Abstract
Anthropogenic land-use changes pose significant threats to the diversity and occurrence of wildlife species around the world. We investigated how land-use and environmental factors affect the richness and occurrence of carnivore species in the Faragosa-Fura Landscape of the Southern Rift Valley of Ethiopia. We used the line transect method to collect data, combining three complementary field survey techniques: sign survey, camera trapping, and opportunistic sighting surveys. We confirmed the presence of 12 carnivore species belonging to six families, including the vulnerable Felidae species Panthera pardus. More species were found in the family’s Felidae and Herpestidae, while Hyaenidae and Mustelidae were composed of a single species each. The two large-sized species identified were Panthera pardus and Crocuta crocuta. The species richness was the highest in wetlands, while it was the lowest in the settlement. The occurrence of most carnivores was negatively associated with agricultural land and settlements, while they were positively associated with wetlands and altitude. Genetta genetta had the highest occurrence, while Panthera pardus had the lowest in the area. We concluded that of the studied habitats, wetlands are the most important, and anthropogenic land-uses have a negative impact on species richness. Our findings provide valuable baseline data for stakeholders making critical conservation decisions as well as researchers conducting related ecological studies in a human-dominated landscape. Based on our findings, we propose a basic approach for integrating land management and wildlife conservation.
Article Highlights
-
Twelve carnivore species belonging to six families were identified; only two were large-sized.
-
The species richness was the highest in wetlands, while it was the lowest in settlements and agricultural land.
-
Most species tended to have positive associations with higher altitudes and wetlands and negative associations with settlements, agricultural land, and roads.
-
Our finding highlights valuable baseline data for critical conservation decisions as well as researchers in a human-dominated landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic land-use changes pose significant threats to the diversity of wildlife species around the world. Recent research has found that anthropogenic and environmental factors can have an impact on the richness and occurrence of carnivore species in various habitats [5, 22]. Agriculture is the backbone of socioeconomic development in developing countries like Ethiopia, but it often comes at the expense of biodiversity and ecosystem services [4].
The two most common conservation strategies are creating protected areas to separate carnivores from human-dominated areas [29] and encouraging human-carnivore coexistence, a sustainable state in which humans and wildlife co-adapt to living in a shared landscape [32, 35]. However, protected areas and native forests are rapidly dwindling as a result of anthropogenic habitat loss, forcing carnivores to local extinction or to share habitats with humans [5, 17, 22]. This sharing of habitat leads to human–carnivore conflicts, which have an impact on both local community livelihood and biodiversity conservation [35, 37].
About 95% of the ranges of carnivore species occur outside protected areas in a human-dominated landscape [27]. Thus, understanding the carnivore species that coexist with humans is important for both carnivore conservation and the local people's livelihood [6, 28]. As a result, for carnivore conservation to be successful, both protected areas and the human-dominated landscape must be addressed [10]. Furthermore, the human-dominated landscape can be very important for the conservation of medium- to small-sized carnivores [3, 6]. As a result, habitats within a human-dominated landscape are becoming increasingly important for carnivore conservation, as protected areas are shrinking.
Carnivora is composed of 290 species, belonging to 16 families. Of these, 72 and 32 different species have been found in Africa [24], and Ethiopia [19, 38], respectively. The six families of mammalian carnivores identified in Ethiopia are Felidae, Viverridae, Herpestidae, Hyaenidae, Canidae, and Mustelidae [38]. The endemic Ethiopian wolf is one of the world's most endangered carnivore species as a result of habitat loss [17]. Furthermore, African lions and leopards are on the verge of extinction in a number of areas [19]. Understanding a region's species richness (number of species) and occurrence is a critical first step toward biodiversity conservation. These two biodiversity attributes have primarily been used in assessing the wildlife conservation potential of areas and developing conservation strategies. A greater number of species and groups indicates a more healthy community, which leads to greater system stability [19, 27].
Carnivores have been extensively studied at the community level in various parts of the world [29]. Nonetheless, carnivore surveys in Africa have focused on single species rather than communities [3, 10]. The same is true in Ethiopia, where no quantitative studies on carnivores have been conducted at the community level, and they only exist at the species level [1]. For example, studies on lions and hyenas [39, 40], leopards [20], African civets [23], white-tailed mongoose [1], and Ethiopian wolves [13] have been conducted.
Most protected areas in Ethiopia are too small to support wide-ranging carnivore species [19]. As a result, carnivores are shifting to a human-dominated landscape and coexisting with humans, implying that biodiversity conservation efforts in shared landscape are necessary. The study of coexisting carnivore species with humans at the community level in the human-dominated landscape, on the other hand, is largely unknown.
