxMnO3 (with x = 0, 0.1 and 0.2) under an applied magnetic field change of 0—5 T [12]. Also, a large MCE value is reported in the Sm0.55Sr0.45MnO3 polycrystalline sample (−ΔSmax ≈8.48 Jkg−1 K−1 for ΔH = 6 T) and a very large relative cooling power value (RCP ≈ 574 J/kg at ΔH = 6 T) at TC = 145 K [13]. Among manganite families, the La1–xSrxMnO3 perovskite is one of the most attractive manganites. Different studies showed that the ferromagnetic interaction becomes strongest at a concentration of x = 0.3 in La1–xSrxMnO3 [14]. Therefore the giant magnetocaloric effect is usually recorded around this concentration [7]. Hence, this perovskite has been of huge interest to scientists and engineers. La0.7Sr0.3MnO3 has been synthesized with many techniques such as conventional solid-state reaction [15, 16], conventional ceramic technique [17], the auto-combustion process [14], high energy ball milling [18], and the sol–gel method [9, 19, 20]. In this perovskite, partial substitution of lanthanum by other rare earth (Pr, Dy, Nd, Tb, Eu, … etc.) generates interesting physical phenomena. The difference in the average ionic radius between these elements will enhance the structural and magnetic disorders in the lattice with no effect on the amounts of Mn3+ and Mn4+ ions [8, 21]. The ionic size of the Pr3+ ion is smaller than the ionic size of the La3+ ion. In addition, Pr has a magnetic moment value of 3.58 µB compared to La3+, which has zero magnetic moment. These parameters will strongly affect the structure and magnetic properties of the perovskite.
Barik and Mahendiran [22] observed the magnetoresistance of ∼100% MR and magnetic hysteresis in Bi-doped La0·7Sr0·3MnO3 compounds, which are due to the co-existing of ferromagnetic (FM) and antiferromagnetic (AFM) matrices. Pr0.6Sr0.4MnO3 has been reported to experience a structural phase transition from a high-temperature orthorhombic (Pnma space group) to a low-temperature monoclinic (I2/a space group) and create a ferromagnet with a Curie temperature TC ∼ 297 K [23]. Tozri et al. [11] studied the influence of Pr-doping on the magnetic phase transition and the magnetocaloric effect of the La0.7–xPrxBa0.3MnO3 manganite. Zhang et al. [24] used the standard solid state-reaction method to synthesize La0.7–xPrxSr0.3MnO3 (0.02 ≤ x ≤ 0.3) polycrystalline samples. They reported that all the samples are single phase with rhombohedral structure and the doping with Pr3+ leads to a decrease in the lattice parameters, Curie temperature, and the maximum magnetic entropy change. On the other hand, Zhou et al. [25] have reported the synthesis of the perovskites La0.7–xRxA0.3MnO3 (0 < x < 0.7), where R = Pr or Gd and A = Ca, Sr, or Ba by conventional ceramic techniques. They found that Tc is above room temperature in rhombohedral La0.7Sr0.3MnO3 and drops to 260 K in the orthorhombic Pr0.7Sr0.3MnO3. Moreover, the substitution of La3+ by Pr3+ strongly improves the magnetoresistance (MR) and increases the coercive field Hc and the magnetization M. Researchers have found that doping Pr and Nd in La-based manganites causes a larger lattice distortion, which results in the weakness of double-exchange interactions. Hence, the magnetic properties and the MCE of these compounds are sensitive to structural changes [7]. Horng et al. [21] studied the magnetic and magnetoresistive properties of La0.7–xPrxPb0.3MnO3 perovskite polycrystalline and reported that the substitution of La3+ by Pr3+ leads to a structural change from rhombohedral to orthorhombic and the repression of ferromagnetism. FM-PM and metal–insulator transitions are affected by the Pr concentration and the magnetic order shows an incomplete magnetic saturation and varies from the FM long-range order to the short-range one. In addition, there is a decrease in the metal–insulator (MI) transition temperature TP with an increase in the magnetoresistance ratio with Pr doping.
One of the objectives of this study was to synthesize (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) nanoparticles perovskites at low temperatures using sol–gel method. We also studied the influence of the substitution La3+ by Pr3+ on the crystal structure, morphology, magnetic properties, and magnetocaloric effect of (La1–xPrx)0.7Sr0.3MnO3 compounds. X-ray diffraction (XRD) was used to investigate the formation of the samples and their crystal structure parameters. The surface morphology was examined using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). X-ray photoemission spectroscopy (XPS) was employed to determine the oxidization state of the elements and confirmed the existence of the mixed valence of Mn3+ and Mn4+. The magnetic properties of our samples were investigated using a Quantum Design Vibrating Sample Magnetometer (VSM) part of the Physical Property Measurement System (PPMS).
2 Experimental
2.1 Samples preparation
The polycrystalline (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) samples were synthesized by the sol–gel procedure followed by annealing (Fig. 1). In this method, an appropriate stoichiometric amount of lanthanum (III) nitrate hexahydrate (La (NO3)3.6H2O) (99.99%, Alfa Aesar), praseodymium (III) nitrate hexahydrate (Pr(NO3)3.6H2O) (99.99%, Alfa Aesar), strontium nitrate (Sr(NO3)2) (99.97%, Alfa Aesar) and manganese(II) nitrate tetrahydrate (Mn(NO3)2.4H2O) (98%, Alfa Aesar) was dissolved in 100 mL deionized water using the ultra-sonic bath for 5 min. The solution was then placed in a beaker and stirred continuously for 15 min (500 rpm) on a magnetic stirrer. In another process, the desired amount of Citric acid (CA) C6H8O7 (99.5 + %, Alfa Aesar) and ethylene glycol (EG) HOCH2CH2OH (99%, Alfa Aesar) were dissolved in 100 mL deionized water using the ultra-sonic bath for 5 min and then added to the nitrate solution forming in a total of 200 mL solvent. The citric acid was used as a complexation agent, while ethylene glycol was used as a polymerization agent. The ratio of citric acid to ethylene glycol to total metal nitrates was 4:2:1. Ammonium hydroxide NH4OH (24% NH3 in H2O, Fluka) was added slowly to the mixture to adjust the pH value to 8. Then the solution was stirred constantly and set for evaporation slowly at 90–95 °C for about 90 min till a viscous wet gel was formed. The formed gel was dried in an oven at 160 °C for 5 h and then calcinated at 400 °C for 4 h to form brown ash. Finally, the ash was grinded in an agate mortar and annealed in a furnace at 700 °C for 5 h to form the desired phase of (La1–xPrx)0.7Sr0.3MnO3.
3 Characterizations
The XRD patterns in this study were obtained using a Panalytical X-Ray diffractometer (Model: X’Pert Pr) equipped with a Cu-Kα (λ = 1.5405 Å) radiation. The XRD patterns were obtained at 2θ angular ranging 20° – 80° with the scanned steps of 0.0167° and the counting time of 1 s per step. The spectra obtained were analysed using Rietveld refinement methods. The program used is MAUD (Material Analysis Using Diffraction) version (2.93), which can provide many information about the sample such as: lattice parameters, cell volume, preferred orientation, micro-strain, and crystallite size. The morphology and structure of the samples were analysed using a JEOL (1200EX) TEM operating at an accelerating voltage of 120 kV.
ImageJ software was used to estimate the average particle size of all the powdered samples. The structure and elemental distribution of the samples were characterized using a field emission scanning electron microscope FESEM (JSM-7800F JOEL, Japan) equipped with an energy dispersive spectrometer (EDS) at an accelerating voltage of 15 kV and a working distance of 10 mm. The FTIR measurements were carried out using a Perkin-Elmer spectrometer (Perkin-Elmer spectrum BX, Germany) through a wavenumber ranging from 4000 to 400 cm−1 with the background correction and the resolution of 4 cm−1. XPS measurements were performed using a Scienta Omicron multiprobe photoelectron spectroscopy system with Al Kα radiation of hν = 1486.6 eV (under an operating vacuum of 10–9 mbar) to confirm the presence of the different elements and to analyse the chemical states of the desired elements. The C 1 s peak with the binding energy of 284.6 eV was used as a reference to calibrate the binding energies of the XPS spectrums. Magnetic measurements were analysed with Quantum Design PPMS DynaCool magnetometer (Quantum Design PPMS-VSM-USA) in applied magnetic fields from −9 T to + 9 T and temperature range 1.9–400 K. MCE parameters were calculated from the magnetization measurements versus the magnetic applied field at several temperatures using Maxwell relation.
