Abstract
Based on weekly sampling, the present short-term study was set out over a one-year cycle in the Eastern Harbor (Alexandria, Egypt). This study aims to assess water quality monitoring data. Among the 13 indexes used in the present study to define the water quality in the harbor, 6 depend on physical and chemical variables, and the other 6 are biological criteria. Chlorophyll concentrations and phytoplankton cell numbers were also used as indicators for assessing trophic status. The study indicated that water status mesotrophic and poor-bad classes are the major trend in the harbor, but oligotrophic condition dominates in winter, and the eutrophic status and poor class dominate in autumn. Looking for suitable tools to assess the water status in such a highly dynamic marine basin is not a simple task, and identification of a common quality status relying on a single assessment approach is hard to realize. Despite the consensus between some approaches, significant discrepancies were observed in the classification results on temporal scale, and for the inability of some indices to sort all types of water bodies in the harbor. The study might represent an integral part of holistic ecosystem-based management of coastal waters.
Highlights
-
1.
Diatoms are prevailing in harsh dynamic ecosystem
-
2.
Skeletonema costatum is known as an opportunistic species
-
3.
Blooms of Chattonella antiquaoffer evidence as effective biological elements to eutrophication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Eastern Harbor (EH) is among the most important regions over the world from a historical point of view, due to the thousands of ancient Egyptian artifacts found at its bottom. Monitoring and assessment of water quality is a crucial sustainability issue for surface water, [1]. Pollution by numerous anthropogenic chemical and pollutant may not only degrade these ecosystems but also threat public health [2]. Water quality is defined as the suitability of water to sustain various uses or processes [3]. Water trophic conditions vary considerably from region to region [4]. Generally, oligotrophy means nutrient poor (low productivity) and eutrophic means nutrient rich (high productivity) waters. However, few studies are reported about the boundaries between these categories [5]. Regarding the EH’s diversity, Zaghloul [6] investigated the phytoplankton structure, dynamics, and species diversity throughout three separate years in the basin. In addition, its correlation with some environmental parameters. Water quality index (WQI) is a mathematical tool used to resolve the composite influence of large multi-parameters of water quality into single digit scores that simplify the expression of water quality status [7]. It is useful to facilitate information to the public and legislative decision makers for the establishment of a common reference framework for different water bodies [8]. In spite of the efforts done and continuous discussion on indices being used globally, it is extremely difficult to develop a universally acceptable general water quality index [9]. The temporal differences in water quality parameters are critical in recognizing the main contributors in assessment [10].
The present study is the first short-term study to measurements of Secchi disk, nutrient concentrations, and different phytoplankton attributes (floristic composition, blooms, abundance, and biomass), attempts to assess water quality (WQ) status in the harbor. To achieve the purpose, different water quality indexes were also applied. Other goals seek knowledge on the interpretation of interactions of various ecological stressors, the reliance of these methods under different local environmental conditions, and to evaluate their comparative performance. The study, finally, aims to facilitate monitoring the health of the harbor waters, aid in designing specific pollution prevention programs, reduce the uncertainty in regulation and management, and increase the effectiveness of the decisions adopted by managers and policy makers.
2 Materials and methods
2.1 Study area
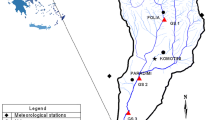
The Eastern Harbor of Alexandria City (long. 29°53ʹ–29°54ʹ-E and lat. 31°12ʹ–31°13ʹ-N, Fig. 1), is a relatively shallow, sheltered, and semi-enclosed marine basin (area 2.53 Km2, water volume 15.2 × l06 m3, average depth 6.5 m). The harbor throughout water exchange with the adjacent Mediterranean Sea receives supply of water masses mainly affected by the inflow from Qaitbay sewer outlet located at its western vicinity. Its western part represents another direct source of pollution as being a site for anchoring fishing vessels and human activities.
2.2 Sampling and measurements
Weekly sampling during a year cycle (2018–2019) was conducted at a fixed station (4 m depth, Fig. 1). The surface water temperature and salinity were measured in field using the water checked physical parameter device HANA, Model HI 9828, and the water transparency by a Secchi disc. Water samples for the chemical analyses were filtered and kept at − 4 °C. The determination of NO3, NO2, NH4, PO4, SiO4 concentrations followed Parsons [11], Oxidizable Organic Matter (OOM) according to FAO, [11], and chlorophyll a, (Chl. a) by Jeffrey and Humphrey, [12] method. Living subsamples were first examined by an inverted microscope for identification of delicate flagellates, and the water samples were then preserved (Ligoul’s acid solution), and counted [13] after sedimentation [14]. The identification followed mainly the taxonomic guidelines of Tomas [15].
