Abstract
Diaion® HP-20 resin passive samplers deployed in water and sediment samples collected from Lake Ziway were analyzed for 30 organochlorine, organophosphorus, carboxamide, and pyrethroid pesticide residues. The samples were collected from purposely selected sampling stations in five sites on Lake Ziway. Levels of selected pesticides were determined by GC–MS/MS in all samples. p,p′DDE and boscalid residues were the only detected pesticides in sediment samples. Similarly, only metalaxyl and boscalid residues were recovered from HP-20 resins. The concentration of p,p′DDE and boscalid in sediment ranged from 0.66–7.23 and 0.1–15.26 ng g−1 dry weight respectively. The presence of p,p′DDE but no other metabolites of DDT in all sediment samples indicated that DDT residues in Ziway Lake were aged and probably originated from the weathered agricultural soils of the surrounding region. The highest level of boscalid was recorded at Site 2 (near the floriculture enterprises) both in sediment and in HP-20 resins with a mean concentration of 11.8 ng g−1 dw and 39.6 ng g−1 disk respectively. However, the concertation of metalaxyl was the highest in the HP-20 resins deployed at Site1 and Site 4 (near the intensive small-scale vegetable farm) with a mean concentration of 54.7 ng g−1 disk and 54.3 ng g−1 disk respectively. Generally, most sampling sites of p,p′DDE were found to have a moderate ecological risk based on levels specified in the sediment quality standards. Moreover, the relatively high boscalid and metalaxyl levels in HP-20 deployed in Lake Ziway would be the result of recent intensive pesticide use by floriculture enterprises and small-scale vegetable farmers in the region. A spatial variation on the accumulation of detected pesticides among the sampling sites depends on the anthropogenic activities, around the lake from the point and non-point sources. Although most of the analyzed pesticides were below the detectable limit, further studies and continued monitoring of currently used pesticide residues in the Lake are highly recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Increasing population size and pesticides utilized in farming have amplified the contamination of the aquatic ecosystem. Pesticides can reach the water bodies from the farm by direct discharge in water bodies where pesticides are converted to different metabolites by biodegradation, photodegradation, chemical hydrolysis, and other forms. These metabolites may have higher toxicity than the parent compounds [1]. Although the use of pesticides in Africa is remarkably low in comparison to the rest of the world, it had been used for quite 50 years for protecting agricultural pests and eradicating disease vectors [2], and also the most common organochlorine compound used for pest control has been the organochlorine pesticides like insecticide p,p′DDT (Dichloro Diphenyl Trichloroethane) and endosulfan. DDT and endosulfan are banned for agricultural use under the Stockholm convention of persistent organic chemicals and signed by Ethiopia.
In Ethiopia, DDT is endorsed only for the use of indoor residual spraying(IRS) for vector control [3]. However, studies report the illegal use of DDT in small-scale irrigated farms in Ethiopia [4, 5]. Moreover, in the Rift valley region of Ethiopia, intensively use of organophosphorus, pyrethroids, carbamides, and other currently used pesticides by small-scale and large-scale floriculture enterprises were common [6, 7]. Moreover, studies conducted along the littoral of Lake Ziway by Megnesite et al. [5] and Mergia et al. [7] reported that lack of knowledge of small-scale farmers regarding pesticide use, pouring leftover pesticides in the field, improper disposal of empty containers directly into the lake water, and overdose of pesticides application in the region. These poor pesticide use practices would contaminate the nearby lake and may produce adverse effects on aquatic organisms.
Pesticides enter the aquatic environment from various sources such as wastewater, rainwater, runoff from agricultural land, erosion, etc. [8]. Most of the pesticide chemicals are sparingly soluble in water and capable of accumulating in an organism’s fat tissue or being adsorbed by suspended solid particles. After settling on the bottom, they will accumulate in the bottom layer organisms and enter the food chain, eventually entering the human body [9]. Sediment pollution has adverse impacts on ecosystems and poses potential risks to macroinvertebrates [10, 11]. Lake sediments are frequently polluted with pesticides as a consequence of such as malaria spray and agricultural wastes [12].
The dependability of taking spot samples of water is uncertain, as there is a risk that probably detrimental pollutants can be missed if a sample is taken at the wrong time over a pollution event. The grab sampling approach is only able to collect pollutants existing in the column of water the moment the sample is taken. Passive sampling is a technique where pollutants are sequestered by an in-situ device over prolonged periods; typically 1–6 weeks [13]. Passive sampling is a new methodology used in monitoring pharmaceutical and personal care products, metabolites, pesticides, and heavy metals in aquatic ecosystems due to the fact it can concentrate different substances present in very low concentrations in water bodies. Passive sampling has been used for more than two decades; the first work about passive sampling for organic micropollutants in water was published in 1987. This technique helps a general understanding of the behavior of pesticides in aquatic ecosystems and allows get values of the time-weighted average (TWA) for different substances [14, 15]. This sampling technique increases the probability of catching different pollution events, whether they are point or diffuse. The technique measures the ‘toxicologically relevant fraction’ of contaminant mixtures and can also indirectly lower analytical detection limits [16].
The Diaion® HP-20 resin has been identified as a suitable resin for effectively accumulating lipophilic toxins from seawater [17]. Furthermore, the HP-20 resin has been used to adsorb a large amount of okadaic acid (OA) and dinophysistoxin 2 (DTX-2) from seawater in Norway [16]. The HP-20 is a porous polystyrene adsorbent resin that, by its relatively large pore sizes (260 Å), makes it suitable for the recovery of contaminants that can be eluted with common organic solvents. In the present study HP-20 passive sampler used pesticide sampling in the lake water.
Much of the knowledge of the Ethiopian rift valley lakes has come from studies on organochlorine pesticides in fish samples from Lake [18,19,20,21]. There are few studies in Ethiopian Lakes on levels of organochlorine in sediment, only one study reported DDTs in sediment from the lake Hawassa [20]. Moreover, little is known about the degree of pollution in these environments by currently used pesticides such as metalaxyl, diazinon, boscalid, etc [6]. Few studies have addressed the bioaccumulation potential of currently used pesticides (CUPs) compared with historic use pesticides (HUPs), likely resulting from a combination of the low octanol–water partition coefficients (log Kow) and short environmental persistence of these chemicals [22]. The toxic effect of CUPs on aquatic organisms is considered to be relatively low,however, studies on their adverse health effects in freshwater aquatic organisms are extremely scarce [23]. These CUPs may be reaching surface waters in significant amounts that could be an exposure risk for invertebrates, amphibians, and fish. Therefore the primary objective of this study was to evaluate the spatial distribution of OCPs and some currently used pesticides (CUPs) at Lake Ziway in the sediment and in lake water (using HP-20 passive sampler).
2 Methods
2.1 Lake Ziway and sampling sites
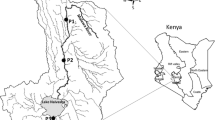
Lake Ziway (Fig. 1), is situated between 7°51–8°7 N and 38°43–38°56 E with an altitude of 1636 m above sea level located about 160 km to the south of Addis Ababa, Ethiopia. The lake covers a surface area of 442 km2 with a catchment area of 7380 km2. It is a shallow freshwater lake with an average depth of 2.5 m. The depth variations are partially explained by seasonal rainfall differences [6]. The perennial Meki River and Katar River flow into the lake, and as an exorheic lake(surface waters ultimately drain to Lakes), Lake Ziway outflows into Lake Abjatavia Bulbula River [24]. Besides the thriving fisheries, the lake is the water supply for the town, irrigation for nearby floriculture farms, and small-scale vegetable farmers near the lake. Sampling sites were selected based on their proximity and vulnerability to shoreline human activities (Table 1).