4 Results and discussion
4.1 Structure and morphology
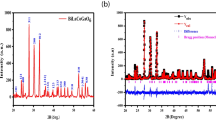
The rietveld refinement of the powder X-ray diffraction data of (La1–xPrx)0.7Sr0.3MnO3 perovskite obtained at room temperature was performed for all the samples, where Fig. 2 illustrates the refinement of the compounds with x = 0.2 and 0.8 as a representative for the samples. The XRD spectra show that all the samples are single-phase perovskite structure with no impurity was observed. The reflection peaks are broad, which indicates that the materials are nanocrystalline in nature. The results obtained from the best refinement evaluated from the goodness of the χ factor are summarized in Table 1. The XRD data for x = 0.0, 0.2 and 0.4 matched the ICSD file 164470 indicating rhombohedral perovskite phase (space group R-3c No.167) and the peaks are indexed according to this structure [18, 26,27,28]. In this setting (La3+, Sr2+, Pr3+) are positioned at 6a (0, 0, 0.5), Mn3+ at 6b (0, 0, 0), and O2− at 18e (x, 0, 0.75). For x = 0.6, 0.8 and 1.0 the XRD data matched the ICSD file168694 indicating orthorhombic perovskite phase (space group Pbnm No.62) and the peaks are indexed according to this structure [3, 29]. In this setting (La3+, Sr2+, Pr3+) are positioned at 4c (x, y, 0.25), Mn3+ at 4b (0.5, 0, 0), O1 at 4c (x, y, 0.25) and O2− at 8d (x, y, z). It can be seen from Fig. 2 that for the sample with x = 0.8 there are extra peaks labelled with (*), which do not belong to the R-3c structure indicating the formation of Pbnm structure, these peaks start to appear at x = 0.6. Figure 3 shows a zoomed region for the angles between 45° and 60° for the two representative samples (x = 0.2 and 0.8), where the extra peaks indicated by the (*) are part of the Pbnm structure. It is worth mentioning here that, for the R-3c structure the different atoms are in hexagonal (h) setting while for the Pbnm structure they are in orthorhombic (o) setting. The volume per formula unit is calculated by dividing the unit cell volume by six for the R-3c structure and by four for the Pbnm structure. For the samples with x = 0.0 and 1.0, the lattice parameters values and the unit cell volumes are almost in agreement with the previously reported values of a = b = 5.4843 nm, c = 13.3306 nm and V = 347.23 Å3 for x = 0.0 [14], and a = 5.4598 nm, b = 5.4681 nm, c = 7.7416 nm and V = 231.1 Å3 for x = 1.0 [30].
Figure 4a, b shows that the lattice parameters a and b and the volume per formula unit of the materials decreased as Pr concentration increased. This is due to the partial substitution of La3+ of a higher ionic radius (1.216 Ả) with Pr3+ of a lower ionic radius (1.179 Ả) [24, 25]. In addition, it can be observed from Table 1 that the average A-site radius \(\langle {r}_{A}\rangle\) of the compounds decreases with the increase in the Pr concentration. The average A-site ionic radius were calculated using 12 coordinated ionic radii for the R-3c structure and nine coordinated ionic radii for the orthorhombic structure given by Shannon [31].
The FESEM images for the (La1–xPrx)0.7Sr0.3MnO3 nanoparticles are represented in Fig. 5a–f. The images show that all the samples consist of agglomerated or interconnected nanoparticles with an anisotropic shape. The SEM micrographs confirm that the average grains distribution remains in the nanometric regime.
The compositional analysis for all the samples was performed using the EDXS and Fig. 6 represents the results obtained for the samples with x = 0.2 and 0.8. The EDXS spectra display the peaks corresponding to La, Pr, Sr, Mn, and O elements, which verifies the presence of these elements in the prepared samples with no additional impurity peaks indicating the purity of the prepared samples. The estimated elemental compositions of the (La1–xPrx)0.7Sr0.3MnO3 samples obtained from the EDXS measurements are illustrated in Table 2. The EDXS estimated ratios of La, Pr, and Sr in the samples are very close to their nominal values within the experimental limits.
The TEM images of the polycrystalline (La1–xPrx)0.7Sr0.3MnO3 are shown in Fig. 7 and the inset indicates the particle size distribution. The TEM images confirm that all the samples are nanoparticles with isotropic shapes of different sizes and present as aggregate fashion. The particle agglomeration could be due to an excess of alcohol used in the preparations of the samples for TEM measurements as reported by Kong et al. [32]. In addition, particle clustering is common in magnetic perovskite nanograins. This could be due to the magnetic dipole–dipole interactions, which cause the nanoparticles to aggregate [33]. Table 3 represents the average particle size (DTEM) calculated from the TEM results and the average crystallite size (DXRD) obtained from the XRD pattern using Scherrer’s equation for all the samples. The average particle size is in the range of 21–44 nm while the average crystallite size is in the range of 22–28 nm. It can be seen from the table that DTEM and DXRD for samples with x = 0.0, 0.6 and 0.8 almost the same within the error for each one indicating that each particle consists of one crystallized grain. On the other hand, the DTEM for samples with x = 0.2, 0.4 and 1.0 are larger than their DXRD indicating that each particle consists of several crystallized grains. It can be seen from Table 3 that La0.7Sr0.3MnO3 (x = 0.0) with the rhombohedral structure has the smallest average particle size while Pr0.7Sr0.3MnO3 (x = 1.0) with the orthorhombic structure has the largest average particle size. The increase in the average particle size as the Pr content increases indicates that substitution of Pr in the La sites improves the grain growth.
Figure 8 shows the FTIR spectra of the (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) compounds. The MnO6 in the manganite structure has an octahedral symmetry with six vibration modes only two of them are IR active at around 400 and 600 cm−1 [19, 26]. The absorption dips observed at the band 425–471 cm−1 are attributed to the bending mode, which is due to the change in Mn–O-Mn bond angle [34]. The absorption dips that occurred in the range of 603–634 cm−1 correspond to the stretching mode of the Mn–O-Mn and Mn–O bonds [26, 35]. They revealed the presence of the MnO6 octahedron in all the samples [36,37,38,39]. The appearance of the bending and stretching modes in the spectra indicates the formation of the perovskite structure, which is in an agreement with the results obtained from the XRD measurements [19, 26, 34, 40, 41]. The two bands ranging between 634 and 425 cm−1 are attributed to metal (M)-oxide (O) bonds where the highest one (603–634 cm−1) attributed to M–O (M = La, Pr, Sr and Mn) stretching vibrations and the lower one at (425–471 cm−1) represents M–O-M Bending vibrations [42,43,44]. It can be seen from the spectra that the absorption bands at (603–634 cm−1) shifted to lower frequency as Pr content increased, indicating the substitution of La3+ ion by Pr3+ ion in the lattice A-site. The shift indicates there is a change in the bond length and the volume of the lattice. Moreover, this shift is due to the change in the lattice constant as reported by Hameda et al. [45]. The FT-IR spectra show that the C = O and C-O stretching vibration band of COOH groups are at 1042 to 1053 cm−1 [46]. The absorption bands at 1384–1386 cm−1 correspond to the asymmetric stretching vibrations of carbonyl group COO− [19], while the dips around 1627–1636 cm−1 assigned to vibrations of H2O absorbed at the surface or to the asymmetric stretching vibrations of C = C bond and symmetric stretching of C = O bond of the COOH groups [33, 38, 47]. The absorption band at 2920 cm−1 is assigned to the symmetric and asymmetric stretching of the CH2 group [38, 47, 48]. In addition, the strong absorption dips observed at the band 3401–3436 cm−1 are attributed to the hydroxyl (O–H) stretching vibrations of H2O molecule absorbed at the surface [34, 47]. The results of FTIR measurements for the whole range are summarized in Table 4.
