Abstract
Simultaneous evaporation of PbS and ZnSe using hot-wall deposition was investigated to prepare nanocomposite thin films. X-ray diffraction patterns indicated that the films formed a phase mixture of ZnSe and PbSe, suggesting that an evaporation source of PbS phase-transformed to PbSe during a film deposition. Wavelength-dispersive spectroscopy indicated that the composite contains a small amount of S below 1 at.%. High-angle annular dark-field scanning transmission electron microscopy and line scan analysis in electron energy-loss spectroscopy indicated that PbSe nanocrystals were dispersed in a ZnSe, while S tended to segregate in ZnSe matrix. Photocurrent spectra indicated that peak position at approximately 460 nm shifted toward a shorter wavelength as Pb concentration increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanocomposite thin films containing nanocrystals (NCs) of narrow-gap semiconductor embedded in a wide-gap material are potential application for photodetectors [1, 2], solar cells [3,4,5,6], and thermoelectric devices [7, 8]. The combination of lead chalcogenide and zinc chalcogenide is one of the candidates for such use due to the reasons: (1) the combination phase-separates without forming a solid solution in thermal equilibrium, (2) band-gap difference between the both is relatively large, and (3) vapor pressures of the chalcogenides are similar and relatively large. Specifically, phase diagram of PbSe and ZnSe system, for instance, indicated that PbSe and ZnSe phase-separated at temperatures below 1283 K in thermal equilibrium [9]. A phase separation provides a compositional steepness at a hetero-interface with a relatively large band-gap difference. Zinc chalcogenides have relatively large band gaps of 3.7 eV in ZnS [10], 2.67 eV in ZnSe [11], and 2.25 eV in ZnTe [12]. In addition, lead chalcogenides have relatively large exciton Bohr radiuses of 18 nm in PbS [13], 46 nm in PbSe [14], 152 nm in PbTe [15]. Hence, composites using the combination are expected to exhibit quantum confinement effects. To prepare such composite film, we employ hot-wall deposition (HWD), which provides a state near thermal equilibrium in a thermal evaporation [16]. Hence, lead and zinc chalcogenides, whose vapor pressure are similar and a relatively large, are useful for simultaneous evaporation. Following the material designs, the simple composites using evaporation sources of selenides (i.e., PbSe and ZnSe) and tellurides (i.e., PbTe and ZnTe) provided a characteristic of phase separation at heterointerface between PbSe NCs and ZnSe matrix or PbTe NCs and ZnTe matrix [17,18,19].
In a next step, solid solution NCs (e.g., PbSe1−xTex) under keeping phase separation between NCs and matrix could offer continuous change in energy band gap of NCs, capable of providing a tunability in wavelength for devices such as infrared detectors. In fact, simultaneous evaporation of PbTe and ZnSe provided a phase transformation from PbTe (i.e., evaporation source) to PbSeTe ternary solid solution in a composite [20]. The nanocomposite formed PbSeTe NCs embedded in a ZnSe matrix (PbSeTe-ZnSe). Energy band gap of the solid solution NCs was able to increase from 0.278 eV in PbSe [21] to 0.31 eV in PbTe [22]. Hence, the combination of lead and zinc chalcogenide and a use of HWD technique provided unique composites. However, it is unclear whether similar phase transformation occurs on a different lead chalcogenide (i.e., PbS) or a specific event on PbSeTe-ZnSe composites. The objective of this work is to totally establish a preparation method of nanocomposite films based on the material designs using phase transformation in lead chalcogenide NCs. The present study focuses on simultaneous evaporation of PbS and ZnSe. PbS has a relatively large vapor pressure [23], capable of simultaneous evaporation with ZnSe in HWD. Although PbSe and PbS formed all proportional solid solution PbSe1−ySy, there was no mutual solubility range between PbS and ZnS or PbSe and ZnSe in thermal equilibrium [9, 24,25,26]. Hence, nanocomposite possibly phase-separated to PbSe1−xSx solid solution NCs dispersed in zinc chalcogenide.