2.3 Water quality indices
Different Water Quality Indexs (WQIs) were applied to assess the water quality status: Shannon’s Index [16]; the diversity index was calculated based on the relative degree of dominance of 32 major contributory species using PRIMER software (Ver. 6.1), applying the formula: Diversity Index H′ = Σ (ni/N log2(ni/N)), wherein, ni is individual sum of i-th species, N is individual sum of all species; the Palmer pollution index [17] was used to assess the harbor's water quality; the 20 algae species that are most resistant to organic pollution: Euglena viridis > Nitzschia palea > Oscillatoria limosa > Scenedesmus quadricauda > Oscillatoria tenuis > Stigeoclonium tenue > Synedra ulna > Ankistrodesmus falcatus > Pandorina morum > Oscillatoria chlorina have tolerance (pollution index) of 6,5,4,4,3,3,3,3,3 respectively; phytoplankton abundance criteria [18] with the criteria as: up to 6 × 103cell−1 for oligotrophic waters, 6 × 103–1.5 × 105 cells l−1 for mesotrophic waters and > 1.5 × 105 cells l−1 for eutrophic waters; the Secchi disk measurements [19,20,21,22] is used to determine the trophic status of surface waters by using the following equation K = l.7/depth; The boundary values of quality classes for nutrient variables [23], [19] and the European Union Water Framework WFD [24], [25] as illustrated in Table 1; the eutrophication scale [26] modified by Simboura [27]; the Estuarine Trophic Status, ASSETS [21]; the Eutrophication Index (E. I.) based on Chl. a and nutrient contents, and the phytoplankton elements-based assessment have been used to develop multi-metric or univariate classification scale for eutrophication assessment tools [28,29,30]. The eutrophication scale is divided into the categories: High quality system (< 0.1 μg l−1), Good or oligotrophic (0.1—0.4 μg l−1), Moderate or mesotrophic (0.4—0.6 μg l−1), Poor (0.6—2.21 μg l−1) and Bad (> 2.21 μg l−1).
The diatom/dinoflagellate index was calculated within the scope of the Marine Strategy Framework Directive MSFD, [31]. The water bodies with algal Shannon-Weiner index are classified following the scale of Wilm and Dorris, [16].
3 Statistical analyses
Pearsons [32] correlation analysis was applied to determine the relation for each pair of the measured water physical, chemical variables and biotic components and different indices.
4 Results and discussion
It is beyond the scope of this paper to discuss other fundamental factors not mentioned in the present article as critical aspects influencing the water quality status. Among the 13 approaches used to assess the water quality status, and as indicators of pollution in the harbor, six different indices rely on physical and chemical variables, and the others are based on biological criteria. Furthermore, basic phytoplankton parameters were used to evaluate and track trends in eutrophication.
Water turbidity affects the clarity of the water [31], and the layer of photic zone that extends here between 1.35 and 9 m, mostly deeper in winter. The Secchi disk measurements (mean 2.1 ± 0.66 m) never fall below the threshold < 0.5 m, which describes the water eutrophic [18] and as being > 0.5 m indicates “Good” water quality [21]. According to the standards of Bricker [20], [21], 96% of the Secchi disk readings lie in the range of 1–3 m, indicating medium turbidity. The Secchi disk measurement, despite being an easy, inexpensive way that simplifies and makes comparison between different temporal trophic situations of a marine system more consistent, is a rather coarse method to determine the trophic status of surface waters. Secchi readings seem to be affected significantly by NH4 (r = − 78, P < 0.01) in winter, and with Chl. a (r = − 0.23, p < 0.05), while the very weak, unexpected negative correlation found with OOM levels (r = − 0.04) and the phytoplankton abundance (r = − 0.14) proves its uncertain evaluation. The inverse relationship with Chl. a is in agreement with other records [33]. However, the proven strong relationship between eutrophication and accelerated organic matter [18] should specify notably this relation as an integral part of holistic ecosystem-based management of coastal seas. Thus, the determination of the general specific status of the harbour water cannot rely on solo Secchi measurements, particularly in estuaries, and coastal eutrophic areas.
