Abstract
In the context of the anaerobic digestion of slowly biodegradable substrates for energy and chemicals production, this study investigated the anaerobic digestion of cellulose without any chemical pre-treatments using open (undefined) mixed microbial cultures. The anaerobic conversion of cellulose was investigated in extended-length (run length in the range 518–734 days) batch and semi-continuous runs (residence time 20–80 days), at high cellulose concentration (20–40 g L−1), at temperatures of 25 and 35 °C. The maximum cellulose removal was 77% in batch (after 412 days) and 60% (at 80 days residence time) in semi-continuous experiments. In semi-continuous experiments, cellulose removal increased as the residence time increased however the cellulose removal rate showed a maximum (0.17 g L−1 day−1) at residence time 40–60 days. Both cellulose removal and removal rate decreased when cellulose concentration in the feed was increased from 20 to 40 g L−1. Liquid-phase products (ethanol and short chain organic acids) were only observed under transient conditions but not at the steady state of semi-continuous runs. Most of the observed results were well described by a mathematical model which included cellulose hydrolysis and growth on the produced glucose. The model provided insight into the physical phenomena behind the observed results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anaerobic digestion (AD) is a widely used biological process with open mixed cultures, typically aimed at producing methane, which is then used for energy generation. However, AD could also be used in a biorefinery context to produce chemicals such as short-chain organic acids, ethanol, and hydrogen, which are intermediates in the digestion process [1, 2]. According to recent studies [3, 4], most of the potential of AD for the generation of energy is still undelivered. Indeed, on a global scale AD generates less than 1% of the electricity it could potentially generate based on the available feedstocks and, in the UK, only 19% of the AD potential is being delivered. Furthermore, there is no currently full-scale production of chemicals using AD.
One of the reasons preventing the achievement of the full potential of AD is the fact that most feedstocks are made of slowly biodegradable substrates, i.e. substrates that need to be hydrolysed before being metabolised by microorganisms, for example lignin and lignocellulosic biomass [5]. As an example of a slowly biodegradable substrate, this study investigates the AD of cellulose with open mixed cultures. Cellulose is the primary structural component of lignocellulosic materials, which makes it the most abundant natural product in the biosphere [6]. The recalcitrance of lignin and cellulose limits the use of lignocellulosic biomass for fuels and chemical production. Most of the hydrolysis-related problems are solved through feed pre-treatments, which often involve high-temperature high-pressure processes such as steam explosion and/or enzymatic hydrolysis [7]. Enzymatic hydrolysis of cellulose requires the synergistic actions of different enzymes, known as cellulases. These cellulases, which are not recoverable from the conventional biological processes, contribute to the increased process costs [8]. The high costs of pre-treatments and enzymes are major constraints for the scale-up and commercialisation of AD and biorefinery processes which use cellulosic or lignocellulosic feedstocks.
Therefore, as an alternative to utilising chemical or physical pre-treatments or external enzymes, this study investigated the anaerobic fermentation of cellulose using open (undefined) mixed microbial cultures without pre-treatment to produce methane and chemicals.
Biodegradation of untreated cellulose has so far mainly been investigated using pure cultures or mixed cultures from specialised inocula, typically rumen contents. Pavlostathis et al. [9] used a pure culture of Ruminococcus albus in continuous fermenters, obtaining cellulose degradation of 30–70% with residence times in the range 0.5–2 days. Hu et al. [10] used rumen microorganisms in batch experiments at various pH values, obtaining cellulose degradation of up to 78%. Zhang et al. [11] observed up to 81% cellulose degradation in batch tests with rumen microorganisms. Singh et al. [12] isolated a novel thermophilic anaerobic bacterium (Clostridium sp. DBT-IOC-C19), which was able to degrade cellulose up to 95%. Relatively little research has been carried out on the anaerobic biodegradation of untreated cellulose using open mixed cultures from non-specialised inocula. Chyi and Dague [13] investigated cellulose degradation in continuous reactors, using inoculum from anaerobic digesters, observing cellulose degradation in the range 17–54%. Ueno et al. [14] investigated the batch degradation of cellulose using anaerobic sludge or sludge compost obtaining up to 58% cellulose digestion. Soundar and Chandra [15] investigated the degradation of cellulose in batch tests using enriched mixed cultures inoculated from different sources, obtaining up to 33% degradation of cellulose. Kinet et al. [16] carried out batch tests with anaerobic sludge observing 85% cellulose degradation, which increased to 97% with the addition of a cellulolytic consortium isolated from composting plants. Siegert and Banks [17] observed up to 66% cellulose degradation in batch tests with anaerobic sludge as inoculum.
The published studies on cellulose degradation by anaerobic mixed cultures from non-specialised inocula, which are the mixed cultures likely to be found in anaerobic digesters, have several limitations (Table 1 shows literature studies on cellulose degradation with mixed and pure cultures):
-
Most of these studies were carried out in batch, while full-scale anaerobic digesters are likely to be operated as continuous or semi-continuous processes;
-
Most of these studies were carried out with relatively low concentration of cellulose in the feed, usually up to 10 g L−1. However, the concentration of slowly biodegradable substrates in biorefinery applications is likely to be higher, to increase the concentration of the products and the productivity of the process;
-
When the studies were carried out in continuous processes, the range of investigated residence times was relatively limited (up to 13 days), while the solids residence time of full-scale digesters is usually longer.