5 XPS results
X-ray photoelectron spectroscopy (XPS) analysis of the (La1–xPrx)0.7Sr0.3MnO3 was performed to evaluate and investigate the chemical compositions, the ionic valence states, and the cation distribution of the elements. The XPS survey spectra of the (La1–xPrx)0.7Sr0.3MnO3 perovskites are presented in Fig. 9. The XPS survey scan reveals the emissions corresponding to La 3d, Pr 3d, Sr 3d, Mn 2p, and O 1 s XPS peaks, indicating the existence of La, Pr, Sr, Mn, and O elements in the (La1–xPrx)0.7Sr0.3MnO3 nanoparticles. The survey shows small traces of adventitious carbon (C 1 s XPS peak), which is probably came from the surface contamination while samples were exposed to the ambient [52].
It is clear from Fig. 9 that as the Pr concentration increases the intensity of the peaks associated to La decrease, while the peaks associated to Pr increase confirming the substitution of La by Pr.
The XPS spectra for La 3d, Pr 3d, Sr 3d, and Mn 2p were further analysed to their individual components using CasaXPS software. A Gaussian–Lorentzian function in a fixed ratio (GL(30)) was used to fit the deconvoluted peaks for each element with the Shirley function for background subtraction. The calibration of the binding energies was performed with respect to adventitious C 1 s binding energy (284.6 eV).
Figure 10 shows the high-resolution core level XPS spectra of selected orbitals in the (La1–xPrx)0.7Sr0.3MnO3 perovskite. It can be seen from Fig. 10a that the La 3d are split into two lines (La 3d5/2 and La 3d3/2) due to spin–orbit interaction and each line is split into two due to a transfer of an electron from oxygen ligands to the La 4f [53, 54]. The double peak structure of the La 3d5/2 and La 3d3/2 orbitals reflects states with the configuration 3d94f0L and 3d94f1L (L attributed to oxygen ligand while L attributed to a hole) [54, 55]. There is no substantial shift but a slight shifting of BE values of the La 3d was observed due to the progressive substitution of La by Pr and as expected, the intensity of the peaks decreases as Pr concentration increases. The binding energies positions of La 3d5/2 (4f0) and La 3d3/2 (4f1) orbitals are found to be at 834.0 eV and 850.8 eV respectively confirming the existence of the La3+ oxidation state. The La 3d orbital splitting is approximately 16.8 eV, which is typical of La3+ compounds and is in an agreement with previous reported studies [55,56,57,58]. In addition, the satellite peaks of La 3d5/2 and La 3d3/2 are located at 838.3 eV and 855.3 eV successively, which are attributed to the charge transfer process between the O 2p orbital and the La 4f orbital according to Kamath et al. and Masteralo et al. [56, 59]. The splitting between the two components of La 3d5/2 is determined to be ΔE/eV = 4.3, which is approximately equal to ΔE/eV = 4.6 and it is significant to La2O3 [60]. The La 3d5/2 and La 3d3/2 were further fitted to three components that are shown in Fig. 11a, b for samples with x = 0.2 and 0.8 as representatives for the series and the positions of the peaks are summarized in Table 5. The components with the lower binding energies centred at 833.8 and 850.7 eV were attributed to La3+ ions in oxide states, while the remaining four with high binding energies were attributed to the satellites peaks [61].
Figure 10b shows that as Pr concentration increases, the intensity of the peaks increases while there was no significant shift in the BE values of Pr 3d but a slight shift was observed as Pr gradually replaced La. The binding energies positions of Pr 3d5/2 and Pr 3d3/2 orbitals were estimated to be 933.5 eV and 953.7 eV respectively and separated by 20.2 eV in good agreement with the values reported in the literature [62, 63]. A shoulder is observed at the lower binding energy sides of the Pr 3d5/2 and Pr 3d3/2 peaks and the separation between the shoulder and the main peak is ≈ 5.5 and 4.0 eV respectively. The XPS spectra of Pr 3d are deconvoluted into three peaks as shown in Fig. 12a, b for samples with x = 0.2 and 0.8 as representatives for the series and the binding energies of the XPS spectra are summarized in Table 5. The peaks on the low binding energy sides of Pr 3d5/2 and Pr 3d3/2 are attributed to the shake-off satellite structures of the Pr3d [64, 65]. These shake-off satellites are due to the charge transfer effects between the localized Pr 4f electrons and the O 2p wave functions [64]. Yagoub et al. reported that these satellites peaks occur from a strong mixing of the two final states of 3d94f2 and 3d94f3 [66]. The main peaks with the highest intensity centred at 933.2–933.4 eV for Pr 3d5/2 and 953.5–953.7 eV for Pr 3d3/2 are assigned to Pr3+ in the perovskites [67, 68], while the peak located at the higher BE side of Pr 3d3/2 main peak is caused by the multiplet effect [66].
Figure 10c illustrates the core-level XPS spectra of Sr 3d3/2 and Sr 3d5/2 orbitals. The appearance of the two doublets of Sr is evidence of the substitution of La by Sr in the A sites, as reported by Villa-Bustamante et al. [54]. The binding energies of Sr 3d3/2 and Sr 3d5/2 orbitals can be attributed to the Sr2+ ions in our samples [53, 69]. The spectra were deconvoluted into three components as can be seen in Fig. 13a, b for samples with x = 0.2 and 0.8 as representatives for the series and the peak positions for the three components are represented in Table 6 for all compounds. The binding energies centred at 132.3–132.5 eV are assigned to the Lattice components, while the one centred at 134.0–134.2 eV can be assigned to the surface components, and both attribute to Sr2+ ions [54, 70, 71]. It is believable that the shoulder peak at 135.2 eV has originated from SrO [53, 72].
Core-level XPS spectra of Mn 3p1/2 and Mn 3p3/2 orbitals, are exhibited in Fig. 10d. The binding energies peaks located at 642.2 eV and 654.0 eV can be assigned as Mn 2p3/2 and Mn 2p1/2 orbitals respectively with spin–orbit splitting of 11.72 eV. There are no changes in the intensities of the peaks as the Pr concentration increases. In addition, there is no detectable shift in the positions of the binding energies peaks. This indicates that MnO bonds in La–O-Mn and Pr–O-Mn exhibit similar ionic/covalent bond characteristic as a result of La and Pr possess almost similar electronegativity values, 1.1 and 1.13 consecutively [73]. The Mn 3p1/2 and Mn 3p3/2 XPS peaks are deconvoluted into two fitted peaks, suggesting mixed valency as illustrated in Fig. 14a, b for samples with x = 0.2 and 0.8 as representatives for the series. These peaks correspond to Mn3+ and Mn4+ ions at different binding energies, as illustrated in Table 7. The non-existence of satellite peaks in the fitted spectra verified the absence of any Mn2+ state [74]. Deconvolution of Mn 3p1/2 and Mn 3p3/2 lines was used to calculate the ratio of Mn4+/ Mn3+ (Table 7). It was found that the ratio of Mn4+/Mn3+ remains almost the same, which is consistent with the fact that substitution of Pr3+ for La3+ would not influence the ratio Mn3+/Mn4+ since Pr3+ and La3+ have the same valence. The split of the Mn 3p1/2 and Mn 3p3/2 lines into two peaks is due to the fact that the manganese has two valences (Mn3+ and Mn4+), which result from the Sr2+ doping. This forms the basis of the double exchange interactions (Mn3+–O2−–Mn4+) that give rise to the ferromagnetic ordering in the system [33, 74]. As we can see from the above result, the substitution of La3+ by Pr3+ does not affects the oxidation state of the elements or effect the Mn3+/Mn4+ ratio. However, it can be seen that the intensity of the Pr 3d increases while the intensity of La 3d decreases implying the substitution of Pr in the A site of our perovskite which is in good agreement with the XRD and the FTIR results which show a distortion on the perovskite structure of our samples due to the substitution of La3+ (large ionic size) by Pr3+ (lower ionic size). Hence altering the M–O bond distance and M–O-M angle.
The XPS deconvoluted peaks of C 1 s and O 1 s core levels for sample with x = 0.2 were used as an example for the whole samples in the (La1–xPrx)0.7Sr0.3MnO3 perovskite since all the other compounds exhibited similar results. Figure 15a illustrates the fitting of the C 1 s core-level spectrum for the x = 0.2 sample. Three major components were identified with binding energies at 284.6, 285.6, and 288.8 eV that were imputed to carbon group (C = C/C–C, CHx), hydroxyl groups (− C-OR) and carboxylic groups (− COOR) respectively [35, 46]. The same results were obtained for all the other samples, which disclose the presence of these functional groups on the surface of our samples in good agreement with the results obtained from the FT-IR technique.