adapted from Girma et al. [25])
Map of Lake Ziway showing sampling sites (
2.2 Passive sampling disks
The HP-20 resin used in the disks has been tested and validated for a range of lipophilic biotoxins by others [17, 27], Studies after Mackenzie et al. [27] focused on the development of alternative sampler designs with DIAION® HP-20, the resin identified as the most efficient for lipophilic toxin tracking. HP-20 devices are conceptually similar to semipermeable membrane devices (SPMD) or polar organic chemical integrative samplers (POCIS) that have already been used for other trace contaminants in water [28]. According to Roué et al. [28], among all the resins tested, the Diaion® HP20 appeared the most versatile and is by far the most commonly used adsorbent substrate. The DIAION® HP20 disks were used in field studies to monitor in situ lipophilic toxin dynamics during mixed algal blooms at Flødevigen in Norway [16]. Passive sampling devices are commonly evaluated by the capacity of the sorbent medium to accumulate the mass of the compounds and the time weight average concentration of each compound.
In 2018 HP 20 disks were found from norwegian national veterinary institute (NVI) to investigate the pesticide contents in lake water from Lake Ziway, the rift valley lake Ethiopia. The samplers were deployed at 0.5 m below the surface for 20 days from November 3 till 24, 2018. The sampler holders at site 3 were lost and not considered for analysis. Passive samplers were constructed from 100 μm nylon mesh (Sefar AG, Heiden, Switzerland) folded in half, a 75 mm diameter plastic embroidery frame (Permin, Copenhagen, Denmark), and HP-20 resin (Diaion® HP-20 resin, Mitsubishi Chemical Corporation, Tokyo, Japan). The discs (8 cm in diameter) contained the absorbent resin (3.0 g HP-20) between two layers of plankton net (95 μm in mesh diameter) and clamped tightly in the embroidery frame to form a thin layer of resin between the layers of mesh. A cord was attached to the outer ring of the embroidery frame to provide a point of attachment during deployment (Fig. 2). The resin was activated by soaking the packed disk in methanol for 15 min and washing it in deionized water, as described in [16]. The activated passive sampling disks were placed in air-tight plastic bags and stored cold (at 4 ℃) before deployment in the Lake. Totally 30 discs were prepared. One blank HP-20 resin was exposed to open air during the deployment and retrieval of the HP-20 resin and was transported and analyzed as the deployed Diaion® HP-20 resin. Procedural blank consisted of HP-20 resin taken through the entire processing and analysis sequence. The disks were rinsed with distilled water and kept in the already marked plastic bags or stored at 4 °C until further preparation.
Fully assembled passive sampling disk E, and its parts: A 100 μm nylon mesh; B Diaion® HP-20 resin; C inner and D outer rings of a 75 mm diameter embroidery ring with F a No. 2 fishing swivel attached (adapted from [16])
2.3 Extraction of pesticides from disks
The extraction was done in the laboratory of the norwegian national veterinary institute (NVI), Norway. The embroidery ring containing contaminated resins were removed from the mesh and inserted into empty solid-phase extraction (SPE) glass cartridges placed on a manifold was opened, and the used resin was quantitatively transferred to a 25 ml varian bond-elute reservoir fitted with a 20 µm nylon frit (Varian, Palo Alto, CA) and washed free of salts with 30–50 ml deionized water. Excess water was drawn from the column by application of a vacuum. A 10 ml acetonitrile was used to elute slowly the resin at ca 0.5–1 drop/s flow rate and the resin was stirred gently then left to stand for 15 min. When the first elute finished, the process was repeated with another 10 ml acetonitrile. Finally, an additional 3 were pushed through to flush the column and the combined eluate. The extracts were transferred into 25 ml volumetric flasks and an additional 2 ml was used for rinsing and to complete up to the mark. Extract aliquots were taken from the volumetric flask, inserted into GC vials, and injected into a GC–MS MS system (Fig. 3).
2.4 Sediment sample collection
A total of 15 surface sediment (top 5 cm layer) samples were collected from the five sites of Lake Ziway in December 2018. Three subsamples were collected in triplicate by using Ekman grab sampler from each sampling site. The subsamples were composited in a bucket, homogenized, and cleaned for stones, pebbles, and any debris. A sediment sample aliquot (500 g) was wrapped with aluminum foil and stored in polyethylene air-tight bags and transported to the laboratory under refrigerated condition, using a cooled ice-box. The samples were kept on ice in the field and later at below −18 ℃ in the laboratory.
2.5 Sediment extraction
The extraction of pesticides from sediment samples was carried out following a modified QuEChERS procedure [29]. First,10 g of was sample was weighed into a 50 ml centrifuge tube and spiked with 50 ng/g decachlorobiphenyl (PCB no.209). Then, the samples were allowed to stand for 30 min at room temperature and then saturated with 10 ml of water. After 20 min, samples were extracted with 10 ml acetonitrile: acetic acid (99:1 v/v). A tube holding pre-weighed amounts of 4 g magnesium sulfate (MgSO4), 1 g sodium chloride, 0.5 g sodium citrate dibasic sesquihydrate, and 1 g sodium citrate tribasic dihydrate (CH3COONa2 H2O), supplied from SUPELCO, Bellefonte, PA, USA, a salt mixture was added and the tube then was shaken for 10 min using an "end over end" device (shaker) and then vortexed to ensure complete dispersion. The mixture was used to help the partitioning of the organic and aqueous phases. After centrifugation (5 min at 3000 rpm) the aliquots of the resulting supernatant was decanted into a new tube containing 900 mg magnesium sulfate and 150 mg primary secondary amine (PSA) which is used to clean organic extract and shaken for 1 min, centrifuged at 3000 rpm for 5 min and 6 mL of the acetonitrile layer was transferred into 50 mL tube. The aliquot of the extract was carefully evaporated to avoid losses of volatile compounds using a nitrogen blow-down concentrator to dryness under vacuum at a temperature −40 °C and reconstituted in acetonitrile (1 ml) for final analysis. Each sample solution was transferred to GC vials for pesticide analysis.
2.6 Pesticides and analysis methods
The pesticides were analyzed at the laboratory of the Norwegian Institute of Bioeconomy Research (NIBIO), Department of Pesticides and natural products chemistry, Norway. Individual standards of 22 OCPs (including lindane (γ-HCH, α-HCH, β-HCH, heptachlor, heptachlor epoxide cis, heptachlor epoxide trans, methoxychlor, Ooychlordane, dieldrin, Hexachlorobenzene (HCB), cis-chlordane, trans-chlordane, α-endosulfan, β-endosulfan, endosulfan sulfate, o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, and p,p′-DDT) and 7 CUPs (including chlorpyrifos, diazinon, deltamethrin, fenarimol, tetradifon, metalaxyl, and boscalid were obtained from the laboratory of the Norwegian Institute of Bioeconomy Research (NIBIO), Department of Pesticides and natural products chemistry, Norway. Reading was performed in a gas chromatograph coupled to a mass spectrometer (GC–MS/MS).