In this study, we aimed at filling the main gaps in the reported literature studies and at extending the range of experimental conditions investigated for the anaerobic degradation of cellulose by open mixed cultures. We investigated higher feed concentrations (up to 40 g L−1) and a wider range of residence times (up to 80 days) than any other published studies. We carried out three extended-length runs (run length in the range 518–734 days) by varying the operating mode (batch and semi-continuous), the residence time and the cellulose concentration and measuring the formation of liquid phase products (organic acids and ethanol), extending the results of our previous study [24]. Furthermore, we used a mathematical model with microbial hydrolysis of cellulose and growth on the produced glucose to describe all the experimental data obtained with the same set of parameters, and we used the model to gain better insight into the reasons for the observed results. To the best of our knowledge, this is the first study that uses this model for the biodegradation of slowly biodegradable substrates in both batch and continuous reactors at different operating conditions.
Overall, this study aimed to provide better insight into the process conditions that affect the biodegradation of slowly biodegradable substrates in a biorefinery context.
2 Materials and methods
2.1 Runs and inoculum
Three experimental runs were carried out (Runs A, B, C, Table 2). Run A was done entirely in batch, Run C entirely in semi-continuous mode. During Run B, the operating mode was changed from batch to semi-continuous. In Run A the inoculum was anaerobic sludge obtained from Gask anaerobic digester (Turriff, Aberdeenshire, UK). The Gask digester treats food industry and agricultural waste and operates at 38–40 °C. The inoculum was stored in fridge (2 °C) in order to minimise its degradation and was maintained at room temperature for 24 h before use. In Runs B and C soil from Craibstone Farm (Aberdeen, Scotland), characterised in other studies [25, 26] and used in previous studies in our group [27, 28], was used as inoculum. The VS and VSS content of the Craibstone soil was 0.13 gVS g−1 and 0.12 gVSS g−1. The reason for choosing both inocula was to use open mixed cultures of microorganisms which were not previously acclimated to cellulosic substrates. The soil was stored at room temperature in airtight Ziploc bags, homogenised manually and sieved with a 150 µm mesh before use.
2.2 Feed preparation
Pure crystalline cellulose (Sigmacell type 20, S-3504, Sigma-Aldrich, UK), insoluble in water, was used as the sole carbon source. The media comprised essential macro-nutrients for the growth of the microorganisms (in 1 L tap water): 20.0 g cellulose, 69.6 g K2HPO4, and 48.0 g NaH2PO4, 2.0 g NH4Cl, 0.125 g MgCl2.6H2O and 0.09 g CaCl2.6H2O. The phosphate salts were added to maintain the pH in the range 6.8–7.0. For the runs with 40 g L−1, the feed was prepared with 40.0 g of cellulose per litre, leaving the concentration of the other macro-nutrients unchanged. The feed bottle was continuously agitated at room temperature to maintain the cellulose in suspension. The feed was prepared fresh every three or four days, the feed vessel was thoroughly cleaned between feeds and no degradation of the feed was observed.
2.3 Reactor set-up
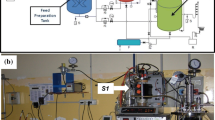
All runs were carried out in glass jacketed vessels of 200 mL working volume. For the batch runs, 200 mL was the volume at the start of the experiment, the volume then decreased due to sampling. All vessels were continuously agitated using magnetic stirrers, with no settling phase, and purged with nitrogen at the start of the runs to ensure anaerobic conditions. The temperature of the reactors was maintained at the desired temperature by water recirculation from a thermostatic bath.
Runs A and C were started up by mixing the inoculum and the feed and the experimental period reported in this study started immediately. In Run B, after inoculation the reactor was operated in semi-continuous mode at 2 days residence time for 28 days. This part of the run was not monitored regularly, but occasional sampling indicated no cellulose removal. After 28 days, Run B was switched to batch mode and the experimental period reported in this study started. The start-up and operating procedure for Run B was chosen based on the following considerations: the reactor was started up under semi-continuous mode at short residence time (2 days) to see whether the unacclimated culture could degrade cellulose under these conditions. Once no degradation of cellulose was observed under these conditions, the operating mode was switched to batch to increase the residence time. Once cellulose degradation was observed, the operating mode was switched to semi-continuous in a range of residence times (20–80 days) to determine the minimum residence time which could sustain cellulose degradation. In the semi-continuous runs (Run C and part of Run B) the reactors were fed once per day with a peristaltic pump controlled by a programmable power management system. The effluent was discharged with an overflow system. The residence time (hydraulic and solids residence time coincided) was controlled by changing the volume fed per day, which was 10, 5, 3.3 and 2.5 mL day−1 for residence times of 20, 40, 60, 80 days, respectively.
2.4 Analytical methods
Samples were drawn from each reactor periodically and analysed for pH, total (TSS) and volatile (VSS) suspended solids, total (TC) and soluble (SC) carbohydrates, short-chain fatty acids and chemical oxygen demand (COD, only the total COD was measured). For each sample, 7 mL were withdrawn from the reactors, and divided into aliquots for TSS/VSS, TC and COD measurements. The measurements on the soluble fraction (acids and carbohydrates) were done on the filtrate produced from the TSS/VSS filtration. TSS and VSS were evaluated according to standard methods [29]. Total COD (TCOD) was analysed using the Spectroquant cell test method (Merck Millipore, method number 114555) and the Spectroquant Nova 60 photometer. Total (TC) and soluble (SC) carbohydrate analysis were carried out using the Anthrone method, and the liquid fermentation products were measured using Gas Chromatography (GC). Details on the analytical procedures are reported elsewhere [30].
