Abstract
Groundwater quality assessment is critical for maintaining freshwater sources in semi-arid and dry environments, which is critical for long-term development. Water quality control authorities can regulate the use of water for agriculture or drinking implementations based on groundwater quality in various areas, as nearly 85% of India's population is reliant on groundwater for domestic and irrigation needs, particularly as a source of drinking in rural areas. From Hunsur taluk, Mysore district, Karnataka, India, this study explores and identifies potential places for pumping groundwater for drinking and irrigation. An analysis of groundwater quality was conducted by analyzing the Water Quality Index (WQI) determined from 30 wells during two seasons of the year 2019, namely Pre-Monsoon and Post-Monsoon. The identification of the quality of water resources for irrigation and drinking purposes can be determined with the help of the WQI. It’s extremely useful for classifying water quality considering the standard-based classification of water as excellent, good, poor, very poor, or unsuitable for drinking. By using the Weighted Arithmetic Index method calculated using the groundwater samples collected data for the study, reported ‘excellent water’ ranges between 80 and 83.33 and ‘good water’ between 20 and 16.67, and never found ‘poor water.’ The IWQ index for the study area has been reported to be in the average range of 17.35–65.64. This paper highlights the physico-chemical parameters assess water quality in comparison with climatology.
Article highlights
-
The major crux of the study was to identify suitable groundwater areas in Hunsur taluk of Mysore district in Karnataka, India that could be exploit to meet the drinking and agricultural requirements of the local people.
-
30 hand pumps samples were collected during pre and post-monsoon seasons of 2019 and their water quality analysis was conducted.
-
The results of our study showed that water samples that were selected areas of the Hunsur taluk were excellent or good or both and could be used for drinking and irrigation purposes and met the Water Quality Index and Sodium Adsorption Ratio limits when compared to the guidelines and standards set by the WHO (2004) and BIS (2012).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water is important for the sustenance of life on Earth. It could be present as surface or groundwater. Groundwater availability is subject to the hydro-geological terrain specifics of a region. India is said to host many aquifer systems formed from the precipitation of surface runoffs in uphill regions and discharged in the lowlands. Renewable water supplies below 1700 m3/capita /year indicate water stress which turns chronic when the figure drops below 1000 m3/capita /year [1]. The natural system of groundwater inflow and discharge has been greatly altered by anthropogenic and geogenic activities in recent decades. The issue of groundwater availability is critical as one-third world population uses it for drinking purposes [2]. Increased population density, industrialization, agricultural practices, disposal of domestic sewage, industrial runoffs all contaminate the groundwater and water resources in general [3, 4]. India’s agricultural and drinking water requirements are partly met by aquifers and groundwater that supply roughly 62% towards irrigation, 85% towards water supply in rural areas, and 45% in urban areas [5]. This dependence, has stressed the groundwater resources immensely [6].
The groundwater aquifers in Karnataka fall geographically in the most predominant hard rock or crystalline zone, where rock porosity is acquired through processes of weathering, fractures, and joints over time. The groundwater tables are declining gradually, leading to the drying up of wells and giving rise to severe water crises. Therefore, understanding the mechanics of aquifers systems and their pollution, ecological sustainability of groundwater is in need due to severe pollution and lack of strict regulations [7].
Assessment of groundwater quality and well locations that are suitable for drinking purpose is imperative to qualify them for drinking purposes. Water studies and researches should assist in highlighting the impending problems with the waters used in the region. Many methods have been employed in the past to test water samples both qualitative and quantitatively [8]. The assessment in the study has been elaborated step by step below which comprises of scrutinising the study location with the help of GIS Maps, physical parameters like pH, Electrical Conductivity (EC), Total Hardness, Colour, and odor. of the water samples have been carried out within 24 hours of collection [9]. Followed by chemical parameters that have been carried out are Total Dissolved Solids (TDS), Chloride, Calcium, Magnesium, Sodium, Potassium, and Fluoride [10]. To show the relationship between the changes observed in the groundwater quality between the pre monsoon and post monsoon seasons statical analysis like Water Quality Index, Pearson and Correlation, Regression analysis, Sodium Absorption Ratio (SAR), and Sodium Percentage [11].