2.7 Quality assurance and quality control
Blanks and spiked samples were analyzed with each batch, with recoveries for all OCPs ranging between 72 and 120%. The extraction efficiency of the internal standard (Decachlorobiphenyl (PCB no.209).) was 93.3 ± 11.8% (n = 3). Reproducibility was typically 15% for spiked recovery studies, with detection limits ranging between 0.42 and 3.1 ng g−1 dry weight (dw). For the passive sampler, field blanks were also included, consisting of samplers that were not deployed but were exposed to air during deployment and recovery of exposed samplers. A rigorous quality assurance program was employed to ensure the quality of the data generated from this study, including the addition of a surrogate standard to account for the recovery in each sample, the addition of isotope-labeled internal standard before injection into GC to compensate for injection variability in each sample, field and procedural blanks to assess contamination, and matrix spikes to quantify method detection limits (MDLs). The recoveries between 70 and 130% were accepted for the concentration correction.
2.8 Data analysis
Initially, a descriptive analysis of the data was applied to each collection point. Analysis of variance (ANOVA) using Tukey’s test at 5% significance was applied in the analysis of spatial distribution. The data were classified by collection points (S1–S5) in the spatial distribution. All tests were considered significant when P < 0.05. Descriptive data analyses were performed using IBM SPSS version 20. In the assessment of possible environmental risks caused by p,p′DDE contamination to organisms in sediments, the concentrations identified in the samples were compared to the guidelines of Long et al. [30], Macdonald et al. [31], and CCME [32]. To assess the ecotoxicological significance of DDTs present in sediments from Lake Ziway, concentrations were compared with Canadian interim sediment quality guidelines (ISQGs), and probable effect levels (PELs) for DDE in freshwater sediments were developed according to the procedures described in CCME [32] and Smith et al. [33]. The effect range low value (ERL) and effect range median value (ERM) guidelines, as well as the threshold effect level (TEL) and probable effect level (PEL) guidelines, were applied to evaluate the possible ecological risk of p,p′DDE in sediments from the Lake Ziway.
3 Results
3.1 Passive sampler device
Among pesticides analyzed boscalid and metalaxyl were recovered from the Diaion® HP-20 rein deployed in the lake. Concentrations of all of the selected organochlorine pesticides such as γ-HCH, α-HCH, β-HCH, heptachlor, heptachlor epoxide cis, heptachlor epoxide trans, methoxychlor, oxychlordane, dieldrin, HCB, cis-chlordane, trans-chlordane, α-endosulfan, β-endosulfan, endosulfan sulfate, o,p′-DDE, o,p′-DDD, p,p′-DDE, p,p′-DDD, o,p′-DDT, and p,p′-DDT were below the detection limit in all HP-20 resin deployed at all study sites. The distribution of boscalid and metalaxyl along the Lake Ziway is shown in Table 2. Both pesticides were quantified in 100% of the samples analyzed. As accurate sampling rates for Diaion® HP-20 resin were not available, levels are presented as ng g−1 disk.
The concentration of boscalid in HP-20 disk deployed at Site 2, Site 1, Site 4, and Site 5 ranged from 18–68 ng g−1 disk, 16–30 ng g−1 disk, 2–22 ng g−1 disk, and 6–16 ng g−1 disk respectively. Unlike boscalid, the higher concentration of metalaxyl was measured in HP-20 resin deployed at the site1 with a mean of 54.7 ng g−1 (ranged 44–70 ng g−1), followed by Site 4 (54.3 ng g−1), Site 5 (53.3 ng g−1), and Site 2 (31.3 ng g−1). The mean concentrations of boscalid were significantly higher in the HP-20 resin deployed at Site 2 (39.7 ng g−1 disk) than in Site1 (21.3 ng g−1 disk, P-value = 0.039), Site 4 (14.3 ng g−1 disk, P-value = 0.003) and Site 5 (11.7 ng g−1 disk, P-value = 0.001). In contrast, the mean concentrations of metalaxyl were significantly lower in the HP-20 deployed at Site 2 (31.3 ng g−1) compared to Site 1 (54.7 ng g−1 disk, P-value = 0.007), Site 4 (54.3 ng g−1 disk, P-value = 0.008), Site 5 (53.3 ng g−1 disk, P-value = 0.011)(Table 2).
3.2 Residue in sediment samples
Pesticides analyzed in sediment samples are described in Table 3. Similar to the Passive sampler except for p,p′-DDE, and boscalid, all other selected pesticides were below the detectable limit in the surface sediments of Lake Ziway. p,p′ DDE presence was identified at all collection points and in 100% of the samples. The concentrations of p,p′-DDE in sediment samples ranged from 0.6 to 7.27 ng g−1 dw. Concentrations of p,p′DDE varied among the location of collection sites. The highest p,p′-DDE concentration was obtained in sediment samples from Site5 (at the entry of Katar River) where concentration ranged from 6.58–7.27 ng g−1 (average of 6.9 ± 0.32 ng/g dry weight), while the lowest concentration was found in samples from Site 4 ( ranged from 0.67–1.15 ng g−1 dw with a mean of 0.82 ± 0.28 ng/g dw) (Table 3). The concentration of p,p′DDE in sediment at Site 5 was statistically showed a significant difference from all other sampling sites (P < 0.001). Similarly, Site 4 was also significantly lower than all other sites. However, no significant differences in mean concentrations between Site 1, Site 2, and Site 3 were observed (Table 3).
The boscalid was detected in all sediment samples and ranged from 0.1 ng g−1 dw to 15.26 ng g−1 dry weight. The highest concentration of boscalid was measured at site 2 (near the floriculture enterprise) with a mean concentration of 11.8 ng/g dw. The order of the concentration of boscalid at different sites of the Lake Ziway surface sediment was: site 2 > site 3 > Site 5 > Site 1 > Site 4 with the mean concentration of 11.78, 2.14, 0.75, 0.68, and 0.2 ng g−1 dw respectively. The concentration of boscalid in sediment in Site 2 was statistically showed a significant difference from all other sampling sites (P < 0.001) (Table 3).
3.3 Comparison of pesticide residues in passive sampler and sediment of Lake Ziway
The majority of the analyzed pesticides did not detect in both sediment samples and HP-20 resin passive sampler deployed in Lake Ziway. A DDT metabolite p,p′DDE was quantified only in sediment samples whereas boscalid was detected in both sediment and HP-20 passive sampler deployed in all study sites. However, metalaxyl was measured only in a passive sampler (Table 4). Chlorpyrifos, diazinon, fenarimol, and deltamethrin were below detectable limit both in sediment and passive sampler even though they have higher octanol/water partition coefficient (log Kow) value compared to boscalid but all have a lower half-life in sediment (Table 4).
4 Discussion
4.1 The concentration of pesticides in the passive samplers
The HP-20 resin used in the disks has been tested and validated for a range of lipophilic biotoxins by others [17, 27], and no attempts were made to perform validation in this study. HP-20 resin uptake kinetics data are required to accurately estimate ambient water concentrations of pesticides. Therefore we couldn’t calculate the time weight average concentration of pesticides in water. Accordingly, we present the concentration of pesticides in the ng g−1 disk. Only two pesticides out of 30 target pesticides were recovered from the HP-20 resins deployed in Lake Ziway from all study sites. At all study sites, metalaxyl and boscalid were detected in 100% of the samples.