According to the Water Framework Directive (WFD, 2000/60/EC) [22] commits to European Union (EU) regulations, data of the concentrations of phosphorus, nitrogen, nutrients ratio, salinity, pH, the Secchi depth, and chlorophyll a are the basic quality elements for the evaluation of the ecological status of the water bodies. The present nutrient concentrations were regularly disturbed by escalating external and internal input of nutrients from anthropogenic activities, nutrient accumulation and retention affected by episodic storm events, and from degradation after algal overgrowth. The TN values (mean 1.17 ± 0.47 µM l−1, range 0.42–3.03 µM l−1) compared with the depleted PO4 (93.88% < 1 µgl−1) affected the stoichiometric ratio, particularly in the autumn (1.44–32.55). The negative significant link between N/P, N/Si and salinity (r = − 0.32 and − 0.48, p < 0.05) signals the influence of discharged water input as a cause of eutrophication [34]. Based on the nutrient concentrations of the European Union Water Framework (WFD) criteria, and according to the boundary values, the discriminant of the water quality classes showed different status (high H-good G—medium M—poor P—bad B) at temporal scale (Table 1). Although this technique is not a computational tool for scaling trophic status, it can characterize water masses by establishing a hierarchy based on variables describing eutrophication [26]. This scaling needs testing and possible adaptations to real conditions [25]. The (H/G) water quality seems to rely on N concentrations, rather than P concentrations, and the concentrations of NH4 seem to have effect on both (G/M), and (M/P). The P levels seem responsible for water degradation in the harbor. The results indicated mesotrophic condition, with no particular temporal trend to be the major, oligotrophic condition dominates in winter, and the eutrophic status accompanied the bloom periods in autumn, spring and summer. The P levels seem responsible for water degradation in the harbour. On contradictory, the other hypothesis assumes different results as the oligotrophic condition in winter, the dominance of eutrophic condition but mostly during the bloom periods in autumn, spring and summer, and the mesotrophic condition to occupy the second contributory level, with no particular temporal trend. This classification seems logic as linked with the variation in phytoplankton abundance.
The pollution index score of Palmer [17], has been assigned to each of the 20 types of algae that are most tolerant to organic pollution. The score is evidence of high organic pollution when the pollution index score is 20 or more, 15–19 probable organic pollution, and 0–1 indicates a lack of organic pollution. The present calculated pollution index score was 26; the score is evidence of high organic pollution. The study includes nine genera from the 20 genera in the list of Palmer [17].
Chlorophyll a concentration as a usual cause of practical problems resulting from eutrophication has been widely utilized to assess the status of aquatic ecosystems and possible human impacts [35]. Based on the (ASSETS) methodology applied comparatively to rank the temporal changes in the eutrophication status of the harbour and based on Chl. a thresholds (µg l−1), the calculated results are given in Table 2. The application of the ASSETS methodology may be applied comparatively to rank the temporal changes in the eutrophication status of estuaries and coastal areas [21], into the four categories: hypereutrophic, high, moderate and low, where moderate ranges between 5–20 μg l−1 and low 0–5 μg l−1.
The major fraction of the trophic status (65.31%) falls within the low status category, and the moderate status is ranked second. The low eutrophic condition was a permanent characteristic feature in winter when all the values of Chl. A were lower than the threshold of 5 μg l−1. The status changed in spring as influenced by the minor spring bloom when moderate status existed by 25%. The maximum value of Chl. A in summer (16.75 μg l−1) accompanying proliferation or blooming of phytoplankton rendered the water into of moderate status most of the time. The effect of high Chl. A in autumn, particularly by the end of September (12.69 μg l−1) was also clear, but, the low status category dominated.
According to the classification scale ranges of Karydis, [26] and Simboura [27] the calculated data illustrated almost similar results with the dominance of the poor and bad status (65.31%), particularly in spring and summer, while the high-moderate status was of insignificant appearance (collectively, 16.3%). Thus, the classification is based on Chl. a data goes in parallel with the above approach as declaring the bad condition in the harbor.
The calculated data following the assessment of trophic status proposed by Primpas [29] classified the water quality into two categories, declaring the E. I. a fundamental index that affects the ecological evaluation of water quality [36]. The calculated E. I. values higher than 1.5 (89.8%, 4 out of 49 observations) and the 5 observations (10.2%) between 0.85 and 1.5, were classified as “affected by eutrophication”, explaining the harbor waters of “Poor”− “Bad” classes. The poor status appeared scattered, but mostly was registered to late autumn-early winter (Fig. 2). The E.I. values varied seasonally (average 2.49 ± 0.80), with the highest accompanying the massive blooms. It appears that the ranges of E. I. tool scales do not properly sort oligotrophic and mesotrophic water bodies, and no “Good” or “High” class level of water was calculated. The high TN (total nitrogen) compared with the depleted PO4 as previously mentioned was a reasonable factor that might explain the observed status, similar to that reported in the NE Mediterranean waters [37]. There was a strong relation of E. I. with salinity and Ph (r = − 0.54 and 0.47, p < 0.05), while both temperature ( +) and transparency (−) seems to affect the eutrophic status of the harbor to a much lesser degree (Fig. 2). These factors are considered primary determinants of the seasonal dynamics of eutrophication level [36]. The calculated data of the E. I. illustrates the high to moderate quality status (oligotrophic-mesotrophic) to be of insignificant appearance. Seasonally the values were relatively lower in winter (average 2.12), and were almost similar during the other seasons. As the nutrient and Chl. a concentrations exhibited significant temporal variations, it is hard to define boundary values discriminating the trophic levels in the harbor.