We conducted this research in the Faragosa-Fura Landscape (hereafter FFL) in the Mirab Abaya district of the Gamo Zone, Southern Ethiopia. The study landscape is diverse and primarily a forest area with distinct carnivore species. The study area is linked to Lake Abaya, the largest lake in the Ethiopian Rift Valley system, which serves as the main water source for carnivores and the lake-created wetlands. Understanding the species richness and occurrence of carnivore species in the area is thus important for immediate management actions. Moreover, there are no earlier ecological studies carried out in the study area vis-à-vis carnivores.
Because of resource differences, land-uses such as forest, grassland, wetland, agricultural land, and human settlement [37] are influential for carnivore species richness and occurrence. Furthermore, altitude and distance from the road [10, 34] are environmental factors that influence the richness and occurrence of carnivore species. We hypothesized that (1) human-dominated landscapes with higher levels of anthropogenic land-uses support a lower species richness and occurrence of carnivores compared to areas with more natural habitats; (2) carnivore species richness is negatively affected by higher levels of anthropogenic land-uses compared with more natural habitats; and (3) the distribution of different carnivore species negatively associated with anthropogenic land-uses at the landscape scale. We therefore (1) compared wild carnivore species richness between higher anthropogenic land-uses (agriculture, settlement) and more natural habitats (wetland, forest, and grassland), (2) examined the effects of land-uses (agriculture, settlement, wetland, forest, grassland, and road) on the species richness, and (3) evaluated the effects of land-uses on the specific carnivore species inhabiting this area.
2 Materials and methods
2.1 Study area
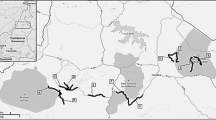
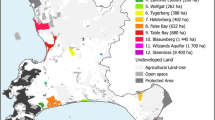
We conducted this research at the FFL in the Mirab Abaya district, Gamo Zone, Southern Ethiopia. The FFL has an area of about 100 km2 and is located between 06°10′12″ and 06°15′00″ N latitude and 37°42′36″ to 37°47′24″ E longitude, with elevations ranging from 1184 to 1795 m a. s. l. (Fig. 1).
The mean monthly temperature and rainfall of the study area range from 14 to 26 °C and 41 mm to 161 mm, respectively [12]. It is bordered to the north by Ankober and Faragosa kebeles (the lowest administrative units), to the east by Done kebele, to the west and southwest by Lake Abaya, and to the south by Umo-Lante and Fura kebeles.
There is one main asphalt road from Addis Ababa to Arba Minch that crosses the study landscape, making it easily accessible. Besides, the area is predominantly rural, characterized by settlements and surrounded by agricultural land and a forested landscape. The FFL is linked with Lake Abaya, the largest lake in the Ethiopian Rift Valley system. Thus, the area is important for wildlife conservation in Ethiopia. The main economic activities are farming, livestock breeding, beekeeping, and the collection of forest products. As a result, anthropogenic activities such as agricultural expansion for vegetables and banana plantations, as well as livestock rearing, are putting pressure on the landscape [21].
The common flora observed in the area were Vachellia and Senegalia spp., Terminalia brownie, Dodonaea angustifolia, Acalypha fruticosa, Maytenus arbutifolia, Olea europaea, Ximenia americana, Bridelia scleroneura, Maytenus undata, Vangueria apiculata, Balanites aygiaptica, Rhus vulgaris, and Ozoroa insigns. The wild animals in the forest and on Lake Abaya include dik dik, oribi, kudus, monkeys, baboons, hippopotami, Nile crocodiles, warthogs, bush pigs, porcupines, leopards, birds, and reptiles [21].
2.2 Study design
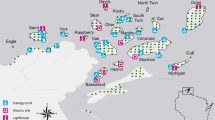
For this study, we used a line transect sampling method. The line transect method is a well-known and cost-effective method for surveying various sized vertebrates in tropical forests and savannas [30]. Our first hypothesis was that a higher level of anthropogenic land-use supports a lower level of species richness than more native habitats. To test this, we divided the landscape into five homogeneous land-uses during reconnaissance using ArcGIS version 10.4.1 from Landsat 8. Then, we obtained five major land-uses. The area for each major land-use was forest (3604 ha), wetlands (1012 ha), grassland (1774 ha), agricultural land (2419 ha), and settlement (323 ha). We further divided each land-use type into spatially isolated sites based on the size of the survey area, yielding 30 sites: 4 wetlands (accounted for 20.00% of total sites), 12 forest sites (accounted for 33.33%), 6 grassland sites (accounted for 16.67%), 6 agricultural lands (accounted for 16.67%), and 2 settlement sites (accounted for 13.33%). Using each spatially isolated site, we established one transect line and a total of 30 transects to collect data (Fig. 1). We limited the distance between adjacent transects and from the habitat edge to a transect to 0.5 km, to avoid double counting and edge effects [34]. The length of each transect was 200 ± 23.34 (SE) m. Furthermore, we measured the altitude and distance from the vehicle road where each piece of evidence was recorded using ArcGIS version 10.4.1 and Garmin GPS. Therefore, we examined the effects of seven factors on carnivore species richness and occurrence in the landscape: five land-uses (forest, wetland, grassland, agricultural land, and settlements) and two environmental factors (altitude and distance from the road).