Figure 15b illustrates the O 1 s XPS spectrum for the x = 0.2 sample. From the figure, it can be seen that the peaks are broad and asymmetric, and the spectrum is fitted with two components with binding energies centred at 529.3 eV and 530.8 eV, respectively. The peak position at 529.3 eV is attributed to the metal–oxygen bonds (crystal lattice Oxygen) of La–O, Mn–O, and Pr–O bonds in the sample. The binding energy at 530.8 eV is assigned to the absorbed oxygen or hydroxyl group on the surface of the perovskite [53, 57, 62, 75, 76].
6 Magnetic properties
Figure 16 demonstrates the temperature dependence of magnetization (M-T) of (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) compounds measured in field warming (FW) and field cooling (FC) at an applied field of 500 Oe in the range (5–400) K. It can be seen from the figure that FW magnetization increases to maximum and then decreases as temperature increases. It is clear from the figure that all samples exhibited a ferromagnetic (FM)-paramagnetic (PM) transition at Curie temperature (TC). The FW-FC curves display λ-shape traces and the shape of the FC curves discloses the predominant ferromagnetic ordering of the magnetic moment caused by the applied magnetic field during the cooling of the sample [77]. There is a large separation between the two magnetization curves, which occurs below the irreversibility temperatures (Tirr) (temperature where the FW and FC diverge as shown in Fig. 16) that are lower than the phase transition temperatures. This large split between FW and FC curves at low temperatures suggests an inhomogeneous ferromagnetic order [26] and Manh et al. related this split to the presence of strong magnetic anisotropy [18]. From Table 8 it is clear that as the Pr content increases the irreversibility temperatures decrease for the samples with x ≤ 0.4 while it remains almost the same within the experimental error for x ≥ 0.6. As seen from Fig. 16, the FW magnetization increases with the increase in temperature and reaches maximum at Tmax then decreases. It is found from Table 8 that Tmax decreases as Pr content increases and then increases for samples with x = 0.8 and 1.0.
Figure 17 shows that the phase transition temperature (TC) shifts to a lower temperature with the increase of the Pr concentration. The Curie temperature is measured from the FC curve using two different methods. One is by calculating the intercept of the extrapolating of the two far ends and the other is by determine the peak of the dM/dT curve as shown in Fig. 18. The broadening in the dM/dT curve is due to the distribution of the particle size, which results in the difference between (TC)dM/dT) and (TC)extrapolation) where the latter is associated to the Curie temperature when all the particles become paramagnetic. From Table 8 it was found that there is a linear relation between the Tc and the concentration of the dopant, as Pr concentration increases TC decreases linearly, which is in agreement with previous studies [78].
To investigate the origin of the decrease in TC let us note that the overall ratio of trivalent rare earth (La3+, Pr3+) to divalent alkaline earth (Sr2+) is preserved in this series of compounds. Therefore, the decrease of TC with increasing Pr content must be related to changes in structural parameters. Zarifi et al. attributed the decrease in TC to the reduction of Mn–O-Mn bond length and the angle in the doped samples [7]. The lattice distortion induces in MnO6 octahedron, which is caused by replacing La3+ of the higher ionic radius with Pr3+ of the lower ionic radius, will affect the double exchange (DE) mechanism that leads to the weakening of the FM interactions and hence the reduction of TC. Khelifi et al. reported that the decrease in the TC is due to the weakening of the double-exchange interaction, which is related to the decrease in the band width [79].
According to many studies [25, 80, 81], the mismatch in the size of the A-cation (size variance (σ2)) influences the magnetic properties of the material. This A-cation size disorder (σ2) is defined as [80]:
where xi is the fractional occupancy of A-site ions, ri is the corresponding ionic radii and \(\langle {r}_{A}\rangle\) is the weighted average radius calculated from the ri values. Using Eq. 1 the calculated values of σ2 are presented in Table 1. The substitution La3+ by Pr3+ leads to a decrease in the average A-site cationic radius and an increase in σ2. This enhancement in σ2 results in a distortion of MnO6 octahedra which causes a decrease in TC as a result of the weakening of the double-exchange interaction.
The La0.7Sr0.3MnO3 sample (x = 0) undergoes FM-PM transition at TC = 358 K, which is almost close to previous values by other researchers [75, 82]. The La0.7Sr0.3MnO3 samples with x = 0.6 and 0.8 have a phase transition temperature close to the room temperature (313 K and 291 K respectively), which makes them a good candidate in terms of the MCE in cooling systems.
Figure 19 represents M versus T curves of (La1–xPrx)0.7Sr0.3MnO3 for x = 0.0 and 1.0 upon warming and cooling in a magnetic field of 0.05 T and 2 T at a temperature range of 5–400 K. As the magnetic field increases, the transition becomes broader and the phase transition temperature shifts to a higher value. The bifurcation between the two curves decreases and disappears at high magnetic fields. All the other samples showed similar behaviour with different magnetization values and transition temperature limits.
Figure 20 illustrates the isothermal magnetization as a function of the applied field for (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) series. As can be seen from Fig. 20, as the temperature decreases the magnetization increases sharply with the applied field signifying the existence of FM behaviour. At high temperatures, the magnetization decreases to a very small value at which the compound behaves like a paramagnetic material. Therefore, it is clear from Fig. 20 that as the temperature increases, there is a steadily decrease in the magnetization, which is caused by the thermal agitation that disturbs the oriented spins leading to an increase in the system entropy according to Villa-Bustamante et al. [54]. Absolute magnetic saturation for all compounds is not attained even at a 9 T magnetic field. This is an intrinsic property of magnetic arrangement in nanoparticles, where FM core is surrounded by so-called magnetically dead layer [83].
Figure 21 represents the magnetization (M) versus the magnetic field (H) for (La1–xPrx)0.7Sr0.3MnO3 compounds (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) at 5 K and 80 K where the inset shows the enlarged view of the middle part of the hysteresis loop. Zhou et al. reported that the substitution of a rare-earth cation having a 4f n configuration with a large orbital angular momentum might increase the coercivity (Hc). Pr 3+ ion with 4 f 2 configuration is considered the largest rare-earth ion, and the Pr4+/Pr3+ redox energy is almost close to the Fermi energy allowing it to influence the magnetic behaviour of the MnO3 array. From Fig. 22a, it is observed that the coercivity at 5 K and 80 K increases as size variance (σ2) increases, which is in agreement with the literature. The rate of increase in the coercivity as function of size variance (σ2) is higher in the Pbnm structure than the R-3c structure. In addition, it was found that the remnant magnetization Mr (for both structures) tends to increase with Pr contents as illustrated in Fig. 22b. Also, the magnetic coercivity (Hc) is found to be small (~ 10 Oe) below TC for each sample. The small values of the coercivity disclose the soft magnetic properties of (La1–xPrx)0.7Sr0.3MnO3, which are preferable for magnetic refrigeration applications.
The saturation magnetization (MS) was estimated by extrapolating linearly the magnetization at high field to zero field. The MS for the undoped sample (x = 0.0) is found to be 81.8 emu/g, which is approximately close to the reported value of 86.67 emu/g for La0.7Sr0.3MnO3 prepared by Manh et al. [18]. It is noted from Fig. 23 that the magnetization (MS) for the samples having orthorhombic structure (x = 0.6, 0.8, and 1.0) increases with Pr content reaching a maximum value of 94.0 emu/g at 5 K for x = 1.0. On the other hand, for the ones with rhombohedral structure (x = 0.0, 0.2, and 0.4) the maximum (MS) is 87.2 emu/g at 5 K is achieved at x = 0.2 as shown in Fig. 23. Hence, the variation in the MS is small for the R-3c structure while it is large for the Pbnm structure as a function of Pr concentration. The exchange bias field HEB of our compounds is very small (−10 to −20 Oe) and negative indicates the strong FM interactions.
To have more insight into the magnetic behaviour of the compounds in the PM region, a deeper study was performed on the inverse magnetic susceptibility dependence on temperature. Figure 24 shows the inverse of the DC magnetic susceptibility (χ−1) versus temperature for (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) series at Happ = 50 Oe. In the PM region, the χ−1 exhibits a linear T dependence and it is usually described by the Curie–Weiss law, which is defined as [84]:
where θP is Paramagnetic Curie temperature (PCT) and C is Curie constant, which is equal to
where kB = 1.38016 × 10–23 J/K is the Boltzman constant and µeff is the effective paramagnetic moment [84]. Therefore, by fitting the PM region (the high temperature linear part of χ-1 versus T), the experimental effective magnetic moment µeff and θP were determined for all compounds.