2.5 Calculations
Removal of VSS, TC and COD was calculated by comparing the measured values in the reactor with the measured values in the feed. For semi-continuous experiments, the average removal was calculated based on the average values measured during the steady-state. The steady state was assumed to be reached when, considering at least five consecutive sampling points, both the maximum deviation from the average of any data points and the ratio between the values of the first and last considered points were both below 15%.
2.6 Mathematical model
The mathematical model (Fig. 1) assumed that cellulose (XS, XS0 being the cellulose concentration in the feed) is hydrolysed by microorganisms (biomass, X) to produce glucose (S) and biomass then grows on the produced glucose. The model also included biomass decay (endogenous metabolism) to produce inert biomass (XI). The model was only aimed to simulate cellulose removal, therefore fermentation products were not included. The variables XS and S were expressed in COD units (gCOD L−1) while X and XI were expressed in g L−1. The biomass X and XI was expressed in the model in g L−1 rather than in gCOD L−1 to be consistent with the most typical method of biomass measurement, which is the gravimetric measurement of the VSS. However, the biomass values calculated by the model can be converted in COD units using the appropriate conversion factor (often taken as 1.42, [31]). The model assumed perfect mixing and the semi-continuous feeding/withdrawal was simulated by continuous feeding/withdrawal. This was acceptable considering that the length of the runs and the residence times used were much longer than the length of one cycle (1 feed per day).
The reaction rates and mass balances [Eqs. (1)–(8)] were based on published models [31]. The hydrolysis kinetics was developed originally [32] to describe microbial growth on soluble substrates. This rate equation has been used in a number of studies [33,34,35] on soluble and insoluble substrates.
Rate of cellulose hydrolysis:
Rate of glucose removal:
Rate of biomass growth on glucose:
Rate of biomass decay (endogenous metabolism):
Cellulose balance:
Glucose balance:
Biomass balance:
Inert biomass balance:
In Eqs. (1)–(8) kh, KX, µmax, KS, b are the kinetic parameters, Q is the feed and effluent flow rate (L day−1) (Q = 0 in batch runs) and V is the reactor volume (L). The simulated profiles of XS, S, X and XI were obtained by solving the mass balances (5)–(8) simultaneously.
The kinetic parameters were adjusted to fit the experimental data of total and soluble carbohydrates collected in Runs A, B, C. The soluble carbohydrates calculated by the model Eq. (9), were considered to be the variable S divided by the COD conversion factor for glucose (1.067 g COD g−1 glucose).
The total carbohydrates calculated by the model, Eq. (10), were considered to be the sum of the Model SC and of the carbohydrates in the variables XS, X, XI. For XS, the carbohydrates were calculated by dividing XS by the COD conversion factor for cellulose (1.185 gCOD g−1 cellulose), for X and XI the carbohydrates were calculated assuming that the microorganisms dry weight is made of 27% carbohydrates [3]:
In Eq. (10), the contribution of the carbohydrates in other biomass components (methanogens) was not considered, since the model only accounted for the microorganisms which metabolised glucose directly. The results of the simulations (Sect. 3) indicated that the carbohydrates from the biomass X always accounted for less than 5%, and in most cases for less than 1% of the Model TC, therefore the inclusion of other biomass components wouldn’t have a significant impact on the modelling results.
Since the experimental data were collected at the temperatures of 25 and 35 °C, the effect of temperature on the kinetic parameters kh, µmax and b was included in the model. The temperature dependence of the hydrolysis process was addressed by an Arrhenius-type relationship, Eq. (11):
with k = kinetic rate constant, in this case kh, µmax and b; A = the pre-exponential factor, in this case called Akh, Aµmax, Ab for the parameters kh, µmax and b respectively; Ea = the reaction activation energy, in this case called Ea,kh, Ea, µmax, Ea,b for the parameters kh, µmax and b respectively; R = the gas law constant (J mol−1 K−−1); T = the absolute temperature (K).
The parameters A, Ea (for kh, µmax and b), KS, KX, YX/S, and the initial biomass concentrations in the three Runs (X0A, X0B, X0C) were adjusted to fit the experimental data of TC and SC. The fitting was done using Microsoft Excel Solver by imposing that the squared difference between the calculated variables and experimental data was minimised. The data from all the experiments were weighed equally. The mass balances were solved by numerical integration in Microsoft Excel, using a first-order finite difference method and using Solver for parameter estimation.
3 Results
3.1 Run A
The results of Run A are reported in Fig. 2. After the first cellulose addition at time 0, total carbohydrates and VSS decreased approximately linearly over the experimental period (412 days). The objective of the second cellulose addition at day 412 was to see whether the removal rate would increase due to the acclimation and growth of the microorganisms. In the second part of the experimental period, VSS and carbohydrates were removed but at a lower rate than in the first part. Indeed, the average removal rate of total carbohydrates after the second addition (0.026 g L−1 day−1) was lower than the average rate after the first addition (0.040 g L−1 day−1). Soluble carbohydrates were detected at very low or undetectable concentrations over all the length of the experiment. Liquid phase products were only observed after the second addition of cellulose. Acetate and propionate were the only detectable products and their concentration reached a maximum at d 700 before decreasing to undetectable levels. However, the large error bars for the liquid products profiles indicate large deviations between the two duplicate experiments. VSS and TC removal were 49 and 77%, respectively, at the end of the experimental period for the first addition of cellulose and were 37 and 49%, respectively, for the second addition of cellulose.