2.3 Carnivore species data collection
We collected data from August to September 2020 during the wet season and from January to February 2021 during the dry season. We carried out carnivore surveys for three consecutive days per month for four months. We used three complementary field survey techniques to collect data along 30 transects: a sign survey, a direct sighting survey, and a camera trapping survey.
2.3.1 Sign and sighting survey along transects
We trained data collectors about the sign and sighting survey protocols and assigned two data collectors for each transect, including the researchers. Sign surveys can improve survey efficiency for mammalian species occurrence [26]. We observed fresh tracks, scats, hair, burrows, odor, and digging along transects [18]. We identified scats of carnivore species in the field based on their morphology, which includes diameter at the widest point, length, shape, color, odor, and disjoint segments following Chame [7]. We marked the signs counted by data collectors and the researcher with a wood stake in order to avoid repeating the same sign during subsequent monthly sampling periods. We did not record signs that were ambiguous. For the direct sighting survey, we counted all opportunistically observed carnivores with the naked eye and Bushnell laser rangefinder binoculars along transects. We used an average sighting distance of 200 m on the right and left sides of each transect for data collection. We did the transect walk between 6:00 and 10:00 a.m. when most animals become more active [16], for three consecutive days per month for four months. As a result, we surveyed each transect line 12 times during the study period. We used body size, coloration, and dominant behavior to identify carnivore species in sightings evidence, using the Kingdon Field Guide to African Mammals [18] and researchers' field experience.
2.3.2 Camera trapping survey
We used infrared digital camera traps (Bushnell Trophy model Cam HD™-119447), which were activated automatically by animal movement. We activated the cameras with a default 10-seconds photographic delay between pictures. We adjusted the time, date, month, year, and auto mode for all camera traps. Then, we fixed one camera trap in each transect by prioritizing signs (tracks and roads) of carnivores. According to the literature, mounting cameras along signs helps to maximize captures within study sites [3]. We kept two to three-meter fixing distances on either side of the trail or road to get identifiable photographs and protect the cameras from animal damage [3]. To maintain a suitable degree, we placed each camera on the flat ground at a height of 30 to 40 cm above the ground. We pointed cameras north or south to reduce false triggers from the rising or setting sun, and we set them parallel to the ground to ensure a direct field of view. Finally, we removed any small vegetation that could interfere with the camera's detection of carnivores, such as long grass and bushes. We checked its proper installation before leaving the fixed site. We fixed each camera for three consecutive days per month for four months on the same day as described for the sign and sighting survey. We used the Kingdon Field Guide to African Mammals [18] and researchers' field experience to identify photographs of carnivores at a species level.
2.4 Data analysis
We used the total number of different species in each habitat as a measure of species richness. We calculated the proportion of occurrence of each species by dividing the number of transects used by each species by the total number of transects surveyed (30 in this study). We used the χ2 to compare the significant difference in carnivore species richness and occurrence between land-uses. We also used Generalized Linear Models (GLM) with (1) Poisson distribution and logit link to test the effect of variables on the species richness because the data was a count, and (2) Binomial distribution and identity link to test the effect of variables on the specific-species occurrence because of presence/absence data (presence = 1, absence = 0). We ran land-uses and environmental factors separately. We checked spatial autocorrelation from model residuals using Moran’s Index and evaluated the collinearity between the predictor variables using variance inflation factors, based on Zuur et al. [41]. We did not find these problems. We interpreted the GLM result based on the value of standardized regression coefficients (β), which were used to compare the direction and magnitude of variable effects. We carried out all statistically significant tests with an alpha level of 0.05. We used Program R version 3.6.1 from the R Development Core Team 2019 [8] for all statistical analysis.
3 Results
3.1 Species taxonomic and body size composition
The FFL contains six families belonging to 12 species (Table 1), including the vulnerable Felidae species, Panthera pardus. The families Felidae and Herpestidae each had three species, while the families Canidae and Viverridae only had two. The other two families were represented by a single species. Of the species identified, two species (Panthera pardus and Crocuta crocuta) were large-sized, six were medium-sized, and the remaining were small-sized carnivores (Table 1).