The temperature dependence of the inverse magnetic susceptibility for (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and1.0) compounds, which provides a further understanding of the magnetic behaviour in the PM region. The solid lines represent the linear fit for the linear part of the curves in PM region
The theoretical (expected) effective magnetic moment \({\mu }_{eff}^{th}\) for (La1–xPrx)0.7Sr0.3MnO3 compounds can be determined using the equation
where µeff (Pr3+) = 3.58 µB, µeff (Mn3+) = 4.90 µB and µeff (Mn4+) = 3.87 µB [85, 86].
Table 9 summarises the magnetic parameters calculated from the fitting of the PM region (the high temperature of χ-1 (T) of our compounds). The experimental effective moment (\({\mu }_{eff}^{exp}\)) for the compounds increases with increasing the Pr concentration (where the rate of increase for the samples with the Pbnm structures is higher than the ones with the R-3c structure) in the compound as shown in Fig. 25, which is in an agreement with the trend of the calculated effective moment. The positive values of θp confirm the existence of a ferromagnetic exchange interaction between the nearest neighbours [87]. From Table 9 it can be observed that θp decreases as Pr content increases, which indicates the weakening of FM interactions. The obtained values are slightly less than TC for samples with rhombohedral structure while a difference of approximately 20 K is noticed for the samples with the orthorhombic structure. It can be concluded that these differences depend on the substance and is related to the presence of short-range magnetic order confirming the presence of a magnetic inhomogeneity [88].
To study the effect of Pr substitution on the magnetocaloric effect of our series and to determine its parameters, the isothermal magnetizations versus the applied magnetic field (M-H) under external magnetic fields vary from 0 to 9 T were measured at different temperatures below and above the transition temperature in the step of 5 K (Fig. 26). The curves show a regular increase of the magnetization without saturation even at a very high field (9 T) for all concentrations. In the higher temperature range (PM range above TC) the magnetization decreases almost linearly with the applied magnetic field, while at a lower temperature FM dependence emerges.
In order to investigate the nature of the magnetic transition in our samples, the Arrott plots obtained from plotting M2 versus H/M are represented in Fig. 27. Banerjee proposed that if there are negative slopes in Arrott plots, the transition is of first order phase transition while Arrott plots of a second order phase transition have positive slopes. Hence, it can be observed from Fig. 27 that all curves possess positive slopes around the TC indicating that the FM to PM transition is of second order for all compounds. The second order magnetic transition is a favorite for active magnetic refrigeration than the first order magnetic transition, which is escorted by hysteretic losses [24].
The magnetocaloric effect is a phenomenon in which magnetic energy is converted to thermal energy [79]. It is defined as the adiabatic temperature change ΔTad or the isothermal entropy change Δsiso due to the application of the magnetic field H at constant pressure [89]. Based on the classical thermodynamic theory and using the magnetic entropy changes ΔSM can be calculated using the following Maxwell’s equation [90]:
where M is the magnetization of the material, H is the applied magnetic field and T is the temperature of the system. The equation can be estimated as follows [90]:
where Mi+1 and Mi are the experimental values of magnetization at Ti+1 and Ti respectively, under a magnetic field Hi.
In addition to ΔSM, the relative cooling power (RCP) is an important parameter in the practical design of magnetic refrigerants. RCP is defined as the measure of the quantity of heat transferred by the magnetic refrigerants to the surrounding [7]. Hence, it is used to evaluate the magnetic cooling efficiency of our samples. Based on the values of ΔSM, the relative cooling power (RCP) is determined using the following equation [7]:
where ΔTFWHM is the full width at half maximum of \({\left|\Delta {S}_{M}\right|}_{max}\) curve (ΔTFWHM = T2 – T1).
Figure 28 shows the temperature dependence of the magnetic entropy changes (−ΔSM) at ΔµoH from 0–9 T for (La1–xPrx)0.7Sr0.3MnO3 perovskites. The magnitude of the magnetic entropy changes increases with increasing magnetic field and attains its highest values near the magnetic phase transition temperatures (TC). One can observe from Fig. 28 that (−ΔSM) peaks are broadened and this broadening is attributed to the enhancement of the temperature range of the heat transfer between the hot and the cold sides leading to the increase of the RCP. The peak position of (−ΔSM) almost does not change and this is due to the second order nature of the FM transition in the compound and the broadening in the (−ΔSM) peaks is a character of the second-order magnetic transition. A series of magnetocaloric materials, which have similar RCP can be used to synthesize a refrigerator that can to function in a large temperature range. The extracted values of the maximum magnetic entropy change (−ΔSM)max, the relative cooling power (RCP), and the temperature position (Tp) of the peak value of (−ΔSM)max for all the samples under a change in the applied magnetic fields of 5 and 9 T are summarized in Table 10.
Figure 29 represents the maximum of the magnitude of the magnetic entropy change (−ΔSM)max and the relative cooling power (RCP) as a function of the change in the applied magnetic field for all samples. It can be seen from the figure that (−ΔSM)max and RCP rise up as the change in the applied magnetic field increases. There is an almost linear increase in (−ΔSM)max values as the change in the applied magnetic field increases for all compounds and the two lines of samples x = 0.2 and x = 0.1 overlap together indicating that both samples have approximately the same (−ΔSM)max values. As illustrated in Fig. 29, there is almost linear increase in RCP values as the change in the applied magnetic field increases for all compounds with Pr0.7Sr0.3MnO3 (x = 1.0) having the largest RCP values compare to the other compounds. In general, with their highest (−ΔSM)max and RCP values these materials can be useful for magnetic refrigeration applications near room temperature.
Table 11 discloses that the compounds under investigation have ((TC)extrapolation) ranges from 358 to 275 K and our values for (−ΔSM)max and RCP at room temperature have high values compared to other compounds. In addition, the compounds with TC below room temperature have even higher values of (−ΔSM)max and RCP compared with the compound with magnetic phase transition near room temperature. Therefore, the compounds may be considered as good refrigerant materials at room temperature or in the vicinity of room temperature.
7 Conclusion
The sol–gel method followed by sintering is a useful technique for preparing perovskites at low temperatures. Single-Phase (La1–xPrx)0.7Sr0.3MnO3 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) perovskites were synthesized at 700 °C for 5 h. The doping of trivalent rare-earth ions (Pr) with lower ionic size into La sites in manganite series can control the structure, magnetic, and magnetocaloric properties of the compounds. Samples with x = 0.0, 0.2 and 0.4 formed a single-phase rhombohedral structure with R-3c space group while the ones with x = 0.6, 0.8 and 1.0 crystallized into orthorhombic structure with Pbnm space group. The lattice parameters a and b are found to decrease as Pr concentration increases and there is a slight decrease in the unit cell volume of the compounds. From XRD analysis, one can conclude that a crystal structure change from rhombohedral to orthorhombic structure, helped to decrease the magnetic transition temperature toward room temperature and increase the effective magnetic moment. The EDXS spectra verify the presence of La, Pr, Sr, Mn, and O elements with no extra peaks indicating the purity of the samples. The XPS measurement reveals the existence of a mixed valence state of Mn4+/Mn3+ and the ratio of Mn4+/Mn3+ remains almost the same. In addition, the oxidation states of Pr and La are 3 + and it is 2 + and 2- for Sr and O respectively. All samples undergo a second order ferromagnetic to paramagnetic phase transition near TC. The Tc is above room temperature in La0.7Sr0.3MnO3 and drops linearly as Pr concentration increase reaching a value lower than room temperature in Pr0.7Sr0.3MnO3. For each sample, the magnetic coercivity (Hc) below TC is found to be small (~ 10 Oe) while the blocking temperature (TB) decreases with increasing the Pr concentration. It was found that the maximum value of magnitude of the magnetic entropy change and the relative cooling power (RCP) for the samples having orthorhombic structure (x = 0.6, 0.8 and 1.0) increases with Pr content reaching a maximum value of 4.67 J/kg.K and 574 J/kg at ΔµoH = 9 T for x = 1.0. On the other hand, the ones with rhombohedral structure (x = 0.0, 0.2 and 0.4) have a maximum (−ΔSM)max and RCP of 4.63 J/kg.K and 472 J/kg at ΔµoH = 9 T for x = 0.2. The experimental effective paramagnetic moment (µeff) has been found to increase with increasing the Pr concentration from 3.99 µB for x = 0.0 to 5.05 µB for x = 1.0, which is in an agreement with the trend of the calculated effective paramagnetic moment (4.62 µB for x = 0.0 to 5.50 µB for x = 1.0) calculated using the free ions moments for Pr3+, Mn3+, and Mn4+. The present study indicates that the substitution of La3+by Pr3+ in the A-site of (La1–xPrx)0.7Sr0.3MnO3 helps to tune the TC towards room temperature with a significant magnetic entropy change and large RCP values making the compounds potential candidates for room temperature magnetic refrigeration applications.