The water quality index (WQI) method that rates water quality effectively in terms of the overall quality of water [12,13,14]. In this regard, WQI data highlights the overall quality of water numerically in simple and easy denominations [15]. WQI technique translates the intricate statistics on water quality derivations into simple ratings in the form of comprehensive conclusions as good, best, poor or excellent for the public assessment. This helps them further to manage their groundwater resources prudently for the near future [16, 17].
The current research work was undertaken to identify groundwater areas that were suitable for pumping to meet the drinking and agricultural requirements in Hunsur taluk of Mysore district in Karnataka, India. 30 hand pumps groundwater samples were collected during pre and post-monsoon seasons during 2019 and analyzed for their physico-chemical composition using the Water Quality Index (WQI) and Sodium Adsorption Ratio (SAR). Structurally the paper is organized into into various heads. Study location and climate that is mentioned under Sect. 2. Section 3 talks about the physico-chemical assessment under headings 3.1.1 and 3.1.2 (physical and chemical parameters) and water quality assessment under Sects 3.2.1 and 3.2.2 (Water Quality Index, Sodium Adsorption ratio) of the water samples. Conclusions were drawn for changes observed in the groundwater quality samples between the pre monsoon and post monsoon seasons using statistical analysis of Correlation, Regression, Water Quality Index, Sodium Absorption Ratio (SAR) and Sodium Percentage. The results of each parameter were compared to the guidelines and standards set by the WHO (2004) and BIS (2012). Holistically our study showed that most of the groundwater samples harvested from the Hunsur taluk study area were excellent or good water in terms of WQI and SAR values and therefore, could be used for drinking and irrigation purposes [18].
2 Study location and climate
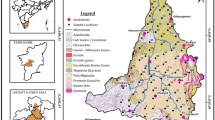
Hunsur taluk, Mysuru District falls in the survey of India topo sheet Nos. 57D/ 3, 4, 7, 8 and 11. The taluk is bounded by north latitudes 12° 05′ 00″–12° 26′ 00″ and east longitudes 76° 05′ 00″–76° 32′ 00″, covering an area of 897 sq km receives an annual average rainfall of 873 mm. This taluk belongs to the Mysuru district of the state of Karnataka and is bordered by Piriyapatna taluk on the north-east, Kodagu district on the south-east, Krishnarajanagara taluk on the north, Mysore taluk on the west, and Heggadadevankote on the south (Fig.1). The rainfall is highly variable in its distribution over space and time. The southwestern monsoon contributes more than 60% of the annual rainfall from June to September. During October and December, the northeastern monsoon and depressions either in the Bay of Bengal or the Arabian Sea bring rainfall to the area. The area is covered with hilly terrain and contains red-shallow gravelly soils. Geologically, the Hunsur area is covered by the Archean basement complex of gneisses, younger granites, and rocks of the Dhawar Group (Fig. 2). Like in other places of India, in Hunsur also, agricultural, domestic and industrial sectors are competing more and more for a limited supply of water. Although the agricultural and industrial demands are increasing as the sectors continue to grow, domestic needs claim only a small portion of the annual water withdrawals, as access to adequate water and sanitation supplies remains low throughout. Greater access and an improvement in the socio-economic situation in Hunsur will result in high demand for water from the domestic sector in the coming years (Figs. 3 and 4) [19].
3 Materials and methods
Groundwater samples collected at 30 hand pumps by simple random sampling technique, Hunsur taluk, Mysore district, Karnataka, India and observed for normalization of the water quality for analysis which is correlated with climatology data obtained. During the pre and post-monsoon seasons of 2019. The primary objective of the study was to explore and identify suitable areas that could be used for pumping groundwater for drinking and agricultural purposes. Water quality was assessed for both physical and chemical parameters of the water, and results were summarized by using the Water Quality Index (WQI), Sodium Adsorption Ratio (SAR), and Pearson’s correlation studies to determine the quality of water.
Samples were either refrigerated at 4 °C or immediately analyzed as per the requirement. Physical or chemical (organic/inorganic) constituents of water were analyzed following protocols from standard procedures [20].