3.2 Species richness and occurrence between land-uses
Wetlands had the most species richness (12), followed by the forest (10), grassland (8), agricultural land (7), and settlement (5). All species recorded in the study area (12) were also recorded in the wetlands. There was a significant difference in species richness between land-uses (χ2 = 19.467, df = 4, P = 0.003). The mean species richness was the highest in wetlands (7.67 ± 0.494(SE)), followed by grassland (6.00 ± 0.548(SE) (Fig. 2)). Overall, the mean richness of the study area was 5.73 ± 0.284(SE).
Out of 30 sampled transects, Genetta genetta was occurred among the 25 transects (the greatest proportion of occurrence = 0.833), followed by Ichneumia albicauda (Fig. 3). Mellivora capensis, caracal caracal, and Panthera pardus were found in the fewest number of sampled transects (Fig. 3). Panthera pardus was detected only at two transects, both in wetlands (the least proportion of occurrence = 0.067).
3.3 Effects of factors on species richness
Overall, species richness was positively associated with distance from the road, forest, grassland, and wetland. The positive association was significant only for wetlands (β = 0.516; P = 0.002; Table 2). However, species richness was negatively associated with settlement (β = − 0.743; P = 0.003; Table 2) and agricultural land (β = − 0.496; P = 0.024). The associations with other variables and species richness were weak (Table 2).
3.4 Effects of factors on the specific-species
The occurrence of seven species was positively associated with the forest, with two of them having statistically significant effects: Galerella sanguinea (β = 0.562; P < 0.0001) and Leptailurus serval (β = 0.943; P = 0.043; Fig. 4A). Civettictis civetta (β = 0.803; P < 0.0001), Galerella sanguinea (β = 0.459; P = 0.01) and Panthera pardus (β = 0.384; P = 0.038) were found to have a significant positive relationship with wetlands (Fig. 4B). Eight species were found to have a positive association with grassland (Fig. 4C). This relationship was found to be significant for Halogale parvula (β = 0.573; P = 0.006) and Civettictis civetta (β = 0.337; P = 0.027). Four species displayed negative associations with grassland, with only Otocyon megalotis (β = − 0.524; P = 0.011) being significant. Four and eight species were positively and negatively associated with the settlement, respectively (Fig. 4D). Only Halogale parvula was found to be significantly and positively associated with settlement (β = 0.572; P = 0.004). Five species showed a positive association with agricultural land, while seven species showed a negative association (Fig. 4E). Three species displayed significant positive associations, whereas two species displayed significant negative associations with agricultural land (Fig. 4E).
The line plus dot diagram showing the response of specific species to different factors output from generalized linear model with binomial distribution on the occurrence of carnivore species in the FFL. Stars indicate significant effect (confidence interval exclude zero), dots indicate coefficient (β) values, solid central axis indicates demarcation (zero point) for positive (avoidance) and negative association (preference), red dot line indicates community effect indicates the mean for the species-specific responses
Six species showed a positive relationship with altitude, with five showing a significant preference for higher altitudes (Fig. 4F). Although six species showed a negative association with altitude, none of them exhibited a significant association (P > 0.05; Fig. 4F). The association of carnivore species with distance from the road was variable: seven species showed a positive association (preferring to be farther away from the road), while five species showed a negative association (Fig. 4G). The two species that were not significantly affected by any of the factors studied were Canis mesomelas and Mellivora capensis (Fig. 4).
Overall, the occurrence of carnivore communities was associated with settlement and agricultural land in a non-significant negative way. However, it did have a positive association with other factors. The carnivore community was found to have a positive and significant relationship with wetlands and altitude (Fig. 4B, F, see red dot).
4 Discussion
Our main findings were that: (1) twelve carnivore species (two of which were large-sized) were identified; (2) the number of species was highest in wetlands (n = 12) and lowest in settlement (n = 5); (3) almost all (83.3%) and more than two-thirds of carnivore species were found to be positively related to wetlands and forest habitats, respectively; and (4) most carnivore species were negatively associated with agricultural land and human settlement, and they tended to avoid roads.
Our findings provide insight into the first quantitative data at the community level of carnivores gathered by using multiple survey techniques in the Southern Rift Valley. Most surveys in Africa focus on single species [3]. Furthermore, our findings suggest that the coexistence of humans and carnivores is possible in a human-dominated landscape. Several earlier studies have also shown humans and carnivores coexist [9, 36]. Since little is known about carnivore occurrences in Ethiopia [11], our survey confirms that FFL is home to at least 12 carnivore species, including the global conservation-concern Panthera pardus, and is one of the most important areas for the conservation of carnivores in Ethiopia.