3.2 Run B
The results of Run B are reported in Fig. 3. During the batch phase, 69% of the initial VSS and 76% of the initial TC were removed, however when the operating mode was switched to semi-continuous with residence time 20 days, VSS and TC removal decreased as shown by the rapid increase in VSS and TC concentration. When the residence time was increased to 80 days, the removal of VSS and TC improved, with the concentrations decreasing until reaching approximately constant values. When the residence time was decreased to 60 days, a slight increase in TC and VSS was observed, indicating a slight decrease in cellulose removal. The decrease in cellulose removal was more evident when the residence time was further reduced to 40 days. Liquid phase products accumulated during the initial batch phase of the run. Acetate was the main product, with ethanol detected at lower concentrations. When the operating mode was switched to semi-continuous, the liquid phase products rapidly decreased to non-detectable levels, which is consistent with the rapid deterioration in cellulose removal. When the residence time was increased to 80 days, acetate and ethanol gradually accumulated in the liquid phase, however this accumulation was transient and their concentrations reduced again to non-detectable levels before the residence time was reduced to 60 days. No detectable concentration of liquid phase products was observed during the rest of the run. At steady state, the removal of VSS, TC and COD was as follows: 54, 60 and 49%, respectively, at residence time 80 days; 45, 53 and 46%, respectively, at residence time 60 days; 19, 30 and 20%, respectively, at residence time 40 days.
3.3 Run C
The results of Run B are reported in Fig. 4. No degradation of cellulose was observed at residence time 20 days, however after the increase in the residence time to 80 days both VSS and TC gradually started to decrease, indicating that cellulose was being degraded. TC and VSS reached approximate constant values between days 300 and 372, when the feed concentration was increased. The increase in feed concentration caused a gradual increase in both TC and VSS concentrations, which reached approximately constant values before the end of the run. No liquid phase products were detected in the initial part of the run, at residence time 20 days, consistently with the observation of no cellulose degradation under these conditions. The only detected liquid phase product, acetate, accumulated when the residence time was increased to 80 days, however, as already observed in Run B, this accumulation was transient and acetate concentration dropped to non-detectable levels at approximately day 300, when the reactor reached steady state. No accumulation of liquid phase products was observed during the rest of the run. The removal efficiency of VSS, TC and COD at steady state was between 20 and 50% and decreased when the cellulose concentration in the feed increased to 40 g L−1.
3.4 Mathematical model
The model generally describes the experimental data well (Fig. 5), except for the first cellulose addition in Run A. The model describes the time evolution of TC and the effect of residence time in semi-continuous reactors well (Run B from day 240 onwards and Run C up to day 372). The model also describes the effect of cellulose concentration well (Run C from day 372 until the end of the run). The simulation of batch experiments is also generally satisfactory for the batch part of Run B and for Run A after the second cellulose spike. Instead, the approximately linear profile of TC in the first part of Run A was not well described, as the model simulates an autocatalytic profile. The values of the adjusted model parameters are reported in Table 3.
4 Discussion
4.1 Rate and extent of cellulose degradation
The obtained experimental data in Runs B and C allow to analyse the effect of the residence time on the steady-state extent of cellulose removal and cellulose degradation rate. Increasing the residence time, the extent of cellulose (measured as TC) removal increases (Fig. 6). Cellulose removal doesn’t occur at residence time of 20 days and then increases up to the longest residence time tested in this study, 80 days. This observation can be explained considering that as the residence time increases, microorganisms are in contact with cellulose for longer and therefore have more time to degrade it. The rate of cellulose removal shows a maximum (approximately 0.17 g L−1 day−1) as a function of the residence time. The reason for this is that, as the residence time increases, the extent of cellulose removal also increases but the volume of feed processed per unit volume of reactor decreases. In our study, there was no solid–liquid separation, so the residence time of the liquid (hydraulic residence time, HRT) and of the solids (solids residence time, SRT) was the same. Based on these results, the introduction of a liquid phase separation which allows to set the HRT and SRT at different values (long SRT and short HRT) is expected to be beneficial. Long SRT would favour high extent of cellulose removal and short HRT would favour high volumetric rate of cellulose removal. Our results in the batch runs (Run A and first part of Run B) generally indicated higher extent of cellulose degradation (up to 77%) than in the semi-continuous runs and lower cellulose degradation rate (the average cellulose removal rate was 0.08 g L−1 day−1 in the batch part of Run B, and 0.04 and 0.03 g L−1 day−1 in the first and second part, respectively, of Run A). These results can be explained considering that batch runs allowed a longer contact time between microorganisms and cellulose (up to 412 days, first part of Run A), which favours high extent of cellulose degradation but, conversely, gives low values of the cellulose degradation rate. The effect of the operating mode (batch vs semi-continuous) and of the residence time on the cellulose degradation is also evident from the cellulose and acetate profiles in Run B. The reason for the increase in cellulose concentration and for the drop in acetate concentration when the operating mode was switched from batch to semi-continuous is that the initial residence time in the semi-continuous mode (20 days) was too short for the growth of the cellulose degrading microorganisms, which instead resumed when the residence time was increased to 80 days.