3.1 Physico-chemical analysis
3.1.1 Physical parameters
pH (using potentiometry, using digital pH meter); Electrical conductivity (EC) (using potentiometry through conductivity meter); total hardness (using EDTA titrimetric) and color, odor through visual inspection.
3.1.2 Chemical parameters
Total dissolved solids (TDS) (using filtration), chloride (argentometry); calcium, magnesium sodium, potassium (flame emission/ flame photometry; fluoride (SPADNS/ spectrophotometry).
3.2 Water quality assessment
3.2.1 Water quality index (WQI Index)
WQI reflects the quality of groundwater for use in domestic and agricultural chores and is calculated employing four steps [12]. In the first step, the weight (Wi) of each water, the quality parameter is calculated by assigning the values between (4-2) depending on the manner in which they would influence the water quality for drinking purpose holistically [21]. The second step involves calculations for the relative weight (Wi) using protocols from [22]. The third step involves measures for the quality rating scale (Qi) for all parameters, and the fourth step is SI determination for the chemical parameters, after which WQI is determined.
Equations used in the WQI calculations for all steps are as following:
Equations for WQI calculations.
-
(1)
Wi = relative weight, wi = weight of the parameter, n = number of parameters.
-
(2)
Qi = quality rating, Ci = concentration of each chemical parameter, Si = drinking water standard as per APHA guidelines.
-
(3)
Si = sub index of ith parameter.
3.2.2 Sodium adsorption ratio (SAR)
Salinity affects water quality negatively making it unfit for domestic and agricultural purposes. Sodium adsorption ratio (SAR) defines the sodium quantity relative to calcium and magnesium ions in groundwater sample. Mathematically SAR can be expressed for all the well water samples following equation where, Na+, Ca2+ and Mg2+ denotes the concentration of sodium, calcium, and magnesium particles in water, respectively as
3.3 Statistics
Samples from 30 hand pumps were reported as Mean ± SD, SAR and WQI values and analyzed statistically using Pearson’s correlation test for multiple-comparisons and interdependence between groups with significance set at p < 0.05. Correlation coefficients ranged between −1 to +1.
4 Results
The analysis of the 30 hand pump samples from groundwater were compared to the water quality guidelines set by WHO (2004) and BIS (2012) for drinking and agricultural purpose (Tables 1 and 2).
4.1 Physico-chemical analysis
4.1.1 Physical parameters
pH of the 30 analyzed samples from handpump showed a minimum pH value of 6.66, maximum of 8.44 and an average of 7.77 with a standard deviation (SD) (0.35) during pre-monsoon and minimum of 6.66, maximum 8.22 and average of 7.05 with SD 0.39 during post monsoon season of 2019 reflecting slightly acidic alkaline nature during post monsoon season.
Electrical conductivity (EC) values of the handpump samples ranged from 676 to 3070 μs/cm with average (1570 μs/cm) during pre-monsoon season and 283 to 2740 μs/cm with average (1580 μs/cm) during post monsoon season.
Total hardness (TH) in handpump samples is in the range 220.22–1299 mg/L and 146.52–2430 mg/L during the pre and post-monsoon season, respectively, with the average value of 677.84 and 644.87 mg/L Table 1 and 2.
4.1.2 Chemical parameters
Ca2+ of the 30 analyzed samples from handpump showed a minimum value of 41.1, maximum of 285 and an average of 148.40 mg/L with SD (61.70) during pre-monsoon and minimum of 33.6, maximum 237.6and average of 124.65 with SD 61.38 during post monsoon season of 2019. Concentration of Mg2+ in the handpump samples ranged from 20.22 to 164.8 mg/L with average (70.72 mg/L) during pre-monsoon season and 15.19 to 121.81 mg/L with average (67.88 mg/L) during post monsoon season. Na+ in handpump samples is in the range 21.3 to 83.8 mg/L and 19.2 to 81.8 mg/L with the average value of 63.73 and 67.45 mg/L during the pre-and post-monsoon season, respectively. K+ concentrations in handpump samples ranges 1.2–38 mg/L and 1–33.9 mg/L with the average value of 8.19 and 7.38 mg/L during the pre-and post-monsoon season, respectively. Cl- in handpump samples ranged from 21.3 to 83.8 mg/L and 67 to 459.4 mg/L with the average value of 179.83 and 149 mg/L during the pre-and post-monsoon season, respectively. Concentrations of F in handpump samples was in the range 0.02–0.58 mg/L and 0.01–0.52 mg/L with the average value of 0.26 and 0.27 mg/L during the pre-and post-monsoon season, respectively. Total dissolved solids (TDS) were in the range 339–1577 mg/L and 181–1789 mg/L with the average value of 793 and 906.8 mg/L during the pre-and post-monsoon season, respectively.