4.1 Taxonomic composition and species richness
In this study, we found all six carnivore families previously identified in Ethiopia [19]. Like this, six families of carnivores were identified in the Serengeti ecosystem, Tanzania [10]. In the current study, the Felidae and Herpestidae were composed of three species each, while the Hyaenidae and Mustelidae were composed of a single species each. The family Herpestidae (mongooses) has a higher species richness. This might be due to their more adaptable nature to different land-uses, diverse foraging behavior (fruits, meat), and high tolerance level for human disturbances [2, 11, 32]. For the Felidae, it could be wild and domestic prey availability, high vegetation cover, and access to water [28]. The family’s Hyaenidae and Mustelidae have low species richness, which could be attributed to anthropogenic pressure in the area.
Out of 32 carnivore species in Ethiopia [38], we documented 12 carnivore species. Out of 12 carnivore species, two were large-sized—Crocuta crocuta and the globally vulnerable Panthera pardus [17]. Large carnivores, in particular, are often used as “umbrella species” in conservation efforts because of their wide area requirements [27]. Also, studies have confirmed that areas containing conservation-concern species are important for conservation practice [26, 35, 37]. Thus, the FFL is one of the most important areas for the diversity of carnivores in Ethiopia.
Our multiple surveys provided the highest number of carnivore species compared to indirect sign surveys and direct visual surveys in different localities in Ethiopia. Qufa and Bekele identified four carnivore species from the Lebu Natural Protected Forest in Southwest Shewa, Ethiopia [26], and Girma and Worku identified six carnivore species from the Nensebo Forest in Southern Ethiopia [16], both of which are lower than the current study. The higher species composition and richness of carnivores in the current study area could be attributed to the use of advanced survey technology (camera trapping), a longer survey period, access to water, and dense vegetation cover.
4.2 Effect of land-uses on carnivores
As expected, wetlands had the highest species richness and almost all (83.3%) carnivore species were positively associated with wetlands, with three species displaying significant association. Given wetlands' smaller area (only 10.12 km2) compared to the forest area (36.04 km2), the highest species richness and strong positive preferences of most species are surprising and contradict the “species-area relationship”. Habitats with a larger area tend to have more species than habitats with a smaller area [33]. Furthermore, wetlands support unique species, specifically the vulnerable Panthera pardus. This could be due to where prey was easier to catch during watering. Prior research has identified water and cover as critical habitat requirements [37]. Thus, the presence of conservation-concerned species, strong preference for most species, and the highest species richness in wetlands demonstrate that wetlands play an important role in wildlife conservation in the FFL and in Ethiopia. This could be due to easier access to water (Lake Abaya) and less anthropogenic pressure.
The forest is the second most abundant in terms of species richness (it contains 10 species) and more than two-thirds of carnivores are positively associated with it. This is possibly due to the high vegetation cover, the cooling effect, and less human disturbances. Observed associations between carnivore species agree with previous research in Ethiopia on Panthera leo, and Crocuta crocuta [40], and Civettictis civetta [23]. This emphasizes the importance of forests in maintaining functional assemblages and/or diversity, as well as ecological integrity, apart from providing basic needs such as food, shelter, and cover [6, 13]. According to studies, the forest is the most important habitat for wildlife and biodiversity conservation worldwide [17, 19, 37].
Although grassland is open and unable to hide elusive carnivore species, it contains eight species and is positively associated with a large number of carnivore species (significant for Halogale parvula, Civettictis civetta, and Genetta genetta). Possibly, they might have been attracted to grassland due to its promotion of ease of movement, access to termites and insects, anti-predator scanning, and prey availability [16]. As a result, the land-uses should be given equivalent conservation attention.
Studies have shown that species richness is likely to be lower in settlement and agricultural lands due to increased anthropogenic activities such as farming, poaching, and controlling carnivores by killing them so as not to harm livestock [11, 32]. As expected, this is supported by our finding that settlements and agricultural land had fewer carnivore species than other land-uses. Furthermore, most carnivore species would have a negative association with these land-uses, which is consistent with the hypothesis of our study. However, this generalization varies between species, most likely due to differences in dispersal, colonization, and stress tolerance [11, 15]. For example, the positive associations were significant only for Halogale parvula, Ichneumia albicauda, and Crocuta crocuta. This might be due to access to domestic prey for three of them, scavenging (Crocuta crocuta) and omnivore behaviors (Halogale parvula, Ichneumia albicauda). The current finding may be indicative of an “ecological trap” (i.e., environmental change causes organisms to prefer to settle in poor-quality habitats) [27]. Agricultural land expansion may result in decreased habitat [25], escalating the conflict between humans and carnivores and posing a threat to biodiversity conservation. Thus, land sharing (wildlife-friendly agricultural system) for agriculturally adapted carnivore species should be implemented to consider carnivore conservation [35].