5 Assessing trophic status using phytoplankton metrics
Phytoplankton, due to its fast population responses to changes in water quality, hydrology, or climate [38], is employed as an indicator of changes in nutrient loads and a key element for assessing eutrophication and quality status in aquatic systems [25], has been also defined in the Water Framework Directive among the biological quality elements for the assessment of the ecological status of water bodies. Different phytoplankton attributes are essential to assess the ecological status [30]; [39]. Since each single phytoplankton metric shows a different response to different pressure and can provide different information on the condition of the aquatic ecosystem, numerous authors have underlined the importance of combining different phytoplankton metrics under the framework of integrative quality assessment to develop robust tools [33]. While metrics based on phytoplankton quantity and production are used indicators of eutrophication in the status assessments of surface waters [40], there is no much work on eutrophication scaling based on phytoplankton cell numbers. However, in published work on spatial analysis it was found that cell number was an efficient variable in studying spatial trends [41, 42].
However, not much work on eutrophication scaling based on phytoplankton cell numbers was conducted. Testing hypothesis [18] for phytoplankton abundance, the oligotrophic condition was scarcely detected (7 observations, 14.28%), restricted mainly to winter, while, the eutrophic condition dominated (24 observations, 48.98%), but mostly during the bloom periods in autumn, spring, and summer. The mesotrophic condition significantly contributed 36.73% (18 observations), without any particular seasonal trend. The results are consistent with that of Chl. a. Shannon’s index is often used to assess water quality in many aquatic ecosystems, with special relevance to coastal marine eutrophication [43]. It is often used in descriptive studies to quantify community diversity, and as a useful indicator for assessing water quality in many aquatic ecosystems, with special relevance to coastal marine eutrophication [43]. Usually, H′ is calculated only from biological taxonomic variables without direct linkage to abiotic variables or functional variables; however, anthropogenic impacts affect both taxonomic and functional diversity [44]. However, several authors pointed out that Shannon’s index does not seem to be appropriate for eutrophication studies for different reasons [45], [46]. The values of H' during a year cycle are shown in Fig. 3. Based on the criteria of Wilm and Dorris [16] the computed index H' on mean values ranged between 0.89 ± 0.4 bits ind−1 in summer and 1.16 ± 0.47 bits ind−1 in fall, while, winter and spring displayed similar patterns (around 0.97 bits ind−1). The values were categorized as 14.5% were < 0.5 bits ind−1, 38.77% between 0.5–1 bits ind−1; were both classified as heavily polluted, and 46.9% of > 1 < 2 bits ind−1 indicating moderate pollution. In contrast, the calculated H' following the criteria of Sun and Liu, [47] did not recognize moderate pollution status, and confirmed the previous results as heaviest pollution representing 46.94% (maximum in winter), and high pollution (53.27%, maximum in summer). Since, different environmental factors driven by natural and human-induced stressors were involved in the temporal variation of H' (Table 3), the assessment of water quality in the harbor seems to be a more complicated problem, and consequently, no specific controlling factor/s could be defined.
The water quality assessment is based on other phytoplankton attributes such as structure, diversity of taxa from different functional groups, variability in dominance, indicator and alien species, frequency, and the intensity of phytoplankton blooms. These attributes constituted an excellent baseline to understand the ecology, water quality status, and degree of eutrophication of the harbor ecosystem, consistent with other observations [48].
The large abundance (mean 227.8 × 103 unit l−1) and number of the recorded diatom species (108 taxa) prevailing in this harsh dynamic ecosystem, their weekly succession, and quick response to abrupt environmental changes offered an advantage to diatoms as valuable indicators of ecological quality [49]. Several recorded diatom species, such as the centric prominent Skeletonema costatum known opportunistically, can benefit from increased nutrients to flourish, indicating signs of eutrophication [39]. This species formed several bloom events in the harbor as previously reported elsewhere under eutrophication conditions [50]. The increased number and recurrent temporal occurrence of the bioindicators; Bacteriastrum spp., Chaetoceros spp., Leptocylindrus spp., Rhisosolenia spp., Cyclotella spp. Pseudonitzschia spp. Thalassionema nithzschioides, and Thalassiosira spp. indicated changeable nutrient loads, the eutrophication process, and organic pollution [40].