Unlike sediment sample p,p′DDE and other organochlorine pesticide residues were not found in the sampler, which is obvious as these compounds have poor water solubility so that adsorbed in the sediment organic matter and showing that the p,p′DDE measured in sediment samples could be from historical use deposition in the area. Contrasting to this study by Deribe [36] reported high concentration DDTs (199.6 ng/SPMD) in Lake Ziway from SPMD passive sampler deployed from September to October 2009. The explanation for the high concentration of DDTs in the SPMD passive sampler in their study might be the illegal use of DDT for agriculture by small-scale farmers in the study area by the time [4]. Metalaxyl is the dominant pesticide recovered from HP-20 resin deployed in our study at all study sites with concentrations ranging from 20 to 74 ng g−1 disk. Previous studies by Jansen and Harmsen [37] reported that metalaxyl in water samples collected from effluent discharge drain of the floriculture enterprise located near the Lake Ziway ranged from 0.18 to 0.51 μgL−1 and water sample at the inflow of Meki river ranged from 0.09 to 0.11 μgL−1. Similarly, a recent study by Loha et al. [38] reported that a high level of profenofos and metalaxyl in water sampling effluents from the floriculture enterprises(located near Lake Ziway) were ranged from 880 μgL−1–1700 μgL−1 and up to 210 μgl−1 respectively. These results clearly show the Lake is contaminated with the waste directly released from the floriculture enterprise, however, in all other sampling locations, residues were less pronounced because these enterprises usually spray other groups of pesticides such as ethirimol and fenarimol [37] for flower and rose productions. Moreover, Loha et al. [38] reported considerable levels of metalaxyl in tomato samples collected from small-scale farmers located around the littoral zone of Lake Ziway. The authors reported that among tomato samples analyzed, 15% and 10% of them had metalaxyl residues exceeding MRL (200 ng g−1).
A survey study conducted along Lake Ziway showed that intensive use of metalaxyl was reported by small-scale farmers [7], which might be a reason for the presence of metalaxyl in such a high concentration. A possible explanation for the occurrence of this fungicide that accumulated on the HP-20 passive sampler could be the spray drift during aerial application of pesticides on vegetables, which is a common practice in the study area. The concentration of metalaxyl in HP-20 resin deployed at sampling site 2 (near floriculture enterprise) was significantly lower than the other sampling sites, where intensive vegetable farming activities by small scale farmers (S1 S4 and S5, sampling sites), this was probably that improper use and over the use of pesticides (including metalaxyl) by small scale farmers in the area [4, 5, 7].
However, metalaxyl was not detected in the sediment samples, this might be due to the rapid degradation of metalaxyl in the environmental matrices. log Kow of metalaxyl is 1.65, therefore if released into water, metalaxyl is not expected to adsorb to suspended solids and sediment based upon the range Koc values. Malhat [39] reported Half-life value (t1/2) for degradation of metalaxyl on tomato fruits was calculated to be 1.81 days which was at the recommended dosage. However, the persistence of metalaxyl has been studied earlier by Hanumantharaju et al. [40] on tomato fruits. They reported a half-life of 5.23–6.95 days, which was higher as compared to those obtained by Malahat and Mahmoud [39]. The half-life of metalaxyl in water is 56 days [35]. This shows that metalaxyl is more stable in the water column than in the sediment phase.
In the present study, the concentration of boscalid extracted from HP-20 resin was ranged from 2–68 ng g−1 disk. There were significant differences in site to site in pesticide occurrence or concentration. There was a significant highest concentration was detected at site 2 (near the floriculture enterprise) compared to other sites (Table 2). The study revealed certain trends about the contaminant profiles in the study area. A spatial variation on the accumulation of boscalid and metalaxyl among the study sites within the lakes depends on the anthropogenic activities around the Lake.
Jansen and Harmsen [37] reported the effluent water discharging to Lake Ziway contained 20 pesticides with a concentration of 0.1 μgL−1 or higher. According to the authors, the concentration of boscalid in the effluent water from floriculture enterprise was ranged from 0.1 to 2.6 μgL−1. This shows that the main source of boscalid could be the floriculture enterprise near the lake. Additionally, surface runoff from contaminated small-scale farm soils into Lake Ziway water is also a potential input pathway of boscalid. The rainy season in the study area coincides with the pesticide application period, which favors an increase in surface runoff [41]. Studies also reported a high concentration of boscalid in water. For example, a study conducted in the largest watersheds along California’s Central Coast of Pajaro, Salinas, and Santa Maria Rivers [42] revealed that among the highest and most frequently detected pesticides in water from all the watersheds was boscalid with a maximum concentration of 36,030 ng L−1.
4.2 Residual of pesticides in sediment samples
Like passive sampler, among 30 monitored pesticides, only two pesticides (boscalid and p,p′-DDE) were detected in sediment samples collected in December 2018 from lake Ziway (Table 1). Boscalid and p,p′-DDE were detected in 100% of the samples from the Lake Ziway surface sediment. All other metabolites including the parent DDT were below the detectable limit in all samples analyzed. p,p′DDE presence was identified at all collection points and it accounted for 100% of the total DDT concentration in the sediment. Maximum concentrations ranged from 1.15 to 7.23 ng g−1 sediment dry weight (p,p′DDE). The highest level of p,p′DDE was recorded at site 5 (7.23 ng g−1 dw). This site is located at the site where the River Kater feeds the lake. Along the Katar river, there are intensively irrigating farming activities [4]. The results showed that such DDTs are from historical rather than newer applications, as a result, a large amount of DDT has been degraded to metabolites like DDE, which is difficult to degrade further [43]. Moreover, Abu-Hilal et al. [44] revealed that p,p′DDE is more stable than DDT and decomposes more slowly by micro-organisms, heat, and ultraviolet rays.
Recent data of DDT contamination in the sediment of the Ethiopian lakes are quite scanty. This is the first study to report the concentrations of organochlorine pesticides in the sediment of LakeZiway's surface sediment. Though, a recent study by Merga et al. [6] monitored endosulfan (ranged from < 0.63–2.9 ng/g dw) in the surface sediment of Lake Ziway. In the present study, however, endosulfan was below the detection limit. In our study, the concentration of p,p′DDE in the sediment (0.66–7.27 ng g−1 dw) was higher than detected in the sediment on the coast of Alicante, Spain (0.01–0.35 ng g−1) and sediment concentration of pp DDE in Volta Lake, Ghana(ranged not detected to 1.39 ng g−1 dw) [45]. However, lower than in the sediment of Ethiopian Rift valley Lake, Lake Hawassa(1.15–18.1 ng g−1 dw) [20]. Similarly, lower than in sediment of River, KwaZulu-Natal, South Africa(20.61–38.72 ng g−1 dw) [46], in sediment of Honghu Lake, China(1.3–22.23 ng g−1 dw) [47], p,p′DDE sediment concentration in Tanzania Rift valley Lake, Lake Natron ranged from 4.6–15.9 ng g−1 dw [48]. In the Tanzanian side of Lake Victoria, the concentrations of ΣDDTs ranged between the detection limit and 12 ng g−1 dw [49], 3.75 to 14.40 ng g−1 dw [50], and 0.03 to 9.67 ng g−1 dw [51] in sediments of Lake Bosomtwi (Ghana) and Lake Victoria (Uganda), respectively.