Despite the fact that the effect was statistically insignificant, eight out of twelve carnivore species had a negative association with human settlements [11]. This might be due to hunting in retaliation for livestock depredation and crop damage [4, 10, 31]. These patterns are shared across East Africa and worldwide, where carnivores avoid human disturbance, except for a few species (e.g., hyenas) that are known to attack livestock inside human settlements at night and are attracted by anthropogenic food sources (e.g., mongooses) [2, 11]. Similarly, previous research indicates that human disturbance has a negative impact on carnivores in southern Ethiopia [14]. The only positive and significant association with the settlement was Halogale parvula, most likely due to the high density of rodents and fruit trees, which may provide easy pickings for this omnivorous species.
4.3 Effects of environmental factors on carnivores
The species richness of carnivores increased significantly as altitude increased. For instance, all carnivore species, with the exception of Panthera pardus, were positively associated with altitude. This is most likely due to higher altitude sites being far removed from human habitation (causing less disturbance), prey cascading (scanning), and lower temperatures at higher altitudes. As temperatures rise in the lowlands, species migrate to higher elevations [10]. This agrees with studies by other scholars elsewhere [11].
The species richness of carnivores was higher farther away from the vehicle road; this could be due to road-kill, vehicle collisions, and human disturbance. Although only one species, Galerella sanguinea, showed a significant negative response, the vast majority avoided roads. The impact of roads on carnivores has previously been documented [24]. The vehicle kills of Genetta genetta, Civettictis civetta, and Canis mesomelas were recorded during the study period. Penetrating natural forests with roads results in opening avenues for human encroachment, road-kill, and species collisions with vehicles [34]. Roads, in general, have been associated with cascading effects like overexploitation, habitat conversion, fire, farming, and invasive species [24, 34].
5 Conclusion
We studied the occurrence and species richness of carnivores by gathering data with multiple survey techniques. This serves as valuable baseline information for stakeholders making imperative conservation decisions and for researchers wishing to conduct related ecological studies in a human-dominated landscape. We found that the FFL is home to 12 carnivore species belonging to six families, including the globally vulnerable Panthera pardus. Of these, Panthera pardus and Crocuta crocuta were large-sized. Wetlands are the most important land-use type for species because of water availability and less human pressure. It provides a tangible target for carnivore conservation in the FFL. A considerable number of species prefer forest and grassland. As expected, many of the carnivore species sampled by the present study require largely less disturbed and forested habitats and may be seriously ill-adapted to agricultural land, settlements, and vehicle roads. As a result, intensifying agricultural production on lands that have already been converted to meet human needs, as well as prioritizing the protection of native habitats, is required in the area for wildlife conservation.
Availability of data and materials
Data are available with the corresponding author and will be provided if needed.
References
Admasu E, Thirgood SJ, Bekele A, Laurenson MK (2004) Spatial ecology of white-tailed mongoose in farmland in the Ethiopian Highlands. Afr J Ecol. https://doi.org/10.1111/j.1365-2028.2004.00498.x
Admasu E, Thirgood SJ, Bekele A, Laurenson MK (2004) A note on the spatial ecology of African civet Civettictis civetta and common genet Genetta genetta in farmland in the Ethiopian Highlands. Afr J Ecol 42:160–162. https://doi.org/10.1111/j.1365-2028.2004.00496.x
Agha M, Batter T, Bolas EC, Collins AC, Gomes da Rocha D, Monteza-Moreno CM, Preckler-Quisquater S, Sollmann R (2018) A review of wildlife camera trapping trends across Africa. Afr J Ecol 56:694–701. https://doi.org/10.1111/aje.12565
Amare Y, Serekebirhan T (2019) Human-wildlife conflict around Midre-Kebid Abo Monastry, Gurage Zone, Southwest Ethiopia. Int J Biodivers Conserv 11:212–229. https://doi.org/10.5897/ijbc2019.1314
Bakala F, Mekonen G (2021) Species diversity and relative abundance of medium and large-sized wild mammals: a study from Adaba Community Forest, West Arsi Zone, Southeast Ethiopia. Afr J Ecol 59:538–543. https://doi.org/10.1111/aje.12827