The temporal changes in the diatom:flagellate ratio, particularly within the bloom periods, served as a potential indicator of eutrophication of worsening environmental status as declared by several authors [31], [41]. Mikhail and Halim [5], offered supporting evidence in the harbor where the progressive long-term increase in anthropogenic nutrients resulted in a decreasing of diatom/flagellate ratio, and recurrent flagellate blooms. This alternative index was able to identify the regime shift occurred sometimes as on 21 September (the ratio at 0. 56), 15 May and 16 June (0.64 and 0.67). However, such reduced ratios did not necessarily mean much lower abundance of diatoms, but could also rely on the sharing of other dominant forms. While the diatom/dinoflagellate ratio was suggested as a pre-core indicator [10], the role of changeable N/P ratio during the present study as a driving factor for community shift is difficult to establish. The study included 28 potentially harmful/toxic dinoflagellates species considered as indicative of eutrophic conditions [42]; among them Alexandrium catenella, A. minutum, Gymnodinium catenatum, Heterocapsa triquetra and Protoperidinium quinquecorne were of noticeable occurrences; the latter species inhabits eutrophic and polluted brackish nutrient-rich environments of organic and inorganic forms [51].
The predominance of diatoms all year round over dinoflagellates detected applying the diatoms/dinoflagellates index [52] was a pre-core indicator for the assessments of the environmental status, offering evidence for good environmental status. According to the index, diatoms dominate the community at the index value > 0.5, and dinoflagellates at < 0.5. However, factors as the sharing of other dominant forms rather than diatoms, and the changes in the nutrient concentrations and stoichiometric composition could modify the relative proportion [53]. The study confirmed the quick changes in species composition i.e. the predominance of a certain species could be replaced by another in a couple of days, signaling symptoms of eutrophication [34]. The predominance of the chlorophycean picoplankton Micromonas pusilla over diatoms, mostly during its bloom periods in early autumn and summer offers an example. These species were previously recorded as dominant in eutrophic waters [54].
The massive blooms of the two raphidophytes species Chattonella antiqua and Heterosigma akashiwo might offer extra evidence as effective biological elements in evaluating responses to eutrophication since close connection of H. akashiwo blooming was found with increased load of DIN and DIP in agreement with others [55]. The present-recorded bloom periods of different species from different classes and of different magnitudes provided important indicators of degraded water quality [56]. The major part of Chl. a production occurred with the massive summer phytoplankton bloom representing a strong indicator of heavy eutrophication and trophic state [56].
The present occurrence of the well-diversified freshwater species of chlorophytes (10 taxa, 5.92% of the total species number), cyanophytes (8 species, 4.73%), euglenophytes (4 species, 2.37%), and raphidophytes (2 species, 1.18%) is considered one of the reliable levels of biological organization that possess many attributes to assess the ecological status of a water body, and represent a useful biological indicator [57]. According to Garcia and Bonel [44], the mere presence of these species is an indication of poor water quality and low salinity. The occurrence of Oscillatoria tenuis and O. limosa was reported to be associated with pollutants of biological origin [58], Euglena species with organically polluted water [59], and the presence of Scenedesmus and Anabaena with nutrient enrichment [52].
The present appearance, even in low counts of species that were previously registered aliens of recent introduction in Alexandria coastal waters during the last two decades [53], might offer extra evidence as effective biological elements in evaluating responses to eutrophication [41]. These species were the diatom Pseudonitzschia pungens, the dinoflagellates Alexandrium ostenfeldii, A. catenella, Katenella mikimotoi, G. catenetum, Oestriopsis cf. ovata and the rapidophycean species H. akashiwo and C. antiqua.
6 Conclusions
Looking for suitable tools to assess water quality in the harbor is not a simple task, and it is hard to rely on specific criteria to identify a common quality status. In this study, phytoplankton is employed as an indicator of changes in nutrient loads and a key element for assessing eutrophication and quality status in aquatic systems, which in turn offers full understanding of the water quality status in the harbor. Mesotrophic and Poor”- “Bad” classes are the major trend in the harbor, but oligotrophic conditions dominates in winter, and eutrophic status and poor class dominate in the autumn. Diatom species are prevailing in this harsh dynamic ecosystem, the harbor includes nine genera of the 20 genera in the list of phytoplankton tolerant to organic pollution and 28 potentially harmful/toxic dinoflagellate species considered as indicative of eutrophic conditions. The study also confirmed that The increased number and recurrent temporal occurrence of; Bacteriastrum spp., Chaetoceros spp., Leptocylindrus spp., Rhisosolenia spp., Cyclotella spp. Pseudonitzschia spp. Thalassionema nithzschioides, and Thalassiosira spp. are considered as bioindicators of changeable nutrient loads, eutrophication process, and organic pollution. Hence, we can assess the state of water quality through biological indicators that in turn contribute to coastal management. The importance of the study is that it is one of the most important short-term scales for identifying potential trends in the degradation or improvement of coastal environments.