Pearson’s Correlation was applied to study the covariance between two consecutive physico-chemical parameters of groundwater, and the data extrapolated was in the range of + 1 to − 1 represented as rat p˃ 0.05. When r ˃ 0.7 there is a strong correlation between variables and moderate with r = 0.5–0.7 and poorly for r < 0.5 as represented by Tables 3 and 4 [23].
For pre monsoon samples, at p < 0.001, strong positive correlation was seen for: Cl with Ca (r = 0.84), TDS with Ca (r = 0.85), EC with Ca (r = 0.85), TH with Ca (r = 0.83), TDS with Cl (r = 0.85), EC with Cl (r = 0.86), EC with EDS (r = 0.99), TH with TDS (r = 0.80), TH with EC (r = 0.80), At p < 0.05, parameters that moderately correlated with each other were: TH with Cl (r = 0.66), EC with K (r = 0.56), TDS with K (r = 0.56), EC with Na (r = 0.61), TDS with Na (r = 0.60), EC with Mg (r = 0.60), TDS with Mg (r = 0.60), K with Ca (r = 0.51), Mg with Ca (r = 0.51). pH was negatively correlated with all the other nine physico-chemical parameters namely, Ca, Mg, Na, K, Cl, F, TDS, EC and TH.
For post monsoon samples, at p < 0.001, strong positive correlation was seen for: TDS with EC (r = 0.92), EC with Cl (r = 0.84), TDS with Cl (r = 0.82), EC with Mg (r = 0.77), TDS with Mg (r = 0.70). At p < 0.05, parameters that moderately correlated with each other were: Ca with Cl (r = 0.56), EC with Ca (r = 0.59), TH with Ca (r = 0.55), TDS with Na (r = 0.51), TH with Cl (r = 0.55), TH with EC (r = 0.61). pH was negatively correlated with all the other nine physico-chemical parameters namely, Ca, Mg, Na, K, Cl, F, TDS, EC and TH.
Note: Ca = Calcium, Mg = Magnesium, Na = Sodium, K = Potassium, Cl = Chloride, F = Fluoride
TDS = Total dissolved solids, EC = Electrical conductivity, TH = Total hardness.
4.1.3 Regression analysis results of pre-monsoon and post-monsoon (Figs. 5 and 6)
4.1.3.1 Pre-monsoon
-
1.
Ca vs EC (R squared -----0.7377; Y = 8.323*X + 335.1)
-
2.
Mg vs Total Hardness (R squared ---- 0.7275; Y = 6.201*X + 239.3)
-
3.
TDS vs Total Hardness (R squared ---- 0.6538: Y = 0.6759*X + 141.6)
-
4.
EC vs Total Hardness (R squared ---- 0.6514; Y = 0.3458*X + 134.8)
-
5.
EC vs TDS (R squared ----0.9985: Y = 0.5121*X − 10.90)
The regression analysis was carried out for the following parameters i.e. Ca vs EC, where Ca was taken on X axis (independent variable) EC on Y axis: Y = 8.323*X + 335.1 was obtained; Mg vs Total Hardness, where Mg was taken on X axis (independent variable) Total Hardness on Y axis: Y = 6.201*X + 239.3 was obtained; TDS vs Total Hardness, where Mg was taken on X axis (independent variable) Total Hardness on Y axis: Y = 0.6759*X + 141.6 was obtained, EC vs Total Hardness, where EC was taken on X axis (independent variable) Total Hardness on Y axis: Y = 0.3458*X + 134.8 was obtained; EC vs TDS, where EC was taken on X axis (independent variable) Total Hardness on Y axis: Y = 0.5121*X - 10.90 was obtained.