2 Materials and methods
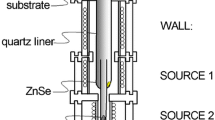
A nanocomposite thin film was prepared using HWD (Fig. 1). Here, PbS and ZnSe were used as evaporation sources with 5 N of purity in ZnSe (Furuuchi Chemical, Tokyo, Japan). PbS was synthesized in a vacuum-sealed quartz tube using elements with 6 N of purity in Pb (Osaka Asahi Metal Mfg., Osaka, Japan) and S (Furukawa, Tokyo, Japan) and then vapor transported repeatedly in an electric furnace with a maximum temperature of 1273 K until disappearing an unreactive S. In a HWD, the two sources were located at different positions in a quartz liner to simultaneously thermal-evaporate on a Si (100) substrate. A background pressure was 4 × 10–4 Pa. The substrate temperature was changed using 2 instruments of water-jacket, which was located upon a substrate, circulating water controlled at temperatures from 280 to 350 K and Peltier devices at temperatures from 370 to 450 K. The temperatures were kept constant at 853 K for a source of ZnSe, and 673 K for the wall, which was located between the source and the substrate with no evaporation sources in the quartz liner. The temperature for a source of PbS was varied to provide different Pb concentrations.
The film was structurally characterized using X-ray diffraction (XRD) with Cu Kα radiation (Rigaku RAD-X, Tokyo, Japan). A symmetric 2θ–θ configuration was used. The composition of the film was analyzed using wavelength-dispersive spectroscopy (WDS) (Oxford Instruments INCA, Abingdon, UK) operating at 15 kV with standard samples of PbS to calibrate the analyzed results for Pb and S and with ZnSe for Zn and Se. The chemical bonding in the films was evaluated using X-ray photoelectron spectroscopy (XPS) (JEOL, JPS-9010MC, Tokyo, Japan) with AlK radiation. The film was directly observed using high-angle annular dark field scanning transmission electron microscopy ((HAADF-STEM) operating at 200 kV (JEOL ARM200F, Tokyo, Japan). Focused ion beam was performed in the sample preparation. Photocurrent spectrum of the film was measured using photocurrent spectrometer (BUNKOUKEIKI, Tokyo, Japan). Prior to characterizing photocurrent spectrum, indium tin oxide (ITO) with a thickness of 100 nm was coated on the film surface as a transparent electrode by radio-frequency (RF) sputtering.
3 Results and discussion
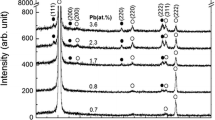
Figure 2a depicts the XRD pattern of the composite thin films with respect to Pb concentration. In this case, the substrate temperature was kept constant at 350 K. The composites form a phase mixture of lead chalcogenide with NaCl structure and zinc chalcogenide with zinc-blende structure with dominant [111] orientation. Any peaks attributing to elements (e.g., Zn and Pb) can’t be seen in the patterns. Figure 2b depicts the enlarged XRD peak at around (220) Bragg reflection of lead chalcogenide with scan ranges from 41° to 44.5°. The dotted lines indicate standard peak positions of PbSe and PbS. The evaporation source is bulk PbS, but the diffraction peak is close to standard PbSe regardless of Pb concentration. This result strongly suggests that an evaporation source of PbS phase-transforms to PbSe during simultaneous evaporation of PbS and ZnSe.
Figure 3a depicts lattice constant of a lead chalcogenide estimated from (220) Bragg reflection and a zinc chalcogenide estimated from (111) Bragg reflection. The lattice constant of the zinc chalcogenide is close to standard ZnSe as indicated by dotted line and almost kept constant regardless of Pb concentration, whereas the lattice constant of the lead chalcogenide is close to PbSe rather than that of PbS. WDS analysis result (Fig. 3b) indicates that a concentration of Pb increases gradually from 1.5 to 3.5 at.% with increasing a source temperature in PbS evaporation-source (Fig. 1), but S varies only from 0.1 to 0.4 at.% in the composites, indicating a considerable deficit in S. Composition ratio in cation with Zn and Pb and anion with Se and S ranges from 0.99 to 1.00 (inset in Fig. 3b), almost remaining a stoichiometric composition. Hence, sublimation of ZnSe should compensate a considerable deficit in S by a dissociated Se in vapor state. Therefore, evaporation sources of PbS and ZnSe provide a composite with PbSe and ZnSe containing a small amount of S.