References
Ustaoğlu F, Tepe Y, Taş B (2020) Assessment of stream quality and health risk in a subtropical Turkey river system: a combined approach using statistical analysis and water quality index. Ecol Ind 113:105815
Egbueri JC (2022) Predicting and analysing the quality of water resources for industrial purposes using integrated data-intelligent algorithms. Groundw Sustain Dev 18:100794
Nasrollahzadeh Saravi H, Pourang N, Foong SY, Makhlough A (2019) Eutrophication and trophic status using different indices: a study in the Iranian coastal waters of the Caspian Sea. Iran J Fish Sci 18(3):531–546
Karydis M, Kitsiou D (2013) Marine water quality monitoring: a review. Mar Pollut bull 77(1–2):23–36. https://doi.org/10.1016/j.marpolbul.2013.09.012
Mikhail SK, Halim Y (2009) Phytoplankton diversity and limiting resources in a semi-enclosed marine ecosystem (Alexandria, Egypt). Egypt J Aquat Res 35(1):131–147
Zaghloul FA (1995) Comparative study of phytoplankton production, composition and diversity index in the eutrophic eastern harbour of Alexandria Egypt. J High Inst Public Health 25(3):665–678
Agbasi JC, Egbueri JC (2022) Assessment of PTEs in water resources by integrating HHRISK code, water quality indices, multivariate statistics, and ANNs. Geocarto Int. https://doi.org/10.1080/10106049.2022.2034990
Xu M, Wang Z, Duan X, Pan B (2014) Effects of pollution on macroinvertebrates and water quality bio-assessment. Hydrobiologia 729(1):247–259. https://doi.org/10.1007/s10750-013-1504-y
Bordalo AA, Nilsumranchit W, Chalermwat K (2001) Water quality and uses of the Bangpakong River (Eastern Thailand). Water Res 35:3636–3642. https://doi.org/10.1016/S0043-1354(01)00079-3
Wu Z, Wang X, Chen Y, Cai Y, Deng J (2018) Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci Total Environ 612:914–922
FAO. (1976) Manual of methods in aquatic environmental research part I: permanganate value (oxidability) of organic matter in natural waters. FAO Fish. Tech. Pap.137: 169. http://www.egyseb.net/fulltext/3-1430777406.pdf
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194. https://doi.org/10.1016/S0015-3796(17)30778-3
Hasle GR (1978) ‘The inverted microscope method’ and ‘Identification problems General recommendations’ In: Sournia A (ed) Phytoplankton manual. UNESCO Monogr Oceanogr Method. Paris 6: 88–97
Utermöhl H (1958) Zur Ver vollkommung der quantitativen phytoplankton-methodik. Mitteilung Internationale vereinigung fuer theoretische unde amgewandte. Limnologie 9:39. https://doi.org/10.1080/05384680.1958.11904091
Tomas CR (1997) Identifying marine phytoplankton. Academic Press, San Diego
Wilm JL, Dorris TC (2007) Species diversity of benthic macroinvertebrates in a stream receiving domestic and oil refinery effluents. In: S.M. ISLAM. Phytoplankton diversity index with reference to mucalinda serovar Bodh-Gaya, 12th World Lack Conference. Taal, India, Am. Midl. Nat. 427–449.