4.1.3.2 Post-monsoon
-
1.
Mg vs TDS (R squared -----0.5025; Y = 10.71*X + 179.9)
-
2.
Cl vs EC (R squared- 0.7117: Y = 6.310*X + 638.3)
-
3.
TDS vs EC (R squared--0.8541; Y = 1.535*X + 189.3)
-
4.
TDS vs Cl (R squared-0.6861; Y = 0.1839*X − 17.38)
The regression analysis was carried out for the following parameters i.e. Mg vs TDS, where Mg was taken on X axis (independent variable) TDS on Y axis: Y = 10.71*X + 179.9 was obtained; Cl vs EC, where Cl was taken on X axis (independent variable) EC on Y axis: Y = 6.310*X + 638.3 was obtained; TDS vs EC, where TDS was taken on X axis (independent variable) EC on Y axis: Y = 1.535*X + 189.3 was obtained, TDS vs Cl, where TDS was taken on X axis (independent variable) Cl on Y axis: Y = 0.1839*X − 17.38 was obtained.
4.2 Water quality parameters
4.2.1 WQI index
Water quality index describes water quality most explicitly. The WQI values of hand pump samples ranged from 19.5 to 74.1 (avg. 41.2) in the pre-monsoon season and 15.24 to 60 (avg. 37.2) in the post- monsoon season. According to WQI classification water is classified into five categories based upon the WQI ranges namely: excellent for < 50, good for 50.1–100, poor for 100.1–200, very poor for 200.1–300 and unfit for drinking at > 300.1. The higher WQI values were present in the post monsoon season as compared to the pre-monsoon season as shown in Tables 5 and 6 and Fig. 7.
4.2.2 SAR
Sodium adsorption ratio (SAR) defines the irrigation potential of sodium affected water and soils sources. The SAR values of handpump samples ranged from 0.720 to 2.35 (avg. 1.6) in the pre-monsoon season and 0.6 to 1.9 (avg. 1.3) in the post-monsoon season. The higher SAR value of the handpump water was present in the pre monsoon season as compared to the post-monsoon season as shown in Tables 7, 8, 9 and Fig. 8. According to SAR (meq/l) classification, <10 is considered as excellent, between 10-18 SAR (meq/l) as good, between 18-26 SAR (meq/l) as fair and >26 as poor.
5 Discussion
The current research throws light on the quality of handpump water suitability for drinking and agricultural purposes during pre and post monsoon season in 2019 in Hunsur taluk of Karnataka, India. Handpump samples were taken from 30 destinations and thoroughly investigated for their physico- chemical properties [24]. The results were represented in terms WQI and SAR scores for analysis. All water parameters were compared to the standard guidelines set by the WHO (2004) and BIS (2012) to derive at relevant conclusions [25].
The pH of water indicates the equilibrium between bicarbonate, carbon-di-oxide, carbonate of a water sample and therefore reporting its status is of utmost importance. In our study, pH of the 30 handpump samples showed an average shift from 7.77 to 7.05 between pre to post monsoon seasons reflecting that they were within the permissible limits (6.5–8.5) of the BIS (2012) and (7-8.5) of the WHO (2004) guidelines [26]. conducted a similar assessment on physicochemical properties of the groundwater of Bokaro district, India. They found that during the pre to post monsoon season, there was a slight acidic pH observed in their 51 water samples [27]. It is also assessed the pH of groundwater for drinking and irrigation purposes of Shalmala river sub-basin, in Dharwad district of Karnataka.
EC indicates the presence of ionized substances in a water sample that help in passage of current. The EC values in our study averaged from 1570 to 1580 μs/cm during pre to post monsoon season and showed that the major study locations had higher EC values (> 1000 μS/cm) and that were slightly above the BIS range (1400 μS/cm)
Total hardness in handpump samples was in the range 677.84 and 644.87 mg/L during the pre-and post-monsoon season, respectively. These values were above the acceptable and permissible limits of WHO (100 to 500) and BIS values (100-600). The decrease in hardness of water from the pre to post monsoon seasons, could be attributed to the dilution of the cationic content. Also surface runoffs due to rain, increase in Ca2+ influences the total hardness of water quality [5]. A study also evaluated the seasonal ground water physico-chemical parameters responsible for groundwater degradation through implementation of the water quality Index [15].