References
Phan M-H, Yu S-C (2007) Review of the magnetocaloric effect in manganite materials. J Magn Magn Mater 308(2):325–340
Thaljaoui R, Boujelben W, Pękała M, Szydłowska J, Cheikhrouhou A (2012) Effect of sol-gel method on structural and electron magnetic resonance properties of Pr0.6Sr0.4MnO3manganite. EPJ Web Conf 29:50
Mleiki A, Othmani S, Cheikhrouhou-Koubaa W, Koubaa M, Cheikhrouhou A, Hlil EK (2015) Effect of praseodymium doping on the structural, magnetic and magnetocaloric properties of Sm0.55−xPrxSr0.45MnO3 (0.1⩽x⩽0.4) manganites. J Alloy Compd 645:559–565
Barik SK, Krishnamoorthi C, Mahendiran R (2011) Effect of Fe substitution on magnetocaloric effect in La0.7Sr0.3Mn1−xFexO3 (0.05≤x≤0.20). J Magn Magn Mater 323(7):1015–1021
Ben Jazia Kharrat A, Bourouina M, Chniba-Boudjada N, Boujelben W (2019) Critical behaviour of Pr0.5–xGdxSr0.5MnO3 (0≤x≤0.1) manganite compounds: correlation between experimental and theoretical considerations. Solid State Sci 87:27–38
Franco V, Conde A (2010) Scaling laws for the magnetocaloric effect in second order phase transitions: From physics to applications for the characterization of materials. Int J Refrig 33(3):465–473
Zarifi M, Kameli P, Ehsani MH, Ahmadvand H, Salamati H (2017) Effects of rare earth ions substitution on the magnetocaloric and critical behavior of La0.6A0.2Sr0.2MnO3 (A=Pr, Nd, Ce) manganite. J Alloy Compd 718:443–452
Solanki S, Dhruv D, Boricha H, Zankat A, Rathod KN, Rajyaguru B, Trivedi RK, Joshi AD, Mukherjee S, Solanki PS, Shah NA (2020) Charge transport mechanisms and magnetoresistance behavior of La0.6Pr01Ca0.3MnO.3 manganite. J Solid State Chem 288:121446
Cheng ZX, Zhen HF, Li AH, Wang XL, Kimura H (2005) CMR La0.7Ca0.3MnO3 and La0.7Sr0.3MnO3 thin films fabricated by sol-gel method. J Cryst Growth 275(1–2):e2415–e2419
Ezaami A, Sfifir I, Cheikhrouhou-Koubaa W, Koubaa M, Cheikhrouhou A (2017) Critical properties in La0.7Ca0.2Sr0.1MnO3 manganite: a comparison between sol-gel and solid state process. J Alloy Compd 693:658–666
Tozri A, Khelifi J, Dhahri E, Hlil EK (2015) Influence of Pr-doping on magnetic phase transition and magnetocaloric effect of La0.7−xPrxBa0.3MnO3 manganite. Mater Chem Phys 149–150:728–733
Sekrafi HE, Ben Jazia Kharrat A, Chniba-Boudjada N, Boujelben W (2020) Impact of B-site doping on magnetic and magnetocaloric effect of Pr0.75Bi0.05Sr0.1Ba0.1Mn1–xTixO3 (0≤x≤0.04) manganites. Solid State Sci 105:106274
Giri SK, Dasgupta P, Poddar A, Nigam AK, Nath TK (2014) Field induced ferromagnetic phase transition and large magnetocaloric effect in Sm0.55Sr0.45MnO3 phase separated manganites. J Alloy Compd 582:609–616
Kumar D, Verma NK, Singh CB, Singh AK (2018) Crystallite size strain analysis of nanocrystalline La0.7Sr0.3MnO3 perovskite by Williamson-Hall plot method
Qixiang S, Guiying W, Guoqing Y, Qiang M, Wenqi W, Zhensheng P (2008) Influence of the substitution of Sm, Gd, and Dy for La in La0.7Sr0.3MnO3 on its magnetic and electric properties and strengthening effect on room-temperature CMR. J Rare Earths 26:821–826
Zhao WHSBC, Ma YQ, Zhang RL, Yang J, Sheng ZG, Lu WJ, Dai JM, Sun YP (2005) Magnetic and transport properties of the Co-doped manganite La0.7Sr0.3Mn1–xCoxO3 (0 ≤x ≤ 0.5). Phys Status Solidi (b) 242(8):1719–1727
Seo YJ, Kim GW, Sung CH, Lee CG, Koo BH (2011) Magnetic and electrical transport properties on (La0.7Sr0.3MnO3)1–x/(CuFe2O4)xcomposites. J Phys Conf Ser 266:012106
Manh DH, Phong PT, Nam PH, Tung DK, Phuc NX, Lee I-J (2014) Structural and magnetic study of La0.7Sr0.3MnO3 nanoparticles and AC magnetic heating characteristics for hyperthermia applications. Phys B 444:94–102
Ravi S, Karthikeyan A (2014) Effect of calcination temperature on La0.7Sr0.3MnO3 nanoparticles synthesized with modified sol-gel route. Phys Procedia 54:45–54
Shlapa Y, Solopan S, Belous A, Tovstolytkin A (2018) Effect of synthesis method of La1 – xSrxMnO3 manganite nanoparticles on their properties. Nanoscale Res Lett 13(1):13
Horng L, Kang PC, Young SL (2003) Synthesis characterization of magnetotransport properties in Pr-doped La0.7Pb0.3MnO3 Compounds. Japanese J Appl Phys 42(Part 1, No. 5A):2685–2689
Barik SK, Mahendiran R (2010) Effect of Bi doping on magnetic and magnetocaloric properties of La0.7−xBixSr0.3MnO3 (0≤x≤0.4). J Appl Phys 107(9):093906
Rößler S, Nair HS, Rößler UK, Kumar CMN, Elizabeth S, Wirth S (2011) Ferromagnetic transition and specific heat of Pr0.6Sr0.4MnO3. Phys Rev B 84(18):184422
Zhang Y, Lampen PJ, Phan T-L, Yu S-C, Srikanth H, Phan M-H (2012) Tunable magnetocaloric effect near room temperature in La0.7–xPrxSr0.3MnO3 (0.02 ≤ x ≤ 0.30) manganites. J Appl Phys 111(6):063918
Zhou JP, McDevitt JT, Zhou JS, Yin HQ, Goodenough JB, Gim Y, Jia QX (1999) Effect of tolerance factor and local distortion on magnetic properties of the perovskite manganites. Appl Phys Lett 75(8):1146–1148
Moradi J, Ghazi ME, Ehsani MH, Kameli P (2014) Structural and magnetic characterization of La0.8Sr0.2MnO3 nanoparticles prepared via a facile microwave-assisted method. J Solid State Chem 215:1–7
Zhou Y, Zhu X, Li S (2017) Structure, magnetic, electrical transport and magnetoresistance properties of La0.67Sr0.33Mn1−xFexO3 (x=0–0.15) doped manganite coatings. Ceram Int 43(4):3679–3687
Ashutosh Kumar HS, Tomy CV, Thakur AD (2016) Extreme sensitivity of magnetic properties on the synthesis routes in La0.7Sr0.3MnO3. AIP Conf Proc 1728(1):20494
Mleiki A, Othmani S, Cheikhrouhou-Koubaa W, Cheikhrouhou A, Hlil EK (2016) Normal and inverse magnetocaloric effect and short-range ferromagnetic interaction in (Pr, Sm)0.5Sr0.5MnO3 phase separated manganite. J Alloy Compd 688:1214–1224
Kostogloudis GC, Vasilakos N, Ftikos C (1997) Preparation and characterization of Pr1–xSrxMnO3±8 (x = 0, 0.15, 0.3, 0.4, 0.5) as a potential sofc cathode material operating at intermediate temperatures (500–700°C). J Eur Ceram Soc 17:1513–1521
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767
Kong LB, Zhu W, Tan OK (2000) Preparation and characterization of Pb(Zr0.52Ti0.48)O3 ceramics from high-energy ball milling powders. Materials Lett 42:232–239
Kumar S, Dwivedi GD, Kumar S, Mathur RB, Saxena U, Ghosh AK, Joshi AG, Yang HD, Chatterjee S (2015) Structural, transport, and optical properties of (La0.6Pr0.4)0.65Ca0.35MnO3 nanocrystals: a wide band-gap magnetic semiconductor. Dalton Trans 44(7):3109–3117