Results obtained revealed differences in pollutant profiles between the study sites investigated. p,p′-DDE concentrations showed a clear difference between sites, with concentrations at S5 significantly higher than concentrations at all other sites (P < 0.005). p,p′-DDE concentration at site 4 is also significantly different from Site 1, Site 2, and Site3. Spatial variations in p,p′-DDE concentrations can be attributed to previously used IRS and illegal agricultural use of DDT which reflect the persistent nature of p,p′-DDE within respective catchment areas. Site 2 are considered low-risk malaria areas, while outbreaks of malaria have historically been more common around Mike River (Site 4) and Katar River (Site5) [5].
Boscalid, a carboxamide fungicide registered in Ethiopia is a systemic fungicide used on a wide variety of food crops and ornamental plants [4]. It is stable to hydrolysis and also photolysis in soil and water. In aquatic systems, boscalid is not significantly transformed under aerobic or anaerobic conditions but is relatively rapidly transferred from the water phase to the sediment phase through sorption to sediment (dissipation half-life of < 2 weeks [52]. Moreover from the European database (https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/86.htm, degradation half-life is estimated to be 545 days in the water–sediment phases, while metalaxyl is 32 days which was not detected in all sediment samples. In the present study regarded to currently used pesticides, boscalid was the most frequently detected pesticide in all study sites. To our knowledge, this is the first study that has reported boscalid detections in the sediment of Ethiopian lakes. The frequent application of fungicides by small scale vegetable farmers around lake Ziway (typically 15–20 applications per season [5, 7]) is reflected by the consistent presence of boscalid creating a relatively constant source of these chemicals to Lake Ziway that receive drainage or irrigation return flow from treated vegetable farmlands along the littoral of Lake Ziway. Few studies have addressed the bioaccumulation potential of currently used pesticides (CUPs) compared with historic use pesticides (HUPs), likely resulting from a combination of the low octanol–water partition coefficients (log Kow) and short environmental persistence of these chemicals.
The higher concentration of boscalid in sediment was measured at site 2 ranged from 8.87–15.26 ng g−1 dw and was significantly different from all other study sites. These differences are likely due to differences in the types and application intensity of pesticides used in the areas closer to the sampling sites. The highest incidence of contamination (boscalid) at S2 occurred possibly due to the location near the flower farm discharge into the lake. Malefia [26] reported this point at the lake is subject to a greater accumulation of sediments/pesticides from the floriculture enterprise due to the wastewater flowing directly into the lake. The detection of the boscalid residues in sediment samples implied recent application since they are environmentally unstable. These findings are consistent with those observed in a study conducted in tomato fields in Owiro Estate, Tanzania where the detection of chlorpyrifos was related to the recent use [53]. This indicates that the sediment of the lake is probably contaminated with other pesticides released from the surrounding agricultural and floricultural activities. At Site 3 the second-highest concentration of boscalid was recorded, this is probably the small-scale farmers near the lake who used the boscalid that is illegality bought from a floriculture campy situated near Lake Ziway. A survey study conducted in the rift valley region by Negatu et al. [4] reported the use of boscalid by small-scale vegetable farmers around the study area.
In the present study, the other targeted currently used pesticides such as chlorpyrifos, diazinon, deltamethrin, dupirimate, and tetradifon were below the detectable limit both in sediment and passive sampler even though they have higher octanol/water partition coefficient (log Kow) than boscalid and metalaxyl (Table 4). However, other factors also influence pesticide accumulation such as the half-life, application moment, and the time that elapses before the next major storm event [54]. For example, the half-life of boscalid in sediment (545 days) is much higher compared to the other currently used pesticides (Table 4). Ccanccapa et al. [54] also reported that ubiquitous pesticides are those with long half-lives. However, a recent study conducted by Merga et al. [6], reported currently used pesticides in Lake Ziway sediment. These pesticides include diazinon concentrations ranged from > 0.36–0.74, chlorpyrifos ranged from 0.71–0.88 ng g−1 dw, dimethoate ranged from not detected to 0.32 ng g−1 dw and γ-cypermethrin ranged from > 0.71–1.97 ng/g dw were frequently detected (degree of frequency > 30%)in the sediment samples. Moreover, a study in Kenya reported the concentration of diazinon (0.56–1.08 ng g−1 dw) and dimethoate (0.2–0.29 ng g−1 -dw) in the Nyando-Sondu-Miriu River sediment [55], at locations closer to smallholder farms, the point of effluent from the floriculture and the point of inflow of the rivers. This is following the present study that the sediment of the lake is probably contaminated with pesticides released from the surrounding agricultural and floricultural activities. Merga et al. [6] observed high concentrations and the number of pesticides in the wet season sediment samples is likely a result of the high load of pesticides adsorbed to sediments via runoff [56] from the agricultural area in the catchment of the studied lake.
4.3 Comparison of pesticide residues in passive sampler and sediment of Lake Ziway
The passive sampler method allows for the detection of chemical pesticides directly in the water column and offers numerous advantages over monitoring using organisms sampling techniques (such as fish testing) and sediment sampling [57]. Moreover, passive samplers don’t move and metabolism/excretion of sampled chemicals is not an issue. Furthermore no need to analyze multiple tissues to obtain the full picture of exposure to dissolve phase chemicals [16]. However, the disadvantages include passive sampler does not assess exposure to particle-sorbed chemical via e.g., feeding, and do not indicate historical contamination [58]. In the present study for example p,p′ DDE was not detected in the passive sample but detected in 100% of sediment samples. The reason could be the DDE detected was from historical use and adsorbed to organic particles. Metalaxyl was prominent in the passive sampler. This pesticide is water-soluble and mobile can be easily accessed by the passive sampler from the water column.
Although HP-20 Passive sampler is mainly known for absorbing hydrophobic nonpolar chemicals with a log Kow of > 3 [16] metalaxyl accumulated in the passive sampler than other quantified pesticides. The reason for the high accumulation of metalaxyl in the HP-20 Passive sampler deployed in Lake Ziway than other pesticides is unclear, needs further study. Probably it could be the high concentration of metalaxyl due to the intensive and overuse of the metalaxyl by small-scale frames around the watershed of Lake Ziway [5, 7]. The other reason may be metalaxyl has a longer half-life in water (56 days) than boscalid and p,p′DDE (Table 4). So that it is easily accessible by the passive sampler.
Monitoring pesticides in sediment samples is important because of many contaminants and their largely hydrophobic nature; they are known to associate strongly with natural sediments and dissolved organic matter [59]. Pesticides are adsorbed onto the organic matter incorporated in sediments and the persistence depends on their physicochemical properties. Lake sediments are effective supervising tools to appraise pollution episodes, are singular in furnishing historical contamination, and reflect the quality of surface water [60]. In the present study, this is confirmed that the metabolite of DDT (p,p´DDE) which is the most persistent in the environment was the prominent organochlorine pesticide in the sediment samples of Lake Ziway. Boscalid was also quantified in the sediment but metalaxyl was not.