The pre to post monsoon Ca2+ concentration in our study of 30 analyses showed a decrease in the cationic concentration from 148.40 to 124.65 mg/L. Concentration of Mg2+ also followed similar trends with a decrease in concentration from 70.72 to 67.88 mg/L. These values were a bit higher to the acceptable but within the permissible limits of WHO (75–200) and BIS values (75–100) for Ca2+concentration. For Mg2+ these values were again a bit higher to the acceptable but within the permissible limits of WHO (50 to 150) and BIS values (30–100) range. These results correlate with increased total hardness of the 30 samples which could be attributed to the rise in marginally increased values of Ca2+ and Mg2+ ion concentrations as TH are directly related to them.
Concentrations of the cations like Na+ and K+ were well within the permissible limits set by standards WHO and BIS of (100 and 10 mg/L for Na+ and K+). Similarly, Cl- concentrations were within the permissible range of 250 mg/L, identical to the F with 1 mg/L legal limits. The water quality index for groundwater of southern part of Bangalore city [3]. A studied suitability of groundwater quality for drinking purposes in Ratmao-Pathri Rao watershed, Haridwar district, India [21]. The influence of agricultural practices in semi-arid agro-climatic zone the rate of percolation shows a higher concentration of the dissolved elements during post-monsoon and hence the WQI comparatively more than pre-monsoon. The irrigation suitability of the excess CO3 2− and HCO3− in groundwater over the sum of Ca2+ and Mg2+ ions reduces water infiltration into the soil surface so more sodium absorption ratio (SAR) [28]. Concentrations of TDS in handpump samples averaged from 793 and 906.8 mg/L during the pre-and post-monsoon season, respectively. Although these values were on the higher end as compared to the acceptable range of 500 mg/L, they were in the permissible range set by WHO and BIS from 1500 to 2000 mg/L. TDS and EC are directly correlated in theory since higer concentration of cationic and anionic salts and their individual components in the water body cause an increase in TDS quality of water [21]. Present investigation shows that TDS hikes the EC of the water bodies. Therefore, our results of TDS are in agreement with the values obtained with the EC values of the handpump samples [29].
The results from Pearson’s correlation coefficient indicate that there is a strong correlation and direct proportionality between TH and cations like Ca2+ and Mg2+, TH and TDS and TDS and EC both pre and post monsoon seasons. Majorly all physico-chemical parameters correlated well with each other either at p< 0.05 or < 0.001 attributing that presence of specific pollutants would influence other assessed pollutants in the handpump water samples (Figs. 3 and 4). Results of Pearson’s correlation coefficient highlight pre and post monsoon data show a similar trend that gradually decreases during post monsoon season [30]. The difference in values could be attributed to dilution effect caused by rains during post monsoon season (Tables 3 and 4).
Water quality index describes water quality most explicitly. The WQI values of hand pump samples ranged from. 41.2 to 37.2 from pre to post--monsoon season 2019. The higher WQI value of the hand pump water was present in the post monsoon season as compared to the pre-monsoon season as shown in Table 6 and Fig. 7. During the pre-monsoon season 24 handpump samples fell in the excellent category with WQI range < 50. This accounted for 80% of the samples. Similarly, just 20% of the samples that is 6 fell into the good water category, with WQI values ranging from 50.1 to 100. Similarly, 25 handpump samples from the post-monsoon season fell into the good category, with WQI scores of 50 or higher. This accounted for 83.33% of the samples. Five samples identified under good water category with WQI range 50.1 – 100 accounting for just 16.67% of the samples. The higher values of WQI during pre-monsoon season could be due to the higher concentrations of TDS, TH, Ca2+, Mg2+, Cl− and EC in the handpump samples which could be attributed to higher natural and anthropogenic activities during pre-monsoon season. Moreover, lower values of WQI during post-monsoon season could be due to dilution affect.[26] Holistically WQI results of handpump samples indicate that they are categorically excellent or good and suitable for drinking purposes.