Only very limited investigation has been reported (Table 1) on the effect of residence time in the anaerobic fermentation of cellulose with open mixed cultures. Chyi and Dague [13] investigated the residence time in the range 1–3 days with a feed cellulose concentration of 7 g L−1. They reported cellulose conversion in the range 17–54%, increasing with the residence time. Noike et al. [19] worked with 5 g L−1 cellulose in the feed observing cellulose degradation in the range 2–54% and increasing as the residence time increased in the range 1.9–13.3 days. Chiy and Dague [13] also investigated the effect of cellulose concentration, in the range 3.6–13.8 g L−1 at the fixed residence time of 2 days, observing a virtually constant cellulose conversion (39–44%) and, correspondingly, an increase in residual cellulose concentration as the cellulose concentration in the feed increased. Due to the shorter residence time, the cellulose conversion rates reported in these studies were higher than in our study, up to 3.0 g L−1 day−1 [13], while the maximum cellulose degradation was lower than the maximum value observed in our semi-continuous processes. The effect of residence time was also investigated with pure cultures of cellulose-degrading microorganisms, with feed cellulose concentrations in the range 3.7–10.9 g L−1 and cellulose removal of up to 87%. The batch pure culture study by Desvaux et al. [20] worked with different initial cellulose concentrations, observing, in qualitative agreement with the present study, a decrease in the extent of cellulose degradation when the initial cellulose concentration increased. Compared with these literature studies, our study investigated higher cellulose concentrations (up to 40 g L−1) in a wider range of residence times (up to 80 days), and with a longer length of the runs (up to over 700 days). The maximum extent of cellulose degradation reported for the semi-continuous runs in this study (60%) is among the highest reported in the literature for continuous or semi-continuous cellulose-fed reactors with open mixed cultures. However, this maximum cellulose degradation was observed for significantly longer residence times than other literature studies. This is probably due to the higher cellulose concentration used in this study. The comparison of reactor performance observed in this study when the feed concentration was increased from 20 to 40 g L−1 (Run C) and the study by Desvaux et al. [20] confirms the negative effect of increasing cellulose concentration on cellulose removal. In agreement with the results of the present study, the highest extent of cellulose removal in literature studies was observed in batch runs rather than in continuous runs, while the cellulose removal rates were generally higher in continuous studies.
4.2 Mathematical model
To the best of our knowledge, this is the first time that a mathematical model has been used to describe cellulose degradation in wide range of operating conditions (batch and continuous mode, different residence time and cellulose concentration) with the same set of parameters. Although complex models of anaerobic digestion have been proposed and used successfully in a number of studies with real feedstocks (e.g. [36, 37]), those studies usually calibrated the models with a single type of experiments, only batch [36] or only continuous [37], but did not use the same set of parameters to describe different experiments run under different conditions (batch and semi-continuous, different residence times) as we did in this study. The fact that most of the observed results can be described well by the model indicates that this model is a powerful tool for the simulation and design of anaerobic processes for cellulose degradation. One important value of the modelling exercise is that the model can help explain some of the observed experimental behaviours. For example, the model explains why the cellulose degradation rate after the second addition in Run A is not higher than after the first addition. It was expected that, due to microbial growth on cellulose after the first addition, the cellulose degradation rate in the second part of Run A would be higher than in the first part, however this was not observed experimentally (Fig. 2). We can explain this unexpected evidence based on the concentration of active biomass in Run A simulated by the model (Fig. 7a). Active biomass concentration is very low at the start of Run A and then increases due to growth on the glucose from cellulose hydrolysis. However, due to the low rate of cellulose hydrolysis, microbial growth rate is also low and is counterbalanced by the endogenous metabolism (cell death) which causes the concentration of active biomass to reach a peak and then decrease. When the second addition of cellulose is made at day 412, the activate biomass concentration is very low and so the rate of cellulose removal in the second part of the run is also very low. The simulated biomass concentration in Runs B and C (Fig. 7b, c) shows how biomass concentration evolves as a function of batch and continuous conditions, residence time and cellulose concentration. Another unexpected behaviour simulated well by the model is the increase in the steady-state cellulose concentration when the cellulose concentration in the feed is increased from 20 to 40 g L−1. For a soluble substrate and microorganisms growing with Monod kinetics, it is known [31] that the effluent substrate concentration is only dependent on the kinetic parameters and on the residence time, but it is independent of the influent substrate concentration. However, the results of this study and the mathematical model show that this is no longer valid for slowly biodegradable substrates like cellulose. This can be shown by analysing the mass balance for glucose [Eq. (6)], which at steady state can be re-arranged to give:
where τ is the residence time V/Q. In the practical cases of our Run C at 80 days residence time at steady state, the term \(\frac{S}{X \cdot \tau }\) in Eq. (12) is always much lower than the term \(\left( {\frac{1}{\tau } + b} \right)\frac{1}{{Y_{X/S} }}\). For example, for Run C the term \(\left( {\frac{1}{\tau } + b} \right)\frac{1}{{Y_{X/S} }}\) is equal to 0.42 gCOD g−1 day−1 while the term \(\frac{S}{X \cdot \tau }\) is equal to 0.0006 and 0.0004 gCOD g−1 day−1 when the feed concentration is 20 and 40 g L−1, respectively. Therefore, under the conditions of Run C, the ratio \(k_{h} \frac{{\frac{{X_{S} }}{X}}}{{K_{X} + \frac{{X_{S} }}{X}}}\) is essentially independent of the feed cellulose concentration. This means that the ratio \(\frac{{X_{S} }}{X}\) is essentially constant as the feed cellulose concentration increases from 20 to 40 g L−1. Since the active biomass concentration increases as the feed concentration increases (with the values of Run C, X was calculated by the model to be 0.38 and 0.70 g L−1 with feed of 20 and 40 g L−1, respectively) it follows that XS, i.e. the cellulose concentration at steady state, has to increase as the feed concentration increases, which is what we observed experimentally. Physically, this means that, as the biomass concentration increases, the cellulose concentration in the reactor must increase so that all microorganisms remain attached to the cellulose particles. This observation has not, to the best of our knowledge, described by models before and can have important effects in the design of anaerobic processes for cellulose (and other insoluble substrates) degradation.