a Lattice constant of the composite films as a function of Pb concentration. The dotted lines indicate the lattice constant of standard ZnSe, PbS, and PbTe. b WDS analysis result of composite films as a function of source temperature of PbS. Inset indicates composition ratio of anion with Se and S to cation with Zn and Pb
Figure 4a shows the XPS spectrum of the composite film with a Pb concentration of 3.5 at.%. The main peak is located at a binding energy of 88.9 eV, which is assigned to ZnSe with the 3p3/2 spin state of Zn [27]. The spectral peak with the 3d5/2 spin state of Se (Fig. 4b) also assigned to ZnSe at 54.4 eV [28] and PbSe at 54.1 eV [29]. The results well correspond to the XRD result (Fig. 3a). However, the spectral peak of the 4f7/2 spin state of Pb (Fig. 4c) is close to several binding energies of 137.6 eV in PbSe and PbS [30, 31] and 139.2 eV in ZnSe with the 3 s spin state of Zn [27]. WDS analysis (Fig. 3b) indicated that incorporated S is considerably small (0.4 at.%). Hence, the spectral peak of 2p spin state of S (Fig. 4d) is dominated by the 3p3/2 and 3p ½ spin states of Se. Therefore, it is unclear how S exists in the composite.
Figure 5a presents HAADF-STEM cross section image of the composite film containing a Pb concentration of 3.5 at.%. Slightly bright area corresponds to lead chalcogenide, typically forming two shapes of a grain-shape at the bottom area (i.e., at the beginning of deposition) and a columnar-shape near the surface. The different morphologies should be caused by thermal radiation from electric furnaces in the HWD apparatus. Specifically, HWD provides a state near thermal equilibrium during a film deposition, since a deposition and re-evaporation are continuously repeated on the film surface [16]. In the HWD apparatus, a mechanical shutter, which was located between a substrate and a quartz liner, suppresses a thermal radiation from the furnaces toward the substrate. At the beginning of the film deposition by opening the shutter, re-evaporation from a film surface is considered to be weak (i.e., deposition to a substrate is dominant), experimentally resulting in a grain-shape in NCs. At middle and the end of the film deposition, deposition and re-evaporation is considered to be reached a state near thermal equilibrium, experimentally providing a columnar shape. An enlarged image of the bottom area is presented in Fig. 5b. The areas surrounded by white and black lines are enlarged in Figs. 5c and d. Lattice fringe is clearly observed in these images. Fast Fourier Transform (FFT) analysis (Fig. 5e and f) of the areas denoted by circle in Fig. 5c and d indicates that the spots are assigned to cubic PbSe and cubic ZnSe. Hence, the bright area (Fig. 5a and b) corresponds to PbSe and the dark area corresponds to ZnSe. Figure 5g shows electron energy-loss spectroscopy (EELS) line scan analysis for elements of Se, S, Zn, and Pb along the white line denoted in Fi5b. The heterointerface between dark area (i.e., ZnSe) and bright area (i.e., PbSe) at approximately 12 nm clearly changes the detection from Zn to Pb. The intensity of S tends to slightly decrease at the heterointerface, suggesting that S tends to segregate in ZnSe matrix (ZnSe:S), although S distributes in whole area. This result indicates that the evaporation source of PbS is phase-transformed to PbSe with a slight inclusion of S (PbSe:S). PbSe:S NCs and ZnSe:S matrix phase-separates at the heterointerface without forming a solid solution such as Zn1–xPbxSe following the material design. It is therefore concluded that nanocomposite forms PbSe:S NCs embedded in ZnSe:S matrix (PbSe-ZnSe:S) using evaporation sources of PbS and ZnSe. The phase transformation may occur during vapor phase reaction, which needs a relatively large Se-pressure in vapor state. Evaporation source of ZnSe should dominate the Se pressure. Specifically, in a thermal equilibrium, PbS dominantly forms binary compound [32] (i.e., PbS(s) = PbS(g)), while ZnSe dissociate each element [23] (i.e., ZnSe(s) = Zn(g) + 1/2Se2(g)). Vapor state mainly contains PbS, Zn, and Se. Assuming PbS and Se are chemically equilibrium with PbSe and S (i.e., PbS(g) + 1/2Se2(g) = PbSe(g) + 1/2S2(g)), this reaction could provide PbSeS ternary solid solution in solid state, while a small amount of S is incorporated into ZnSe.