Soleymani M, Edrissi M, Alizadeh AM (2015) Thermosensitive polymer-coated La0.73Sr0.27MnO3 nanoparticles: potential applications in cancer hyperthermia therapy and magnetically activated drug delivery systems. Polym J 47(12):797–801
Zheng M, Zhang H, Gong X, Xu R, Xiao Y, Dong H, Liu X, Liu Y (2013) A simple additive-free approach for the synthesis of uniform manganese monoxide nanorods with large specific surface area. Nanoscale Res Lett 8(1):166
McBride K, Cook J, Gray S, Felton S, Stella L, Poulidi D (2016) Evaluation of La1−xSrxMnO3 (0 ≤ x < 0.4) synthesised via a modified sol–gel method as mediators for magnetic fluid hyperthermia. CrystEngComm 18(3):407–416
Wenwei W, Jinchao C, Xuehang W, Sen L, Kaituo W, Lin T (2013) Nanocrystalline LaMnO3 preparation and kinetics of crystallization process. Adv Powder Technol 24(1):154–159
Abdel-Latif IA, Ismail AA, Bouzid H, Al-Hajry A (2015) Synthesis of novel perovskite crystal structure phase of strontium doped rare earth manganites using sol gel method. J Magn Magn Mater 393:233–238
McBride K, Partridge N, Bennington-Gray S, Felton S, Stella L, Poulidi D (2017) Synthesis, characterisation and study of magnetocaloric effects (enhanced and reduced) in manganate perovskites. Mater Res Bull 88:69–77
Rostamnejadi A, Salamati H, Kameli P (2009) Particle size effect on magnetic properties of interacting La0.67Sr0.33MnO3 nanoparticles. cond-mat.mtrl-sci, p 26
Chen Y, Wang Y, Wang P, Ma T, Wang T (2018) Hyperthermia properties of hyaluronic acid-coated La0.7Sr0.3–xBaxMnO3 nanoparticles. J Mater Chem B 6(14):2126–2133
Çoban Özkan D, Türk A, Celik E (2021) Synthesis and characterizations of LaMnO3 perovskite powders using sol–gel method. J Mater Sci Mater Electronics 32(11):15544–15562
Sajawal A, Ishfaq M, Murtaza G, Habib I, Muhammad N, Sharif S (2018) Half metallic ferromagnetism in PrMnO3 orthorhombic stable phase: an experimental and theoretical investigation. Mater Res Exp 5(11):116103
Gómez-Cuaspud JA, Valencia-Ríos JS, Carda-Castelló JB (2010) Preparation and characterization of perovskite oxides by polymerization-combustion. J Chil Chem Soc 55(4):445–449
Hemeda OM, Barakat MM, Hemeda DM (2003) Structural, electrical and spectral studies on double rare-earth orthoferrites La1−xNdxFeO3. Turk J Phys 27:537–549
Han T, Wei Y, Jin X, Jiu H, Zhang L, Sun Y, Tian J, Shang R, Hang D, Zhao R (2019) Hydrothermal self-assembly of α-Fe2O3 nanorings@graphene aerogel composites for enhanced Li storage performance. J Mater Sci 54(9):7119–7130
Rajan A, Sharma M, Sahu NK (2020) Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci Rep 10(1):15
Joshi A, Datar S, Kale SN (2017) Observation of magnetism in La0.7Sr0.3MnO3—graphene nanoribbons complex: a probable magnetoelectronic material study. Mater Res Exp 4(7):075050
Thorat ND, Otari SV, Bohara RA, Yadav HM, Khot VM, Salunkhe AB, Phadatare MR, Prasad AIR, Ningthoujam S, Pawar SH (2014) Structured superparamagnetic nanoparticles for high performance mediator of magnetic fluid hyperthermia: synthesis, colloidal stability and biocompatibility evaluation. Mater Sci Eng C Mater Biol Appl 42:637–646
Lv H, Zhao H, Cao T, Qian L, Wang Y, Zhao G (2015) Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J Mol Catal A Chem 400:81–89
Gholamrezaei S, Salavati-Niasari M (2018) Sonochemical synthesis of SrMnO3 nanoparticles as an efficient and new catalyst for O2 evolution from water splitting reaction. Ultrason Sonochem 40(Pt A):651–663
Xia W, Wu H, Xue P, Zhu X (2018) Microstructural, magnetic, and optical properties of Pr-doped perovskite manganite La0.67Ca0.33MnO3 nanoparticles synthesized via sol-gel process. Nanoscale Res Lett 13(1):13
Wu Q-H, Liu M, Jaegermann W (2005) X-ray photoelectron spectroscopy of La0.5Sr0.5MnO3. Mater Lett 59(16):1980–1983
Villa-Bustamante RA, Jativa JA, Ospina R, Londono-Menjura RF, Jurado JF, Quintero JH, Restrepo-Parra E, Mello A (2018) Thermal treatment influence on structural, morphological and magnetic properties of La1–xSrxMnO3 powders produced by the hydrothermal technique. Contemp Eng Sci 11(81):4021–4039
Popescu DG, Barrett N, Chirila C, Pasuk I, Husanu MA (2015) Influence of hole depletion and depolarizing field on the BaTiO3/La0.6Sr0.4MnO3 interface electronic structure revealed by photoelectron spectroscopy and first-principles calculations. Phys Rev B 92(23):235442
Gunasekaran N, Rajadurai S, Carberry JJ, Bakshi N, andAlcock, C.B., (1994) Surface characterization and catalytic properties of La1–xAxMO, perovskite type oxides. Part I. Studies on La0.95Ba0.05M03 (M = Mn, Fe or Co) oxides. Solid State Ionics 73:289–295
Blackmore RH, Rivas ME, Tierney GF, Mohammed KMH, Decarolis D, Hayama S, Venturini F, Held G, Arrigo R, Amboage M, Hellier P, Lynch E, Amri M, Casavola M, Eralp Erden T, Collier P, Wells PP (2020) The electronic structure, surface properties, and in situ N2O decomposition of mechanochemically synthesised LaMnO3. Phys Chem Chem Phys 22(34):18774–18787
Beyreuther E, Grafström S, Eng LM, Thiele C, Dörr K (2006) XPS investigation of Mn valence in lanthanum manganite thin films under variation of oxygen content. Phys Rev B 73(15):9
Mastelaro VR, Lisboa-Filho PN, Neves PP, Schreiner WH, Nascente PAP, Eiras JA (2007) X-ray photoelectron spectroscopy study on sintered Pb1−xLaxTiO3 ferroelectric ceramics. J Electron Spectrosc Relat Phenom 156–158:476–481
(08–06–2022). Lanthanum X-ray photoelectron spectra, lanthanum electron configuration, and other elemental information. Available: https://www.thermofisher.com/om/en/home/materials-science/learning-center/periodic-table/lanthanide-rare-earth/lanthanum.htmlLanthanum
Hao P, Song P, Yang Z, Wang Q (2020) Porous LaFeO3 microspheres decorated with Au nanoparticles for superior formaldehyde gas-sensing performances. J Mater Sci Mater Electron 31(6):4632–4641
Jiang N, Zhou X, Jiang Y-F, Zhao Z-W, Ma L-B, Shen C-C, Liu Y-N, Yuan C-Z, Sahar S, Xu A-W (2018) Oxygen deficient Pr6O11 nanorod supported palladium nanoparticles: highly active nanocatalysts for styrene and 4-nitrophenol hydrogenation reactions. RSC Adv 8(31):17504–17510
Golden TD, Shang Y, Wang Q, Zhou T (2015) Electrochemical synthesis of rare earth ceramic oxide coatings. Adv Ceramic Process