4.4 Potential ecological risk assessment
No environmental standards have been established for OCPs in sediments in Ethiopia. The possible ecological risk of p,p′DDE in sediments from Lake Ziway was summarized in Table 5. The level below the ERL value indicates that adverse biological effects of aquatic biota would be rarely observed, and the level above the ERM value represents that adverse effects would frequently occur. The TEL and PEL were considered to be reliable when the incidence of adverse biological effects of aquatic biota below the TEL was 25% or less and above the PEL was 50% or greater [31, 33]. Accordingly, criteria such as the interim sediment quality guideline (ISQG) and the probable effect level (PEL) for p,p′DDE were compared with their respective concentrations at different sites of lake Ziway sediments. Compared to the criteria for p,p′DDE, (ISQG: 1.42, in ng g−1 dw,), all sediment samples in the present study exceed the TEL values for p,p′DDE at all study sites. This result indicated the medium risk of DDE in the Lake Ziway sediment. 100% of sediment samples analyzed at S1, S2, and S5 in this study revealed concentrations exceeded ER-L values for p,p′DDE whereas most (67%) of the sediment sample at S3 exceeded ER-L values, indicating that severe biological effects related to p,p´DDE would occur occasionally at these sampling locations in the Lake Ziway. However, none of the samples at S4 exceeded the ER-L values, indicating that this pp DDE might possess rare adverse biological effects on local aquatic organisms. The concentrations of p,pʹDDE, at all sites were lower than their respective ER-M and guidelines(Table 5), indicating that p,p′DDE do not likely to pose detrimental biological effects. The high concentrations detected in sediments from Site 5 have the potential to cause adverse biological effects. With regards to boscalid, the sediment quality guideline was not available.
Early studies revealed that DDT and its metabolites could affect the ecology of the organism living in the sediment. The abundance of several groups of benthic invertebrates, including chironomids, gastropods, and amphipods, was reduced in Midland Bay, Lake Huron, at concentrations of DDE ranging from 2.39 to 2.63 ng g−1, which are above the freshwater ISQG of 1.42 ng g−1 dw [60, 61]. Similarly, benthic species richness was low in sediments from Toronto Harbour, Ontario, with 11.5 μg·kg1 of DDE, which is above the freshwater PEL of 6.75 μg·kg−1. However, sediments containing DDE concentrations of 0.625 ng g−1, below the freshwater ISQG, exhibited a high degree of species richness [61, 62]. Organochlorine pesticides(OCPs) such as DDT, have been linked to several toxicological effects in various bird species, including, great white pelicans, hamerkop, African sacred ibis, and marabou stork in Ethiopia [63], and white stork nestlings in Spain [64]. In South Africa, elevated levels of DDE (up to 300 ng g−1 wet weight) have been linked to eggshell thinning in African darter populations.
5 Conclusion
This study has confirmed the presence of pesticides in Lake Ziway from local agricultural activities and floriculture enterprises located near the lake. Except for p,p′DDE all selected organochlorine were below the detectable limit in the sediments of Lake Ziway. The dominance of sediment metabolites p,p′DDE depicted that DDT contamination was mainly from the historical usage and significant degradation has occurred. In comparison to published guidelines, p,p′DDE levels were at moderate levels, which may cause adverse biological effects to aquatic animals. Only boscalid and metalaxyl were quantified in passive sampler HP-20 resin deployed in the Lake. Moreover, the absence of organochlorine pesticides in HP-20 resin, indicates a decreasing trend of the use of legacy pesticides by farmers around the study area. Moreover, The high detection frequency of fungicides(boscalid and metalaxyl) in this study is representative of Lake Ziway is influenced by small-scale agriculture and floriculture industries in the area. The study showed certain trends in the contaminant profiles in the study area. A spatial variation on the accumulation of p,p′DDE in sediment, boscalid, and metalaxyl both in sediment and HP-20 resin among the sampling sites within the lake depends on the anthropogenic activities around the Lake Ziway.
Data availability and materials
All data generated or analyzed during this study are included in the manuscript and supplementary information.
References
Majumder SP, Das AC (2016) Phosphate-solubility and phosphatase activity in Gangetic alluvial soil as influenced by organophosphate insecticide residues. Ecotoxicol Environ Saf 126:56–61. https://doi.org/10.1016/j.ecoenv.2015.12.018
Mansour SA (2009) Persistent organic pollutants (POPs) in Africa: Egyptian scenario. Hum Exp Toxicol 28(9):531–566. https://doi.org/10.1177/0960327109347048
Biscoe ML, Mutero CL, Kramer RA (2005) Current Policy and Status of DDT Use for Malaria Control in Ethiopia, Uganda, Kenya, and South Africa Colombo, Sri Lanka: International Water Management Institute (IWMI). http://ageconsearch.umn.edu/bitstream/92015/2/WOR95.pdf. Accessed 27 Feb 2021
Negatu B, Kromhout H, Mekonnen Y, Vermeulen R (2016) Use of Chemical Pesticides in Ethiopia: a cross-sectional comparative study on Knowledge, Attitude, and Practice of farmers and farmworkers in three farming systems. Ann Occup Hyg 60(5):551–566. https://doi.org/10.1093/annhyg/mew004
Mengistie BT, Mol AP, Oosterveer P (2017) Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley. Env Develop Sustain 19(1):301–324. https://doi.org/10.1007/s10668-015-9728-9
Merga LB, Mengistie AA, Alemu MT, Van den Brink PJ (2021) Biological and chemical monitoring of the ecological risks of pesticides in Lake Ziway, Ethiopia. Chemosphere 266:129214. https://doi.org/10.1016/j.chemosphere.2020.129214
Mergia MT, Weldemariam ED, Eklo OM, Yimer GT (2021) Small-scale farmer pesticide knowledge and practice and impacts on the environment and human health in Ethiopia. J Health Pollut 11(30):210607. https://doi.org/10.5696/2156-9614-11.30.210607
Schwarzenbach RP, Egli T, Hofstetter TB, Von Gunten U, Wehrli B (2010) Global water pollution and human health. Annu Rev Environ Resour 35:109–136. https://doi.org/10.1146/annurev-environ-100809-125342
Zehra A, Katsoyiannis A, Schuster JK, Moeckel C, Jones KC, Malik RN (2015) Environmental monitoring of organo-halogenated contaminants (OHCs) in surface soils from Pakistan. Sci Total Environ 506–507:344–352. https://doi.org/10.1016/j.scitotenv.2014.10.055
Ahmed G, Anawar HM, Takuwa DT, Chibua IT, Singh GS, Sichilongo K (2015) Environmental assessment of fate, transport, and persistent behavior of dichlorodiphenyltrichloroethanes and hexachlorocyclohexanes in land and water ecosystems. Int J Environ Sci Technol 12:2741–2756. https://doi.org/10.1007/s13762-015-0792-3