As far as the values of the kinetic parameters are concerned, kinetic parameters of AD reported in the literature fall within a very wide range. For example Kalyuzhnyi [38] reported literature values for µmax on glucose in the range 1.2–43 day−1, for b in the range 0.01–0.4 day−1 and for YX/S in the range 0.03–0.16 g g−1. Borja et al. [39] obtained values for µmax in the range 0.1–0.3 day−1. Yang et al. [40] reported values of the growth yield in the wide range 0.02–0.3 g gCOD−1. Vavilin et al. [35] reported values for the hydrolysis parameters in the range 1.5–12 day−1 for kh and 5–30 g g−1 for KX. Gavala et al. [41] reported values for kh for cellulose in the range 0.03–1.95 day−1, depending on the temperature and on the residence time. In the temperature range considered in this study, the kinetic parameters µmax and kh are expected to increase as the temperature increases [42]. Generally, our values in Table 3 fall within the lower range of the reported literature values for µmax and kh, while they are higher than the reported range for YX/S. The effect of temperature for our estimated parameters is higher for kh than for b and is almost negligible for µmax. The higher value of YX/S obtained in this study compared to other literature studies may be due to the fact that in this model only the microbial population that metabolises glucose was considered, while in reality the products of glucose fermentation are metabolised further by other microorganisms (e.g. methanogens) not included in the model. In our model all the microorganisms are lumped together in one single variable X, with one growth yield YX/S and this may give higher values of YX/S than the true value which corresponds to the metabolism of glucose-degrading microorganisms. Any differences between the parameter values estimated in this study and the literature range may also be due to the behaviour of the microbial communities used in this study. However, due to the large number of parameters adjusted in this study, it is possible that correlation effects between parameters are also present, preventing the reliable estimation of individual parameters, as it is the case for many literature studies [43]. It is important to observe that the aim of our modelling study was to prove the applicability of the considered kinetic model to describe our experimental result, rather than to estimate the values of the parameters accurately. More experimental study in a wider range of conditions is needed to improve the accuracy of parameter estimation. While the values of the kinetic parameters estimated in this study are only valid for the microbial communities we used, we believe that the general trends simulated by the model, e.g. the effect of residence time and of substrate concentration, are also valid for other microbial populations, although further study is needed in this area. In addition to the kinetic parameters, the initial biomass concentration was also an estimated parameter for each of our experiments. Experiments B and C were both inoculated with the same soil, however microorganisms in experiment B were subject to a pre-cultivation of 28 days under CSTR conditions (Table 2), where no significant cellulose degradation was observed, which likely caused a wash-out of the microorganisms, explaining the lower initial biomass concentration in experiment B than in C. The lower active biomass concentration in experiment A, inoculated with anaerobic digester sludge, is probably due to the low activity of this inoculum, which came from an anaerobic digester operated at long SRT (50 days).
In summary, the mathematical model developed in this study showed the ability to describe the main features of cellulose biodegradation adequately. The model was able to simulate batch and continuous experiments and to explain the experimentally observed behaviour, i.e. the approximately linear cellulose degradation in batch tests and the effect of residence time and of feed cellulose concentration in continuous reactors. After further validation and calibration with other experimental data, the model could be used to simulate, design and optimise AD processes treating cellulosic and slowly biodegradable feedstocks.
4.3 Liquid-phase products
Liquid-phase products only accumulated during transient phases, but they were never found at steady state in continuous runs. In Run B, acetate and ethanol accumulated in the batch part of the run and, in the semi-continuous part of the run, when the residence time was changed from 20 to 80 days, which triggered the start of cellulose removal. A similar observation was made in Run C, when a change in the residence time from 20 to 80 days triggered the start of cellulose removal and a transient acetate accumulation. However, in all continuous runs the products were completely removed from the liquid phase as the run progressed towards steady state. The observed removal of COD at steady state was always close to the observed TC removal (Figs. 3, 4), which is an indirect indication, using the COD balance, that most of the removed cellulose was converted to methane. Although our experimental set-up was not aimed at measuring the production of biogas and its composition, the indirect evidence of methane production comes from the observed removal of the total COD. Indeed, in anaerobic digestion any reduction in the total COD of the effluent compared to the feed can be attributed to the COD of the biogas [31]. Since carbon dioxide does not give any contribution to the COD, the only species in the biogas that can contribute to the COD are hydrogen and methane. However, under the conditions of this study, a significant hydrogen concentration in the biogas at steady state is unlikely, due to the long residence times and to the fact that hydrogen production can only justify a relatively small fraction of the observed COD removal [44, 45].