Bauer H, Chardonnet B, Scholte P, Kamgang SA, Tiomoko DA, Tehou AC, Sinsin B, Gebresenbet F, Asefa A, Bobo KS, Garba H, Abagana AL, Diouck D, Mohammed AA, Sillero-Zubiri C (2021) Consider divergent regional perspectives to enhance wildlife conservation across Africa. Nat Ecol Evol 5:149–152
Chame M (2003) Terrestrial mammal feces: a morphometric summary and description. Mem Inst Oswaldo Cruz 98:71–94. https://doi.org/10.1590/S0074-02762003000900014
Core Development Team R (2020) A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2. https://www.R-project.org
Dorresteijn I, Hanspach J, Kecskés A, Latková H, Mezey Z, Sugár S, von Wehrden H, Fischer J (2014) Human-carnivore coexistence in a traditional rural landscape. Landsc Ecol 29:1145–1155. https://doi.org/10.1007/s10980-014-0048-5
Durant SM, Craft ME, Foley C, Hampson K, Lobora AL, Msuha M, Eblate E, Bukombe J, Mchetto J, Pettorelli N (2010) Does size matter? An investigation of habitat use across a carnivore assemblage in the Serengeti, Tanzania. J Anim Ecol 79:1012–1022. https://doi.org/10.1111/j.1365-2656.2010.01717.x
Easter T, Bouley P, Carter N (2020) Intraguild dynamics of understudied carnivores in a human-altered landscape. Ecol Evol 10:5476–5488. https://doi.org/10.1002/ece3.6290
ENMSA (2019) Ethiopia National Meteorological Service Agency, Data base for metrological data source (2009–2019) of Mirab Abaya District, Ethiopia
Estifanos TK, Polyakov M, Pandit R, Hailu A, Burton M (2020) Managing conflicts between local land use and the protection of the Ethiopian wolf: Residents’ preferences for conservation program design features. Ecol Econ. https://doi.org/10.1016/j.ecolecon.2019.106511
Gebresenbet F, Baraki B, Yirga G, Sillero-Zubiri C, Bauer H (2018) A culture of tolerance: Coexisting with large carnivores in the Kafa Highlands, Ethiopia. Oryx 52:751–760. https://doi.org/10.1017/S0030605316001356
Gebresenbet F, Bauer H, Vadjunec JM, Papeş M (2018) Beyond the numbers: Human attitudes and conflict with lions (Panthera leo) in and around Gambella National Park. Ethiopia PLoS One. https://doi.org/10.1371/journal.pone.0204320
Girma Z, Worku Z (2020) Large mammal diversity in nensebo forest, Southern Ethiopia. Int J Zool. https://doi.org/10.1155/2020/8819019
IUCN (2015) The IUCN Red List of Threatened Species. In: Version 2015. 1
Kingdong J (2015) Kingdon Field Guide to African Mammals-Bloomsbury Natural History(2nd)
Lavrenchenko LA, Bekele A (2017) Diversity and conservation of Ethiopian mammals: what have we learned in 30 years? Ethiop J Biol Sci 11:1–20
Lin B, Foxfoot IR, Miller CM, Venkatamaran VV, Kerby JT, Bechtold EK, Kellogg BS, Nguyen N, Fashing PJ (2020) Leopard predation on gelada monkeys at Guassa. Ethiopia Am J Primatol. https://doi.org/10.1002/ajp.23098
MAW-RDO (2019) Mirab Abaya Woreda rural development office report, Annual report of the office, Mirab Abaya Woreda, Birbir
Mekonen S (2020) Coexistence between human and wildlife: the nature, causes and mitigations of human wildlife conflict around Bale Mountains National Park, Southeast Ethiopia. BMC Ecol 20:1–9. https://doi.org/10.1186/s12898-020-00319-1
Mullu D, Balakrishnan M (2014) Ecology of African Civet (Civettictis civetta) in Arba Minch Forest, Arba Minch, Ethiopia. Sci Technol Arts Res J 3:99. https://doi.org/10.4314/star.v3i3.16
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Díaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DLP, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, MacE GM, Scharlemann JPW, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. https://doi.org/10.1038/nature14324
Penjor U, Wangdi S, Tandin T, Macdonald DW (2021) Vulnerability of mammal communities to the combined impacts of anthropic land-use and climate change in the Himalayan conservation landscape of Bhutan. Ecol Indic. https://doi.org/10.1016/j.ecolind.2020.107085
Qufa CA, Bekele A (2019) A preliminary survey of medium and large-sized mammals from Lebu Natural Protected Forest, Southwest Showa, Ethiopia. Ecol Evol 9:12322–12331. https://doi.org/10.1002/ece3.5733
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ (2014) Status and ecological effects of the world’s largest carnivores. Science (80-.) 343:12414841–124148411
Rodrigues P, Dorresteijn I, Guilherme JL, Hanspach J, De Beenhouwer M, Hylander K, Bekele B, Senbeta F, Fischer J, Nimmo D (2021) Predicting the impacts of human population growth on forest mammals in the highlands of southwestern Ethiopia. Biol Conserv. https://doi.org/10.1016/j.biocon.2021.109046