Bai J, Lu Q, Zhao Q, Wang J, Gao Z, Zhang G (2015) Organochlorine pesticides (OCPs) in wetland soils under different land uses along a 100-year chronosequence of reclamation in a Chinese estuary. Sci Rep 5:17624. https://doi.org/10.1038/srep17624
Unyimadu JP, Osibanjo O, Babayemi JO (2019) Concentration and distribution of organochlorine pesticides in sediments of the niger river, Nigeria. J Health Pollut 9(22):190606. https://doi.org/10.5696/2156-9614-9.22.190606
Namieśnik J, Zabiegała B, Kot-Wasik A, Partyka M, Wasik A (2005) Passive sampling and/or extraction techniques in environmental analysis: a review. Anal Bioanal Chem 381(2):279–301. https://doi.org/10.1007/s00216-004-2830-8
Vrana B, Allan IJ, Greenwood R, Mills GA, Dominiak E, Svensson K, Morrison G (2005) Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem 24(10):845–868. https://doi.org/10.1016/j.trac.2005.06.006
Booij K, Vrana B, Huckins JN (2007) Theory, modeling, and calibration of passive samplers used in water monitoring. Compr Anal Chem 48:141–169. https://doi.org/10.1016/S0166-526X(06)48007-7
Rundberget T, Gustad E, Samdal IA, Sandvik M, Miles CO (2009) A convenient and cost-effective method for monitoring marine algal toxins with passive samplers. Toxicon 53:543–550. https://doi.org/10.1016/j.toxicon.2009.01.010
Fux E, Marcaillou C, Mondeguer F, Bire R, Hess P (2008) Field and mesocosm trials on passive sampling for the study of adsorption and desorption behavior of lipophilic toxins with a focus on OA and DTX1. Harmful Algae 7(5):574–583. https://doi.org/10.1016/j.hal.2007.12.008
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Eklo OM (2013) Biomagnification of DDT and its metabolites in four fish species of a tropical lake. Ecotoxicol Environ Saf 95:10–18. https://doi.org/10.1016/j.ecoenv.2013.03.020
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Eklo OM (2014) Organochlorine pesticides, and polychlorinated biphenyls in fish from Lake Awassa in the Ethiopian Rift Valley: human health risks. Bull Environ Contam Toxic 93(2):238–244. https://doi.org/10.1007/s00128-014-1314-6
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake—Lake Ziway. Ecotox and Environ Saf 106:95–101. https://doi.org/10.1016/j.ecoenv.2014.04.014
Teklu BM, Hailu A, Wiegant DA, Scholten BS, Van den Brink PJ (2018) Impacts of nutrients and pesticides from small-and large-scale agriculture on the water quality of Lake Ziway. Environ Sci and Poll Res 25(14):13207–13216. https://doi.org/10.1007/s11356-016-6714-1
Nogueira EN, Dores EF, Pinto AA, Amorim RS, Ribeiro ML, Lourencetti C (2012) Currently used pesticides in water matrices in Central-Western Brazil. J Braz Chem Soc 23(8):1476–1487. https://doi.org/10.1590/S0103-50532012005000008
Hoferkamp L, Hermanson MH, Muir DC (2010) Current use pesticides in Arctic media; 2000–2007. Sci Total Environ 408(15):2985–2994. https://doi.org/10.1016/j.scitotenv.2009.11.038
Ayenew T (2007) Water management problems in the Ethiopian rift: Challenges for development. J Afr Earth Sci 48:222–236. https://doi.org/10.1016/j.jafrearsci.2006.05.010
Girma H, Hugé J, Gebrehiwot M, Van Passel S (2021) Farmers’ willingness to contribute to the restoration of an Ethiopian Rift Valley lake: a contingent valuation study. Environ Dev Sustain 23(7):10646–10665. https://doi.org/10.1007/s10668-020-01076-3
Malefia T (2012) Environmental impacts of floriculture industries on Lake Ziway: Pollution profiles of Lake Ziway along with floriculture industries. LAP LAMBERT Academic Publishing, Germany. Available at: https://www.amazon.com/Environmental-Impacts-Floriculture-Industries-Ziway/dp/3848444178. Accessed 20 Sept 2020
MacKenzie L, Beuzenberg V, Holland P, McNabb P, Selwood A (2004) Solid-phase adsorption toxin tracking (SPATT): a new monitoring tool that simulates the biotoxin contamination of filter-feeding bivalves. Toxicon 44(8):901–918. https://doi.org/10.1016/j.toxicon.2004.08.020Get
Roué M, Darius HT, Chinain M (2018) Solid phase adsorption toxin tracking (SPATT) technology for the monitoring of aquatic toxins: A review. Toxins 10(4):167. https://doi.org/10.3390/toxins10040167
Norli HR, Christiansen A, Deribe E (2011) Application of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography-mass spectrometry. J Chromatogr 1218(41):7234–7241 https://doi.org/10.1016/j.chroma.2011.08.050
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19(1):81–97. https://doi.org/10.1007/BF02472006
Macdonald DD, Carr RS, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5(4):253–278. https://doi.org/10.1007/BF00118995
Canadian Council of Ministers of the Environment (CCM) (1995) Protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. Vol. 1 Canadian Council of Ministers of the Environment. Available at: https://www.ccme.ca/en/res/mercury-canadian-sediment-quality-guidelines-for-the-protection-of-aquatic-life-en.pdf. Accessed 20 Sept 2020
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Jay Field L (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystem. J Great Lakes Res 22(3):624–638. https://doi.org/10.1016/S0380-1330(96)70985-1
The United States Environmental Protection Agency (USEPA) (2008) Regulatory determinations support document for selected contaminants from the second drinking water contaminant candidate list (CCL2) Available at: https://www.epa.gov/sites/production/files/2014-09/documents/chapter_5_dde.pdf. Accessed 2 Oct 2020
EUPPDB: Pesticide Properties Database. Available at: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/445.htm. Accessed 25 Mar 2021
Deribe E (2018) Investigating the Spatial Trends in the Level of Organic Contaminants in the Ethiopian Rift Valley Lakes Using Semipermeable Membrane Devices. Bull Environ Contam Toxico 101:20–25. https://doi.org/10.1007/s00128-018-2358
Jansen HC, Harmsen J (2011) Pesticide monitoring in the Central Rift Valley 2009–2010: ecosystems for water in Ethiopia. No. 2083. Alterra, 2011. Available at: https://library.wur.nl/WebQuery/wurpubs/fulltext/173773
Loha KM, Lamoree M, de Boer J (2020) Pesticide residue levels in vegetables and surface waters at the Central Rift Valley (CRV) of Ethiopia. Environ Monit Assess 192:546. https://doi.org/10.1007/s10661-020-08452-6
Malahat FM (2017) Persistence of metalaxyl residues on tomato fruit using high-performance liquid chromatography and QuEChERS methodology. Arab J Chem 10:S765–S768. https://doi.org/10.1016/j.arabjc.2012.12.002
Hanumantharaju TH, Awasthi MD, Siddaramappa R (2002) Persistence of fungicides in tomato following the application of metalaxyl-mancozeb combined formulation. Pestic Res J 14:268–275
Otieno PO, Schramm KW, Pfister G, Lalah JO, Ojwach SO, Virani M (2012) Spatial distribution and temporal trend in concentration of carbofuran, diazinon and chlorpyrifos ethyl residues in sediment and water in Lake Naivasha, Kenya. Bull Environ Contam Toxico 88(4):526–532. https://doi.org/10.1007/s00128-012-0529-7