Schroeder T, Costina I, Storck P, Wilke A, Seifarth O, Giussani A, Mussig HJ, Zaumseil P (2008) Lattice engineering of dielectric heterostructures on Si by isomorphic oxide-on-oxide epitaxy. J Appl Phys 103(8):084102
Mekki A, Ziq KA, Holland D, McConville CF (2003) Magnetic properties of praseodymium ions in Na2O–Pr2O3–SiO2 glasses. J Magn Magn Mater 260(1–2):60–69
Yagoub MYA, Swart HC, Bergman P, Coetsee E (2016) Enhanced Pr3+ photoluminescence by energy transfer in SrF2: Eu2+, Pr3+ phosphor. AIP Adv 6(2):025204
Glisenti A, Natile MM, Galenda A (2009) PrMnO3 prepared by the citrate gel method, studied by XPS. Surf Sci Spectra 16(1):67–74
Shamshi Hassan M, Shaheer Akhtar M, Shim KB, Yang OB (2010) Morphological and electrochemical properties of crystalline praseodymium oxide nanorods. Nanoscale Res Lett 5(4):735–740
Wang C, Jiang SP (2015) Mechanism of SO2 poisoning on the electrochemical activity of LSCF and LSM electrodes. ECS Trans 68(1):1023–1029
Lee JWHW, Chen Y, Cai Z, Yildiz B (2013) Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J Am Chem Soc 135(21):7909–7925
Liu Y, Zhou F, Chen X, Wang C, Zhong S (2021) Enhanced electrochemical activity and stability of LSCF cathodes by Mo doping for intermediate temperature solid oxide fuel cells. J Appl Electrochem 51(3):425–433
Opitz CRAK, Kubicek M, Rupp GM, Nenning A, Gotsch T, Blume R, Havecker M, Knop-Gericke A, Rupprechter G, Klotzer B, Fleig J (2018) The chemical evolution of the La0.6Sr0.4CoO3-delta surface under SOFC operating conditions and its implications for electrochemical oxygen exchange activity. Top Catal 61(20):2129–2141
Lee H-S, Choi SG, Yeom GY, Park H-H (2014) The effect of Gd substitution in perovskite lanthanum strontium manganite films for use in resistive switching devices. J Ceram Soc Jpn 122(1428):P8-1-P8-4
Ghorai S, Skini R, Hedlund D, Strom P, Svedlindh P (2020) Field induced crossover in critical behaviour and direct measurement of the magnetocaloric properties of La0.4Pr0.3Ca0.1Sr0.2MnO3. Sci Rep 10(1):19485
Deshmukh AV, Patil SI, Bhagat SM, Sagdeo PR, Choudhary RJ, Phase DM (2009) Effect of iron doping on electrical, electronic, and magnetic properties of La0.7Sr0.3MnO3. J Phys D Appl Phys 42(18):185410
Wenger JDCh, Zaumseil P, Sorge R, Formanek P, Lippert G, Müssig HJ (2004) First investigation of metal–insulator–metal (MIM) capacitor using Pr2O3 dielectrics. Mater Sci Semicond Process 7(4–6):227–230
Thaljaoui WBR, Pękała M, Pękała K, Fagnard JF, Vanderbemden P, Donten M, Cheikhrouhou A (2014) Magnetocaloric effect of monovalent K doped manganites Pr0.6Sr0.4−xKxMnO3 (x=0 to 0.2). J Magn Magn Mater 352:6–12
Xingrui Pu HL, Dong G, Chu K, Zhang S, Liu Y, Xiaohan Yu, Liu X (2020) Electrical transport properties of (Pr1–xLax )0.7Sr0.3MnO3 (0 ≤ x ≤ 0.3) polycrystalline ceramics prepared by sol-gel process for potential room temperature bolometer use. Ceram Int 46(4):4984–4991
Khelifi J, Tozri A, Dhahri E, Hlil EK (2014) Influence of Pr-doped manganite on critical behavior of La0.7−xPrxBa0.3MnO3 (x=0.00, 0.1, 0.2). J Magn Magn Mater 349:149–155
Oumezzine M, Sales HB, Selmi A, Hlil EK (2019) Pr3+ doping at the A-site of La0.67Ba0.33MnO3 nanocrystalline material: assessment of the relationship between structural and physical properties and Bean-Rodbell model simulation of disorder effects. RSC Adv 9(44):25627–25637
Vanitha AAPV, Santhosh PN, Rao CNR (2000) Effect of cation size and disorder on the structure and properties of the rare earth cobaltates, Ln0.5A0.5CoO3 (Ln ) Rare Earth, A ) Sr, Ba). Chem Mater 12:1666–1670
Cherif R, Hlil EK, Ellouze M, Elhalouani F, Obbade S (2015) Critical phenomena in La0.6Pr0.1Sr0.3MnO3 perovskite manganese oxide. J Solid State Chem 229:26–31
Jirák Z, Hirschner J, Kaman O, Knížek K, Levinský P, Maryško M, Hejtmánek J (2017) Structure and transport properties of La1−xSrxMnO3granular ceramics. J Phys D Applied Phys 50(7):075001
Mazumdar D, Das K, Sen P, Das I (2020) Impact of weak ferromagnetism on the magnetocaloric properties of A-site-doped PrMnO3 compound. J Mater Sci Mater Electron 31(14):11714–11719
Skini R, Ghorai S, Ström P, Ivanov S, Primetzhofer D, Svedlindh P (2020) Large room temperature relative cooling power in La0.5Pr0.2Ca0.1Sr0.2MnO3. J Alloys Comp 827:154292
Dhahri A, Jemmali M, Dhahri E, Valente MA (2015) Structural characterization, magnetic, magnetocaloric properties and phenomenological model in manganite La0.75Sr0.1Ca0.15 MnO3 compound. J Alloy Compd 638:221–227
Bouzaiene E, Dhahri AH, Dhahri J, Hlil EK, Bajahzar A (2019) Effect of A-site-substitution on structural, magnetic and magnetocaloric properties in La0.7Sr0.3Mn0. 0.1O3 manganite. J Mag Magnet Mater 491:165540
Cherif R, Hlil EK, Ellouze M, Elhalouani F, Obbade S (2014) Magnetic and magnetocaloric properties of La0.6Pr0.1Sr0.3Mn1−xFexO3 (0≤x≤0.3) manganites. J Solid State Chem 215:271–276
Basso V (2013) Basics of the magnetocaloric effect. cond-mat. matrl-Sci
Fan J, Pi L, Zhang L, Tong W, Ling L, Hong B, Shi Y, Zhang W, Lu D, Zhang Y (2011) Magnetic and magnetocaloric properties of perovskite manganite Pr0.55Sr0.45MnO3. Phys B: Condensed Matter 406(11):2289–2292
Daivajna MD, Rao A (2016) Magnetocaloric effect in pristine and Bi-doped Pr0.6Sr0.4MnO3 manganite. Solid State Commun 245:65–69
Kallel N, Kallel S, Hagaza A, Oumezzine M (2009) Magnetocaloric properties in the Cr-doped La0.7Sr0.3MnO3 manganites. Phys B 404(2):285–288
Banik S, Das K, Das I (2019) Enhancement of magnetoresistance and magnetocaloric effect at room temperature in polycrystalline Pr0.8–xLaxSr0.2MnO3 (x = 0.2) compound. J Mag Magnet Mater 490:165443
Acknowledgements
We would like to thank the following from Sultan Qaboos University: Mr. Abdul Rahman Al- Nabhani from the Electron Microscopy Unit in the College of Medicine and Health Sciences for the help in TEM measurements, Dr. Htet Htet Kyaw from the Nano-center at for the FT -IR measurements, and the CAARU team for the XRD measurements.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Shahumi, T.M., Al-Omari, I.A., Al-Harthi, S.H. et al. Synthesis, structure, morphology, magnetism, and magnetocaloric-effect studies of (La1−xPrx)0.7Sr0.3MnO3 nanocrystalline perovskites. SN Appl. Sci. 5, 121 (2023). https://doi.org/10.1007/s42452-023-05328-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05328-5