These dynamics of product formation and removal can be explained with the dynamics of acidogenic and methanogenic microorganisms. When cellulose starts being removed, acidogenic microorganisms grow faster than methanogens and liquid-phase products accumulate. However, if the residence time is long enough, as time progresses methanogens slowly grow and convert the liquid-phase products into methane. The slow growth of methanogens can also explain why liquid-phase products were observed in the batch phase of Run B (average cellulose removal rate 0.08 g L−1 day−1) but not in the first phase of Run A (average cellulose removal rate 0.04 g L−1 day−1). Indeed, the lower cellulose removal rate in Run A gave enough time to the methanogenic microorganisms to grow and to convert the liquid phase products into methane.
Little experimental evidence has been reported on the production of organic acids or alcohols from anaerobic fermentation of cellulose with mixed cultures. Chyi and Dague [13] observed that organic acids were the main fermentation products from cellulose in continuous reactors at steady state. This was due to the combination of low pH and short residence time, which favoured wash-out of methanogenic microorganisms.
5 Conclusions
Open mixed cultures have a promising potential in the context of biorefineries of lignocellulosic waste because they can convert pure cellulose without any chemical or physical pre-treatments. In semi-continuous processes, the conversion of high-concentration (20 g L−1) cellulose by mixed cultures under anaerobic conditions was found to be greatly influenced by the residence time, with cellulose removal of 60% at residence time 80 days. However, cellulose removal was negatively affected as the cellulose concentration in the feed increased from 20 to 40 g L−1. Under steady state conditions, indirect evidence from the COD balance showed that cellulose was mainly converted into methane, with liquid-phase products only accumulating under transients. Under batch conditions, cellulose removal of up to 77% was observed, however the cellulose removal rates were lower than in semi-continuous processes. A mathematical model which includes cellulose hydrolysis and microbial growth on glucose was found to describe most of the observed effects well, and to provide insight into the reasons for the observed behaviours.
References
Dionisi D, Silva IMO (2016) Production of ethanol, organic acids and hydrogen: an opportunity for mixed culture biotechnology? Rev Environ Sci Biotechnol 15(2):213–242
Kleerebezem R, Joosse B, Rozendal R, Van Loosdrecht MCM (2015) Anaerobic digestion without biogas? Rev Environ Sci Bio Technol 14(4):787–801
Dionisi D, Bolaji I, Nabbanda D, Silva IMO (2018) Calculation of the potential production of methane and chemicals using anaerobic digestion. Biofuels Bioprod Biorefining 12(5):788–801
ADBA (Anaerobic Digestion and Bioresources Association) (2020) Biomethane: the pathway to 2030. Report, London, UK
Achinas S, Achinas V, Euverink GJW (2017) A technological overview of biogas production from biowaste. Engineering 3(3):299–307
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol 96:2026–2032
Bommarius AS, Katona A, Cheben SE, Patel AS, Ragauskas AJ, Knudson K, Pu Y (2008) Cellulase kinetics as a function of cellulose pretreatment. Metab Eng 10(6):370–381
Pavlostathis SG, Miller TL, Wolin MJ (1988) Fermentation of insoluble cellulose by continuous cultures of Ruminococcus albus. Appl Environ Microbiol 54(11):2655–2659
Hu Z, Wang G, Yu H (2004) Anaerobic degradation of cellulose by rumen microorganisms at various pH values. Biochem Eng J 21(1):59–62
Zhang L, Jongshik C, Qingqing J, Rui S, Jian Z, Yingjuan Z, Nanqi R (2017) Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values. RSC Adv 7(64):40303–40310
Singh N, Mathur AS, Tuli DK, Gupta RP, Barrow CJ, Puri M (2017) Cellulosic ethanol production via consolidated bioprocessing by a novel thermophilic anaerobic bacterium isolated from a Himalayan hot spring. Biotechnol Biofuels 10(1):73
Chyi YT, Dague RR (1994) Effects of particulate size in anaerobic acidogenesis using cellulose as a sole carbon source. Water Environ Res 66(5):670–678
Ueno Y, Kawai T, Sato S, Otsuka S, Morimoto M (1995) Biological production of hydrogen from cellulose by natural anaerobic microflora. J Ferment Bioeng 79(4):395–397
Soundar S, Chandra TS (1990) Anaerobic digestion of cellulose by pure and mixed bacterial cultures. J Ind Microbiol 5(5):269–276
Kinet R, Destain J, Hiligsmann S, Thonart P, Delhalle L, Taminiau B, Daube G, Delvigne F (2015) Thermophilic and cellulolytic consortium isolated from composting plants improves anaerobic digestion of cellulosic biomass: toward a microbial resource management approach. Bioresour Technol 189:138–144
Siegert I, Banks C (2005) The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Proc Biochem 40(11):3412–3418
Gadow S, Li Y, Liu Y (2012) Effect of temperature on continuous hydrogen production of cellulose. Int J Hydrogen Energy 37(20):15465–15472