Smalling KL, Orlando JL (2011) Occurrence of pesticides in surface water and sediments from three Central California coastal watersheds, 2008–09. US Geological Survey. Data Series 600 70. Available at: https://pubs.usgs.gov/ds/600/. Accessed 5 Apr 2021
Pereira VJ, da Cunha JP, de Morais AR, de Oliveira JPR, de Morais JB (2016) Physical-chemical properties of pesticides: concepts, applications, and interactions with the environment. Biosci J 32(3). https://doi.org/10.14393/BJ-v32n3a2016-31533
Abu-Hilal AH, Badran M, Vatigelas J (1988) Redistribution of trace elements in Callichirus laurae burrows (Crustacea, Thalassinidea), Jordan Gulf of Aqaba (Red Sea). Mar Environ Res 25:233–248. https://doi.org/10.1016/0141-1136(88)90014-1
Gbeddy G, Glover E, Doyi I, Frimpong S, Doamekpor L (2015) Assessment of organochlorine pesticides in water, sediment, African cat fish and nile tilapia, consumer exposure and human health implications, Volta Lake. Ghana. J Environ Anal Toxicol 5(4):297. https://doi.org/10.4172/2161-0525.1000297
Gakuba E, Moodley B, Ndungu P, Birungi G (2018) Partition distribution of selected organochlorine pesticides in water, sediment pore water, and surface sediment from Mngeni River, KwaZulu-Natal, South Africa. Water. https://doi.org/10.4314/wsa.v44i2.09
Yuan L, Qi, S, Wu, X, Wu, C, Xing, X, Gong X (2013) Spatial and temporal variations of organochlorine pesticides (OCPs) in water and sediments from Honghu Lake, China. J Geochem Explorat 132:181–187. https://doi.org/10.1016/j.gexplo.2013.07.002
Bettinetti R, Quadroni S, Crosa G, Harper D, Dickie J, Kyalo M, Mavuti K, Galassi S (2011) A preliminary evaluation of the DDT contamination of sediments in Lakes Natron and Bogoria (Eastern Rift Valley, Africa). Ambio 40(4):341–350. https://doi.org/10.1007/s13280-011-0142-8
Kishimba MA, Henry L, Mwevura H, Mmochi AJ, Mihale M, Hellar H (2004) The status of pesticide pollution in Tanzania. Talanta 64(1):48–53. https://doi.org/10.1016/j.talanta.2003.11.047
Darko G, Akoto O, Oppong C (2008) Persistent organochlorine pesticide residues in fish, sediments, and water from Lake Bosomtwi, Ghana. Chemosphere 72:21–24. https://doi.org/10.1016/j.chemosphere.2008.02.052
Wasswa J, Kiremire BT, Nkedi-Kizza P, Mbabazi J, Ssebugere P (2011) Organochlorine pesticide residues in sediments from the Uganda side of Lake Victoria. Chemosphere 82(1):130–136. https://doi.org/10.1016/j.chemosphere.2010.09.010
The United States Environmental Protection Agency (USEPA) (2005) Overview of ecological risk assessment process in the office of pesticide programs, U.S Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Washington, DC. Available at: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/ecological-risk-assessment-pesticides-technical. Accessed 22 Oct 2020
Kihampa CH, Mato RR, Mohamed H (2010) Residues of organochlorinated pesticides in soil from tomato fields, Ngarenanyuki, Tanzania. J Appl Sci Environ Manag 14:3. https://doi.org/10.4314/jasem.v14i3.61462
Ccanccapa A, Masiá A, Andreu V, Picó Y (2016) Spatio-temporal patterns of pesticide residues in the Turia and Júcar Rivers (Spain). Sci Total Environ 540:200–210. https://doi.org/10.1016/j.scitotenv.2015.06.063
Musa S, Gichuki JW, Raburu PO, Aura CM (2011) Risk assessment for organochlorines and organophosphates pesticide residues in water and Sediments from lower Nyando⁄Sondu-Miriu river within Lake Victoria Basin, Kenya. Lakes Reservoirs Res Manag 16:273–280. https://doi.org/10.1111/j.1440-1770.2011.00486.x
Papadakis EN, Tsaboula A, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E (2018) Pesticides in the rivers and streams of two river basins in northern Greece. Sci Total Environ 624:732–743. https://doi.org/10.1016/j.scitotenv.2017.12.074
Kianpoor KY, Jabbarian AB, Huang B, Henareh KA, Hu W, Gao H, Thompson ML (2019) Methods for sample collection, storage, and analysis of freshwater phosphorus. Water 11(9):1889. https://doi.org/10.3390/w11091889
Regan F, Jones L, Ronan J, Crowley D, McGovern E, McHugh B (2018) Role of Passive Sampling in Screening and Monitoring of New and Emerging Chemicals. Available at: http://www.epa.ie/researchandeducation/research/researchpublications/researchreports/Research_Report_243.pdf. Accessed 25 Mar 2021
Rocha AA, Monteiro SH, Andrade GC, Vilca FZ, Tornisielo VL (2015) Monitoring of pesticide residues in surface and subsurface waters, sediments, and fish in center-pivot irrigation areas. J Braz Chem Soc 26(11):2269–2278. https://doi.org/10.5935/0103-5053.20150215
Miranda K, Cunha ML, Dores EF, Calheiros DF (2008) Pesticide residues in river sediments from the Pantanal Wetland, Brazil. J Environ Sci Health B 43(8):717–722. https://doi.org/10.1080/03601230802388843
Jaagumagi R (1988) The in-place pollutants program. Volume V, Part B. Benthic invertebrates studies results. Ontario Ministry of the Environment. Water Res Branch, Aquatic Biol Sect, Toronto
Jaagumagi RD, Persaud TL (1989) The in-place pollutants program. Volume V, Part A. A synthesis of benthic invertebrate studies. Ontario Ministry of the Environment. Water Res Branch, Aquatic Biol Sect, Toronto
Yohannes YB, Ikenaka Y, Nakayama SM, Ishizuka M (2014) Organochlorine pesticides in bird species and their prey (fish) from the Ethiopian Rift Valley region, Ethiopia. Environ Pollut 192:121–128. https://doi.org/10.1016/j.envpol.2014.05.007
de la Casa-Resino I, Hernández-Moreno D, Castellano A, Pérez-López M, Soler F (2015) Chlorinated pollutants in blood of White stork nestlings (Ciconia ciconia) in different colonies in Spain. Chemosphere 118:367–372. https://doi.org/10.1016/j.chemosphere.2014.10.062
Acknowledgements
The lab work was assisted by staff members at NIBIO, Pesticide, and natural products chemistry, Norway. We thank Marianne Stenrød the head of the Pesticide and natural product chemistry section, Roman Florinski, Agnethe Christiansen, and Ellen Aarrestad for their help during laboratory work. The Norwegian National Veterinary Institute is acknowledged for providing the HP-20 sampler used in this study. The authors are extremely grateful to Morten Sandvik (Norwegian National veterinary institute) for technical support during the extraction of pesticides from the sampler. We thank Ziway Fishery research center staff especially, Mathios Hauilu, Alemu, Ashu Beteshi, and Abraham G /Tsadik for their help during sample collection.
Funding
This research was funded by the Institutional Collaboration program between Hawassa University and the Norwegian University of Life science (NMBU-HU ICOP –IV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors declare that there is no conflict of interest associated with the subject of the article.
Consent of publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mergia, M.T., Weldemariam, E.D., Eklo, O.M. et al. Pesticide residue levels in surface water, using a passive sampler and in the sediment along the littoral zone of Lake Ziway at selected sites. SN Appl. Sci. 4, 83 (2022). https://doi.org/10.1007/s42452-022-04966-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04966-5