Palmer CM (1969) Composite rating of algae tolerating organic pollution. J Phycol 5(1):78–82. https://doi.org/10.1111/j.1529-8817.1969.tb02581.x
Kitsiou D, Karydis M (2001) Marine eutrophication: a proposed data analysis procedure for assessing spatial trends. Environ Monit Assess 68(3):297–312
Ignatiades L, Karydis M, Vounatsou P (1992) A possible method for evaluating oligotrophy and eutrophication based on nutrient concentration scales. Mar Pollut Bull 24(5):238–243. https://doi.org/10.1016/0025-326X(92)90561-J
Bricker SB, Clement CG, Pirhalla DE, Orlando SP, Farrow DRG (1999) National estuarine eutrophication assessment. effects of nutrient enrichment in the Nation’s Estuaries. NOAA, National Ocean Service, Special Projects Office, and National Centers for Coastal Ocean
Bricker SB, Ferreira JG, Simas T (2003) An integrated methodology for assessment of estuarine trophic status. Ecol Model 169:39–60. https://doi.org/10.1016/S0304-3800(03)00199-6
U.S. EPA (2001) Nutrient criteria technical guidance manual—estuarine and coastal marine waters. Office of Water, Washington DC
OSPAR (2003) OSPAR Integrated report 2003 on the eutrophication status of the OSPAR maritime area based upon the first application of the comprehensive Procedure. OSPAR Commission, London
Gamito S (2008) Three main stressors acting on the Ria Formosa lagoonal system (Southern Portugal): Physical stress, organic matter pollution and the land-ocean gradient. Estuar Coast Shelf Sci 77:710–720. https://doi.org/10.1016/j.ecss.2007.11.013
Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, Silva MC, Garcés E, Heiskanen AS, Humborg C, Ignatiades L, Lancelot C, Menesguen A, Tett P, Hoepffnerm N, Claussen U (2011) Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar Coastal Shelf Sci 93(2):117–131. https://doi.org/10.1016/j.ecss.2011.03.014
Karydis M (2001) Assessing levels of eutrophication: a short review on quantitative methodology. Biolo Gallo-Hell 27:135–144
Simboura N, Panayotidis P, Papathanassiou E (2005) A synthesis of the biological quality elements for the implementation of the European Water Framework Directive in the Mediterranean ecoregion: the case of Saronikos Gulf. Ecol Indic 5:253–266. https://doi.org/10.1016/j.ecolind.2005.03.006
HELCOM (2009) Eutrophication in the Baltic Sea an integrated thematic assessment of eutrophication in the Baltic Sea region In: Baltic Sea Environmental Proceedings No. 115B. Helsinki Commission, p 148
Primpas I, Tsirtsis G, Karydis M, Kokkoris GD (2010) Principal component analysis: development of a multivariate index for assessing eutrophication according to the European water framework directive. Ecol Indic 10(2):178–183. https://doi.org/10.1016/j.ecolind.2009.04.007
Andersen JH, Carstensen J, Conley D, Dromph K, Fleming-Lehtinen V, Gustafsson BG (2017) Long-term temporal and spatial trends in eutrophication status of the Baltic Sea. Biol Rev 92:135–149. https://doi.org/10.1111/brv.12221
Wasmund N (2018) The Diatom/Dinoflagellate index as an indicator of ecosystem changes in the Baltic sea. 2. Historical data for use in determination of good environmental status. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00022
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Toronto
Davies-Colley RJ, Smith DG (2001) Turbidity, suspended sediment, and water clarity: a review. J Am water Resour As 37(5):1085–1101. https://doi.org/10.1111/j.1752-1688.2001.tb03624.x
Glibert PM (2017) Eutrophication, harmful algae and Biodiversity-challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124:591–606. https://doi.org/10.1016/j.marpolbul.2017.04.027
Hakanson L, Bryhn AC, Blenckner T (2007) Operational effect variables and functional ecosystem classifications a review on empirical models for aquatic systems along a salinity gradient. Int Rev Hydrobiol 92:326–357. https://doi.org/10.1002/iroh.200610931
Liu X, Zhang G, Sun G, Wu Y, Chen Y (2019) Assessment of lake water quality and eutrophication risk in an agricultural irrigation area: a case study of the Chagan Lake in Northeast China 2019. Water 11:2380. https://doi.org/10.3390/w11112380
Tüfekçi V, Kuzyaka E, Tüfekçi H, Avaz G, Günay AS, Tuğrul S (2013) Determination of limited nutrients in the Turkish coastal waters of the Mediterranean and Aegean Seas. J Black Sea/Medit 19(3):299–311
Devlin M, Barry J, Painting S, Best M (2009) Extending the phytoplankton tool kit for the UK Water framework directive: indicators of phytoplankton community structure. Hydrobiologia 633:151–168. https://doi.org/10.1007/s10750-009-9879-5
Barrera-Alba JJ (2016) Short-term response of phytoplankton community to over-enrichment of nutrients in a well-preserved sub-tropical estuary. Braz J Oceanogr. https://doi.org/10.1590/S1679-87592016115406402