a HAADF-STEM cross section image of the composite film containing a Pb concentration of 3.5 at.%. b Enlarged HAADF-STEM cross section image at a bottom area in Fig. 5a. c Enlarged image denoted by c in Fig. 5b. d Enlarged image of the area denoted by d in Fig. 5b. e FFT analysis result in the circle area in Fig. 5c. f FFT analysis result in the circle area in Fig. 5d. g EELS line scan analysis of Se, S, Zn, and Pb
Next, temperature dependence of phase transformation in lead chalcogenide is investigated. Figure 6a depicts XRD pattern of the composite thin films with respect to substrate temperature. The XRD peak of ZnSe at (111) Bragg reflection is still dominant, while a phase transformation from PbS to PbSe occurs even at relatively low substrate-temperatures, but the peak intensity at (220) in PbSe becomes weak as substrate temperature decreases (Fig. 6b). From direct observation using HAADF-STEM (not shown), PbSe crystallizes even at relatively low temperature of 280 K. The simple composite of selenide (i.e., PbSe-ZnSe) provided amorphous structure in PbSe at the temperature range [19]. Therefore, the low temperature stability in PbSe NCs suggests a slight inclusion of S, well corresponding to the EELS line scan analysis result (Fig. 5g). Similar result was also seen in PbSeTe NCs embedded in ZnSe matrix [20], owing to the stability of PbTe NCs in simple PbTe-ZnTe composite [6].
To clarify characteristics of a slight inclusion of S in the composite, the two composites of PbSe-ZnSe:S and simple PbSe-ZnSe are compared on photocurrent spectra. In this case, ITO electrode was deposited on a film surface using RF sputtering (inset in Fig. 7a). Figure 7a depicts normalized photocurrent spectrum of representative PbSe-ZnSe:S nanocomposite thin film with 1.6 at.%Pb and 0.09 at.%S. For reference, the spectrum of simple PbSe-ZnSe nanocomposite thin film (i.e., using evaporation sources of PbSe and ZnSe) is also presented. The broad spectrum is dominant in the two films with a maximum photocurrent at approximately 850 nm, which is considered to be mainly generated from Si substrate. A peak can also be observed at approximately 460 nm, which is close to the energy band gap of ZnSe as indicated by dotted line. Similar peak is also seen in the PbSe-ZnSe nanocomposite, but the position in the PbSe-ZnSe:S nanocomposite is found to slightly shift toward a shorter wavelength exceeding the band gap of ZnSe. Figure 7b depicts the peak position at approximately 460 nm as a function of Pb concentration. The red dot indicates simple PbSe-ZnSe nanocomposites. The peak positions in the PbSe-ZnSe nanocomposites change from 470 to 475 nm. All the positions located below the energy band gap of ZnSe. In contrast, the positions in the PbSe-ZnSe:S nanocomposites tend to decrease as Pb concentration increases. In PbSe-ZnSe, the spectral peak of ZnSe disappeared at the relatively high Pb concentration exceeding 1 at.%. resulting in clustering the plots in the upper left in Fig. 7b. In contrast, PbSe-ZnSe:S remained the spectral peak until approximately 3at%Pb. The reason for the difference is considered to be related to a crystallinity of composite. In fact, a sight inclusion of S exhibited a low temperature stability in PbSe NCs (Fig. 6). As reasons for the shift toward a shorter wavelength, the two possibilities are considered; (1) Increasing energy band gap due to forming a solid solution of ZnSe1-xSx in a nanocomposite (2) Producing a strain in ZnSe due to a presence of PbSe NCs. In the former, energy band gap of ZnSe1–xSx should increase with increasing S concentration, since the energy band gap of ZnS is larger than that of ZnSe [11]. The inset in Fig. 7b depicts a comparison between the present result and the energy band gap of ZnSe1-ySy solid solution [33]. In this case, the photon energy of the PbSe-ZnSe:S is estimated from the peak position. The present result rapidly increases in comparison with ZnSe1-ySy solid solution, suggesting that the contribution of the solid solution is weak on the peak shift. In the latter, compressive lattice-distortion in ZnSe induced an increase in its energy band gap by shifting and splitting heavy- and light-hole bands [34]. In addition, Raman spectral peak with longitudinal-optical (LO) phonon mode tended to shift toward a longer wavenumber as a thickness of ZnSe epitaxial layer on GaAs substrate decreases (i.e., compressive strain induced the shift) [35]. In the present study, the lattice constant of ZnSe with a slight inclusion of S in the nanocomposites actually changed with respect to Pb concentration, but there was no obvious tendency. In addition, Raman spectral peak with LO phonon mode located at shorter wavelengths regardless of Pb concentration. Therefore, the reason for the peak shift needs further investigation.