Noike T, Endo G, Chang J, Yaguchi J, Matsumoto J (1985) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27(10):1482–1489
Desvaux M, Guedon E, Petitdemange H (2000) Cellulose catabolism by Clostridium cellulolyticum growing in batch culture on defined medium. Appl Environ Microbiol 66(6):2461–2470
Desvaux M, Guedon E, Petitdemange H (2001) Carbon flux distribution and kinetics of cellulose fermentation in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. J Bacteriol 183(1):119–130
Weimer PJ, Shi Y, Odt CL (1991) A segmented gas/liquid delivery system for continuous culture of microorganisms on insoluble substrates and its use for growth of Ruminococcus flavefaciens on cellulose. Appl Microbiol Biotechnol 36(2):178–183
O’Sullivan CA, Burrell PC, Clarke WP, Blackall LL (2006) Comparison of cellulose solubilisation rates in rumen and landfill leachate inoculated reactors. Bioresour Technol 97(18):2356–2363
Dionisi D, Bolaji I (2016) Biorefinery with open mixed cultures for biofuels and chemicals production from organic waste: biodegradation of unpretreated cellulose. Chem Eng Trans 49:157–162
Bartram AK, Jiang X, Lynch MD, Masella AP, Nicol GW, Dushoff J, Neufeld JD (2014) Exploring links between pH and bacterial community composition in soils from the Craibstone experimental farm. FEMS Microbiol Ecol 87:403–415
Gubry-Rangin C, Novotnik B, Mandič-Mulec I, Nicol GW, Prosser JI (2017) Temperature responses of soil ammonia-oxidising archaea depend on pH. Soil Biol Biochem 106:61–68
Dionisi D, Igwe V, Paton G (2019) Effect of the process conditions on the anaerobic fermentation of glucose for the production of chemicals. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00557-4,1-10
Dionisi D, Etteh CC (2019) Effect of process conditions on the aerobic biodegradation of phenol and paracetamol by open mixed microbial cultures. J Environ Chem Eng 7(5):103282
APHA, AWWA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Bolaji IO, Dionisi D (2017) Acidogenic fermentation of vegetable and salad waste for chemicals production: effect of pH buffer and retention time. J Environ Chem Eng 5(6):5933–5943
Dionisi D (2017) Biological wastewater treatment processes: mass and heat balances. CRC Press, Boca Raton
Contois DE (1959) Kinetics of bacterial growth: relationship between population density and specific growth rate of continuous cultures. Microbiology 21(1):40–50
Chen YR, Hashimoto AG (1980) Substrate utilization kinetic model for biological treatment process. Biotechnol Bioeng 22(10):2081–2095
Hill DT (1982) Design of digestion systems for maximum methane production. Trans ASAE 25(1):226–0230
Vavilin VA, Fernandez B, Palatsi J, Flotats X (2008) Hydrolysis kinetics in anaerobic degradation of particulate organic material: an overview. Waste Manag 28(6):939–951
Biernacki P, Steinigeweg S, Borchert A, Uhlenhut F (2013) Application of Anaerobic Digestion Model No. 1 for describing anaerobic digestion of grass, maize, green weed silage, and industrial glycerine. Bioresour Technol 127:188–194
Weinrich S, Nelles M (2015) Critical comparison of different model structures for the applied simulation of the anaerobic digestion of agricultural energy crops. Bioresour Technol 178:306–312
Kalyuzhnyi SV (1997) Batch anaerobic digestion of glucose and its mathematical modelling. II. Description, verification and application of model. Bioresour Technol 59(2):249–258
Borja R, Rincón B, Raposo F, Alba J, Martın A (2003) Kinetics of mesophilic anaerobic digestion of the two-phase olive mill solid waste. Biochem Eng J 15(2):139–145
Yang J, Lunhui L, Wenjuan O, Yao G, Youpeng C, Hua M, Jinsong G, Fang F (2017) Estimation of kinetic parameters of an anaerobic digestion model using particle swarm optimization. Biochem Eng J 120:25–32
Gavala HN, Angelidaki I, Ahring BK (2003) Kinetics and modeling of anaerobic digestion process. In: Ahring BK et al (eds) Biomethanation I. Springer, Berlin, pp 57–93
Donoso-Bravo A, Retamal C, Carballa M, Ruiz-Filippi G, Chamy R (2009) Influence of temperature on the hydrolysis, acidogenesis and methanogenesis in mesophilic anaerobic digestion: parameter identification and modeling application. Water Sci Technol 60(1):9–17
Liu C, Zachara JM (2001) Uncertainties of Monod kinetic parameters nonlinearly estimated from batch experiments. Environ Sci Technol 35(1):133–141
Sivagurunathan P, Kumar G, Bakonyi P, Kim SH, Kobayashi T, Xu KQ, Lakner G, Tóth G, Nemestóthy N, Bélafi-Bakó K (2016) A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int J Hydrogen Energy 41:3820–3836
Silva IMO, Dionisi D (2020) Effect of the operating conditions on the anaerobic digestion of wheatgrass for chemicals and energy production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00735-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bolaji, I.O., Dionisi, D. Experimental investigation and mathematical modelling of batch and semi-continuous anaerobic digestion of cellulose at high concentrations and long residence times. SN Appl. Sci. 3, 778 (2021). https://doi.org/10.1007/s42452-021-04750-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04750-x