Srivathsa A, Puri M, Karanth KK, Patel I, Samba Kumar N (2019) Examining human - Carnivore interactions using a socio-ecological framework: sympatric wild canids in India as a case study. R Soc Open Sci. https://doi.org/10.1098/rsos.182008
Tamrat M, Atickem A, Tsegaye D, Evangelista P, Bekele A, Stenseth NC (2020) The effect of season and post-fire on habitat preferences of the endangered Swayne’s hartebeest (Alcelaphus buselaphus swaynei) in Maze National Park, Ethiopia. BMC Ecol. https://doi.org/10.1186/s12898-020-0275-3
Tamrat M, Atickem A, Tsegaye D, Nguyen N, Bekele A, Evangelista P, Fashing PJ, Stenseth NC (2020) Human-wildlife conflict and coexistence: a case study from Senkele Swayne’s Hartebeest Sanctuary in Ethiopia. Wildlife Biol 2020:1–9. https://doi.org/10.2981/wlb.00712
Teixeira L, Tisovec-Dufner KC, Marin GDL, Marchini S, Dorresteijn I, Pardini R (2021) Linking human and ecological components to understand human–wildlife conflicts across landscapes and species. Conserv Biol 35:285–296. https://doi.org/10.1111/cobi.13537
Udy K, Fritsch M, Meyer KM, Grass I, Hanß S, Hartig F, Kneib T, Kreft H, Kukunda CB, Pe’er G, Reininghaus H, Tietjen B, Tscharntke T, van Waveren CS, Wiegand K (2021) Environmental heterogeneity predicts global species richness patterns better than area. Glob Ecol Biogeogr 30:842–851. https://doi.org/10.1111/geb.13261
Vanthomme H, Kolowski J, Korte L, Alonso A (2013) Distribution of a community of mammals in relation to roads and other human disturbances in Gabon, Central Africa. Conserv Biol. https://doi.org/10.1111/cobi.12017
Wall JL, Loken B, Brodie JF (2021) Reconciling resource extraction and species conservation in a multi-use landscape: immediate and long-term impacts of logging on rainforest mammal diversity. Glob Ecol Conserv. https://doi.org/10.1016/j.gecco.2021.e01642
Western G, Macdonald DW, Loveridge AJ, Dickman AJ (2019) Creating landscapes of coexistence: do conservation interventions promote tolerance of lions in human-dominated landscapes? Conserv Soc 17:204–217. https://doi.org/10.4103/cs.cs_18_29
Wolf C, Ripple WJ (2016) Prey depletion as a threat to the world’s large carnivores. R Soc Open Sci. https://doi.org/10.1098/rsos.160252
Yalden DW, Largen MJ, Kock D, Hillman JC (1996) Catalogue of the mammals of Ethiopia and Eritrea. 7. revised checklist, zoogeography and conservation. Trop Zool 9:73–164. https://doi.org/10.1080/03946975.1996.10539304
Yirga G, Gebresenbet F, Deckers J, Bauer H (2014) Status of Lion (Panthera leo) and Spotted Hyena (Crocuta crocuta) in Nechisar National Park, Ethiopia. Momona Ethiop J Sci 6:127. https://doi.org/10.4314/mejs.v6i2.109714
Yirga G, Leirs H, De Iongh HH, Asmelash T, Gebrehiwot K, Vos M, Bauer H (2017) Densities of spotted hyaena (Crocuta crocuta) and African golden wolf (Canis anthus) increase with increasing anthropogenic influence. Mamm Biol 85:60–69. https://doi.org/10.1016/j.mambio.2017.02.004
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x
Acknowledgements
Our special gratitude goes to the Mirab Abaya Wereda Administrative Office for allowing us to conduct research in the FFL. We also duly acknowledge the Faragosa and Fura Kebeles administrative offices and agricultural extension workers for their assistance during data collection in the habitat types. We also thank the Department of Biology, College of Natural Science, Arba Minch University, for their invaluable logistics and financial support. We are thankful to the Arba Minch College of Teachers’ Education for the logistic support as well as covering the living and other costs of the Ph.D. candidate. We are grateful for the support provided.
Funding
The study was funded by Arba Minch University, Ethiopia, through a grant to the first author with grant number AMU/CNS/Biol/04/2013 to study occurrence and species richness of carnivores in the FFL of Southern Rift Valley.
Author information
Authors and Affiliations
Contributions
All the authors conceptualized the study. BG collected and analyzed the data and wrote the draft manuscript; ST and SS edited and modified the structure of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
We declare that we have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for publication
We all approved the manuscript for its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gebo, B., Takele, S. & Shibru, S. Anthropogenic land-use and environmental factors affecting the species richness and occurrence of carnivores in the Faragosa-Fura Landscape of Southern Rift Valley, Ethiopia. SN Appl. Sci. 4, 46 (2022). https://doi.org/10.1007/s42452-021-04930-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04930-9