Boudjenah M, Mokrane Z, Soualili D (2019) Diversity of Phytoplanktonic populations along the Algerian coastline. Biodivers J 10(2):81–92. https://doi.org/10.31396/Biodiv.Jour.2019.10.2.81.92
Glibert PM (2020) Harmful algae at the complex nexus of eutrophication and climate. Harmful Algae 91:101583. https://doi.org/10.1016/j.hal.2019.03.001
Lemley DA, Adams JB, Rishworth GM (2018) Unwinding a tangled web: A fine-scale approach towards understanding the drivers of harmful algal bloom species in a eutrophic South African estuary. Estuar Coasts. https://doi.org/10.1007/s12237-018-0380-0
Tan X, Sheldon F, Bunn SE, Zhang Q (2013) Using diatom indices for water quality assessment in a subtropical river. China Environ Sci Pollut Res 20(6):414–4175. https://doi.org/10.1007/s11356-012-1343-9
Garcia MD, Bonel N (2014) Environmental modulation of the plankton community composition and size-structure along the eutrophic intertidal coast of the Río de la Plata estuary. Argentina J Limnol 73(3):562–573. https://doi.org/10.4081/jlimnol.2014.911
Karydis M, Tsirtsis G (1996) Ecological indices: a biometric approach for assessing eutrophication levels in the marine environment. Sci Total Environ 186:209–219. https://doi.org/10.1016/0048-9697(96)05114-5
Salas F, Patricio J, Marcos C, Pardal MA, Perez-Ruzafa A, Marques JC (2006) Are taxonomic distinctness measures compliant to other ecological indicators in assessing ecological status. Mar Pollut Bull 52:162–174. https://doi.org/10.1016/j.marpolbul.2005.08.014
Sun J, Liu DY (2004) The application of diversity indices in marine phytoplankton studies. Acta Oceanol Sin 26:62–75
Fu T, Chen B, Ji W, Chen H, Chen W, Dong X, Kuang W, Chen J, Wang J, Lin H (2016) Size structure of phytoplankton community and its response to environmental factors in Xiamen Bay. China Environ Earth Sci 75:734. https://doi.org/10.1007/s12665-016-5552-2
Liu ZL (2013) Evaluation on developing level of unbans agglomeration derived from resources exploration. Res J Appl Sci 13(21):4702–4707
Yamamoto T, Inokuchi Y, Sugiyama T (2004) Biogeochemical cycles during the species succession from Skeletonema costatum to Alexandrium tamarense in northern Hiroshima Bay. J Mar Syst 52:15–32. https://doi.org/10.1016/j.jmarsys.2004.03.004
Gárate-Lizárraga M-Gómez MS (2008) Bloom of peridinium quinquecorne abé in la ensenada de la paz, gulf of california (july 2003). Acta Bot Mex 83:33–47
Zargar S, Ghosh TK (2006) Influence of cooling water discharges from Kaiga nuclear power plant on selected indices applied to plankton population of Kadra reservoir. J Environ Biol 27:191–219
Mikhail SK, Labib W (2014) Red tide outbreaks in Alexandria waters: invasive harmful species In: The 15th Conference on Harmful Algal Blooms. Korea Republic. 29 Oct 2 Nov 2013
Komarek J, Jaroslava K (2002) Two common Microcystis species (Chroococcales, Cyanobacteria) from tropical America, including M. panniformis sp. nov. Cryptogamie. Algol 23(2):159–177
Oshima Y (2011) Growth interactions between raphidophytes Chattonella antiqua and Heterosigma akashiwo. Thalassas 27(1):33–45
Richardson K (1997) Harmful or exceptional phytoplankton blooms in the marine ecosystem. Adv Mar Biol 31:301–385. https://doi.org/10.1016/S0065-2881(08)60225-4
Wang X, Wang Y, Liu L, Shu J, Zhu Y, Zhou J (2013) Phytoplankton and eutrophication degree assessment of Baiyangdian Lake Wetland, China. Sci World J. https://doi.org/10.1155/2013/436965
Mateo P, Leganés F, Perona E, Loza V, Fernández-Piñas F (2015) Cyanobacteria as bioindicators and bioreporters of environmental analysis in aquatic ecosystems. Biodivers Conserv 24(4):909–948. https://doi.org/10.1007/s10531-015-0903-y
Kshirsagar AD (2013) Use of Algae as a bioindicator to determine water quality of river Mula from Pune City, Maharashtra (India). Univers J Environ Res Technol 3(1):79–85
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
WL: Participated in the design of the study, coordination and helped to draft the manuscript. AAEl-D: Approved the final version of manuscript including references. SAS: Approved the final version of manuscript including references.MMI: Participated in the design of the study, the practical work, revision and editing of the manuscript. SH: Participated in taxonomic Identification of phytoplankton samples, helped to draft the manuscript and editing of it. MHD: Field work, collect samples and taxonomic identification of it.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This research did not require Ethics Board approval because it does not involve human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Labib, W., El-Dahhar, A.A., Shahin, S.A. et al. Water quality indices as tools for assessment of the Eastern Harbor’s water status (Alexandria, Egypt). SN Appl. Sci. 5, 89 (2023). https://doi.org/10.1007/s42452-023-05304-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05304-z