Sodium adsorption ratio (SAR) defines the irrigation potential of sodium affected water and soils sources. The SAR values of handpump samples ranged from 0.720 to 2.35 (avg. 1.6) in the pre-monsoon season and 0.6 to 1.9 (avg. 1.3) in the post-monsoon season. The higher SAR value of the handpump water was present in the pre monsoon season as compared to the post-monsoon season as shown in Table 8 and Fig 8. During the pre-monsoon season 18 handpump samples fell in the excellent to good category with sodium percentage (meq/l) in the range < 20. This accounted for 60% of the samples. Twelve samples were analyzed in the good to permissible water category with sodium percentage (meq/l) in range 20–40 accounting for just 40% of the samples. During the post monsoon season 14 handpump samples fell in the excellent to good category with sodium percentage (meq/l) in range < 20. This accounted for 47% of the samples. Sixteen samples categorized in the good to permissible water category with sodium percentage (meq/l) in range 20-40 accounting for 53% of the samples. The higher values of sodium percentage (meq/l) during pre-monsoon season indicate that pre-monsoon handpump water is better for irrigation purposes as compared to post monsoon, which could be attributed to dilution affect (Table 9). Both pre and post monsoon samples fall in the good to permissible range and can be used for agriculture.
Conclusively, our study of 30 handpump samples taken from Hunsur taluk clearly reiterates that Hunsur taluk can be exploited for ground water pumping for domestic, drinking and irrigational purposes based upon the WQI, and SAR analysis of the samples studied there.
6 Conclusion
Our study was conducted with a basic interest to assess the quality of water from Hunsur taluk as very few groundwater water sample have been assessed since 2019. Agriculture remains the major preoccupation of the local inhabitants of the area,that require huge quantities of water for their irrigation and domestic purposes. Based on previous literatures of favorable water quality analysis of different water bodies in areas adjoining the Hunsur taluk, we hypothesized that groundwater contamination might be lesser and could be utilized for irrigation and portability purposes in the region. The major findings from the present study indicated the WQI values were in good range (17.35–65.64) which further indicated that our study samples were of “very good-quality”. However the groundwater of the area does need a certain degree of treatment before its consumption. Major sample studied were classified as “best in quality” for drinking purposes since all the parameters were within the acceptable limits prescribed by BIS. The results of the correlation analysis further indicated a strong positive correlation of magnesium with potassium ions and negative correlation between sodium and bicarbonate ions. Correlation of calcium with Na+, K+, Cl−, SO4, and SO4− was found to be weakly positive and negative for nitrate. Sodium indicated maximum correlation with sulphate, minimum with chloride and bicarbonate and negative with bicarbonate. Chlorides showed a weak positive correlation when compared to sulphate and bicarbonate and negative correlation with nitrate. However analysis of the heavy metal and pesticide contamination were not conducted indicating the limitation of the work. Separate studies for their correlation have been planned for future investigations. Holistically the quality of water from Hunsur taluk had an average WQI range (17.35–65.64) for pre and post monsoon seasons and that the physico-chemical parameters assess were in good correlation for irrigation and domestic use.
Data availability
Normal Word format.