a Photocurrent spectra of PbSe-ZnSe:S nanocomposite film. For comparison, photocurrent spectrum of PbSe-ZnSe nanocomposite film is also presented. Inset indicates schematic mage of the specimen. b peak position of ZnSe in the spectrum as a function of Pb concentration. Inset indicates the peak position in photon energy as a function of S concentration. The dotted line indicates Vegard’s law in ZnS-ZnSe system
In the present work, the evaporation sources of PbS and ZnSe provides a composite with PbSe:S NCs embedded in ZnSe:S matrix, while composition ratio is almost remained at stoichiometry in cation with Zn and Pb to anion with Se and S. Hence, excluded elements are considered to be S and Zn, while vapor state mainly contains PbS, Zn, Se, and substances produced by vapor phase reaction [i.e., PbS(g) + 1/2Se2(g) = PbSe(g) + 1/2S2(g)] (Fig. 8a and b). In a composite with 3.5 at.%Pb and 0.4 at.%S in WDS analysis (Fig. 3b), 3.1 at.%S is excluded from the composite, assuming a stoichiometric PbS is evaporated. This corresponds to a S deficit of 88.6%. In visual inspection of the equipment, some excluded substances were deposited at cold area in quartz liner (i.e., the part of “Wall”) (Fig. 1 and Fig. 8a) with colors of yellow-green and metal. The candidates are ZnSe for yellow-green color and Zn, PbS, and PbSe for metallic color. Hence, almost all excluded S is considered to be exhausted due to a relatively high vapor pressure of S. Similar phase transformation occurred on evaporation sources of PbTe and ZnSe, providing a composite with PbSeTe NCs embedded in ZnSe matrix [20]. Therefore, sublimation of ZnSe dominates the phase transformations regardless of evaporation sources in lead chalcogenides [i.e., PbS for PbSe:S NCs (Fig. 8c), PbTe for PbSe:Te NCs (Fig. 8d), and PbSe for PbSeNCs (Fig. 8e)]. In our further experiments with evaporation sources of ZnTe and lead chalcogenides, Te-rich solid solution in lead chalcogenide NCs (e.g., PbTe:S and PbTe:Se) are produced regardless of lead chalcogenides (i.e., PbS and PbSe). Therefore, substances for a matrix (i.e., zinc chalcogenide) dominate a production of solid solution NCs. This unique method could be applied to another combination with IV–VI, II–VI, I–VI, and III–VI families, providing a tunability in energy band gap of NCs and selective doping into NCs or matrix.
a Picked-p image of quartz liner with evaporation sources of ZnSe and lead chalcogenide (LC) in Fig. 1. b Schematic image of vapor state in quartz liner denoted by red circle in Fig. 8a. c Schematic image of composite with PbSe NCs containing a small amount of S (PbSe:S). d Schematic image of composite with PbTe NCs containing a small amount of Te (PbSe:Te). e Schematic image of composite with PbSe NCs
4 Conclusions
We investigated a simultaneous evaporation of PbS and ZnSe in HWD technique. An evaporation source of PbS phase-transformed to PbSe:S NCs in a nanocomposite. The both PbSe NCs and ZnSe matrix contain a small amount of S below 1 at.% that tends to segregate in ZnSe matrix, while composition ratio was almost remained at stoichiometry in cation with Zn and Pb to anion with Se and S. Such vapor-phase reaction needs a sufficient Se vapor, suggesting that sublimation of ZnSe dominated the phase transformation. Therefore, Se-rich solid solution in lead chalcogenide NCs are produced regardless of evaporations of lead chalcogenides (i.e., PbS and PbTe). The both PbSe:S and ZnSe:S crystallized even at a relatively low substrate-temperature of 280 K. Photocurrent spectral peak at approximately 460 nm ended to shift toward a shorter wavelength as Pb concentration increased, while the inclination of the peak shift was much larger than that of Vegard’s law in ZnS-ZnSe system.