References
Saha D, Ray RK (2019) Groundwater resources of India: potential, challenges and management. Groundwater Development and Management. Springer, pp 19–42
Nickson R et al (2005) Arsenic and other drinking water quality issues Muzaffargarh district, Pakistan. Appl Geochem 20(1):55–68
Ravindra M, Sharda SJM (2015) Water quality index for groundwater of southern part of bangalore city. Magnesium 75:013
Singh S et al (2018) Index-based assessment of suitability of water quality for irrigation purpose under Indian conditions. Environ Monitor Assess 190(1):1–14
Kushtagi S, P Srinivas (2011) Studies on water quality index of ground water of Aland Taluka, Gulbarga District, Karnataka. Int J Appl Biol Pharmaceut Technol, 2(4)
Das A et al (2017) Nutrient dynamics of the Brahmaputra (Tropical River) during the monsoon period. Desalin Water Treat Sci Eng 76:212–224
Singh VK et al (2021) Development of fuzzy analytic hierarchy process based water quality model of Upper Ganga river basin. India J Environ Manag 284:111985
Kumar R, Singh R, Sharma KJC (2005) Water resources of India. pp 794–811
Kereyu DD (2013) A GIS based water quality management-a case study of water supply for the city of Nairobi. 2013, University of Nairobi
Ramakrishnaiah C, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State India. E-J Chem 6(2):523–530
Verma DK et al (2018) Assessment of groundwater quality of the central Gangetic Plain area of India using geospatial and WQI Techniques. J Geol Soci India 92(6):743–752
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Brown RM et al (1970) A water quality index-do we dare. Water Sewage Work 117(10):1–5
Tiwari AK et al (2017) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh, India. Appl Water Sci 7(4):1609–1623
Sirajudeen J, Manikandan SA, Manivel V (2013) Water quality index of ground water around Ampikapuram area near Uyyakondan channel Tiruchirappalli District, Tamil Nadu, India. Arch Appl Sci Resear 5(3):21–26
Poonam T, Tanushree B, Sukalyan CJ (2013) Water quality indices-important tools for water quality assessment: a review. Int J Adv chem 1(1):15–28
Singh S et al (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. Int J Adv chem 57(1):45
Krishan G et al (2021) Role of ion chemistry and hydro-geochemical processes in aquifer salinization—a case study from a semi-arid region of Haryana, India. Water 13(5):617
Sharma G et al (2021) Application of multivariate statistical analysis and water quality index for quality characterization of Parbati River, Northwestern Himalaya, India. Discover Water 1(1):1–20
Hoenig MJ (2001) Preparation steps in environmental trace element analysis—facts and traps. Talanta 54(6):1021–1038
Al-Hadithi M (2012) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Ratmao-Pathri Rao watershed, haridwar district India. J Scient Ind Resear 23:1321–1336
Tiwari J, Manzoor A (1988) Pollution of Indian rivers. Aashish Publishing House, Water quality index for Indian rivers. p. 271–286
Balan IN, Shivakumar M, Kumar PM (2012) An assessment of groundwater quality using water quality index in Chennai, Tamil Nadu, India. Chronicl Young Scient 3(2):146
Singh SK et al (2013) Integrated assessment of groundwater influenced by a confluence river system: concurrence with remote sensing and geochemical modelling. Water Resour Manag 27(12):4291–4313
Singh SK et al (2015) Modeling groundwater quality over a humid subtropical region using numerical indices, earth observation datasets, and X-ray diffraction technique: a case study of Allahabad district India. Environ Geochem Heal 37(1):157–180
Verma P et al (2020) Assessment of groundwater quality status by using water quality index (WQI) and geographic information system (GIS) approaches: a case study of the Bokaro district India. Appl Water Sci 10(1):1–16
Kaur M, Nagpal AK (2017) Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ Sci Pollut Resear 24(23):18881–18895
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7(6):3137–3150
Arain MB et al (2014) Evaluation of water quality parameters in drinking water of district Bannu, Pakistan: Multivariate study. Sustainab Water Qual Ecol 3:114–123
Prokop P, Walanus AJT, Climatology A (2015) Variation in the orographic extreme rain events over the Meghalaya Hills in northeast India in the two halves of the twentieth century. Theoret Appl Climatol. 121(1):389–399
Funding
Miss. Noushin Afshan, would like to acknowledge funding support from Department of Minorities, GOK.DOM, Karnataka, India (Grant No. DOM/FELLOWSHIP/CR-032019–20 Dated: 11–10-2019).
Author information
Authors and Affiliations
Contributions
NA Ideology, Methodology, Field visit, Collection of samples, Lab experiments, Data Analysis and interpretation, Statistical Analysis, Software handling, Drafting the article. DN Guidance, Supervision, Conception, Plagiarism check, Critical revision of the article, Final approval of the version to be published, Analysis tools and lab facilities. BHM Field visit. GDP Software handling.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afshan, N., Nagaraju, D., Bhanuprakash, H.M. et al. Seasonal analysis of groundwater samples to identify water quality index and comparative statistical analysis of Hunsur Taluk, Mysuru, Karnataka, India. SN Appl. Sci. 4, 210 (2022). https://doi.org/10.1007/s42452-022-05102-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05102-z