References
LiuZhangZhengLi FMLDMJGH (2000) X-ray photoelectron and Raman spectroscopy of nanocrystalline Ga0.621n0.38Sb-SiO2 compoiste films. Appl Surf Sci 158:281–286. https://doi.org/10.1016/S0169-4332(00)00022-2
Ma S, Li K, Xu H, Zhu J, Zhu H, Wu H (2019) Lattice-mismatched PbTe/ZnTe heterostructure with high-speed midinfrared photoresponses. ACS Appl Mater Interfaces 11:39342–39350. https://doi.org/10.1021/acsami.9b13154
Nozik AJ (2002) Quantum dot solar cells. Phys E 14:115–120. https://doi.org/10.1016/S1386-9477(02)00374-0
Usui H, Abe S, Ohnuma S (2009) InSb/Al-O Nanogranular films prepared by RF sputtering. J Phys Chem C 113:20589–20593. https://doi.org/10.1021/jp906051b
Zhu K, Shi J, Zhang L (1998) Preparation and optical absorption of InSb microcrystallites embedded in SiO2 thin films. Solid State Commun 107:79–84. https://doi.org/10.1016/S0038-1098(98)00159-8
Dey M, Asha IA, Smita ZT, Dey M, Das NK (2019) Highly efficient ZnTe solar cell with PbTe BSF. In: 2019 5th International Conference on Advances in Electrical Engineering (ICAEE), Dhaka, Bangladesh: 613–616. https://doi.org/10.1109/ICAEE48663.2019.8975498
Ahn K, Shin H, Im J, Park SH, Chung I (2017) ZnTe alloying effect on enhanced thermoelectric properties of p-type PbTe. ACS Appl Mater Interfaces 9:3766–3773. https://doi.org/10.1021/acsami.6b15295
Rawat PK, Paul B, Banerji P (2014) Exploration of Zn resonance levels and thermoelectric properties in I-doped PbTe with ZnTe nanostructures. ACS Appl Mater Interfaces 6:3995–4004. https://doi.org/10.1021/am405410e
Oleinik GS, Mizetskii PA, Nizkova AI (1982) Nature of the interaction between lead and zinc chalcogenides. Inorg Mater 18:734–735
Yamaga S, Yoshokawa A, Kasain H (1988) Electrical and optical properties of donor doped ZnS films grown by low-pressure MOCVD. J Cryst Growth 86:252–256. https://doi.org/10.1016/0022-0248(90)90725-Z
Adachi S, Taguchi T (1991) Optical properties of ZnSe. Phys Rev B 43:9569. https://doi.org/10.1103/PhysRevB.43.9569
Nahory RE, Fan HY (1967) Optical properties of zinc telluride. Phys Rev 156:825. https://doi.org/10.1103/PhysRev.156.825
Zhao YB, Zou JH, Shi WF (2005) In situ synthesis and characterization of lead sulfide nanocrystallites in the modified hyperbranched polyester by gamma-ray irradiation. Mater Sci Eng B 121:20–24. https://doi.org/10.1016/j.mseb.2005.02.051
Kolobkova EV, Lipovskii AA, Petrikov VG, Melekhin VG (2002) Fluorophosphate glasses with quantum dots based on lead sulfide. Glass Phys Chem 28:251–255. https://doi.org/10.1023/A:1019918530283
Murphy JE, Mathew C, Andew BG (2006) PbTe colloidal nanocrystals: synthesis, characterization, and multiple exciton generation. J Am Chem Soc 128:3241–3247. https://doi.org/10.1021/ja0574973
Lopez-Otero A (1978) Hot wall epitaxy. Thin Solid Films 49:3–57. https://doi.org/10.1016/0040-6090(78)90309-7
Abe S (2011) One-step synthesis of PbSe-ZnSe composite thin film. Nanoscale Res Lett 6:1–6. https://doi.org/10.1186/1556-276X-6-324
Abe S (2016) Preparation and low temperature stability of PbTe nanocrystals embedded in ZnTe matrix by hot-wall deposition. Mat Renew Sus Energy 5:9. https://doi.org/10.1007/s40243-016-0073-1
Oba Y, Abe S, Ohnuma M, Sato N, Sugiyama M (2014) Temperature dependence of nanostruc-ture in PbSe–ZnSe composite thin film. J Phys D Appl Phys 47:435102. https://doi.org/10.1088/0022-3727/47/43/435102
Abe S (2015) One-step synthesis of composite thin film with ZnSe and PbTeSe ternary solid solution. Mater Renew Sustain Energy 4:18. https://doi.org/10.1007/s40243-015-0060-y
Preier H (1979) Recent advances in lead-chalcogenide diode lasers. AppI Phys 20:189–206. https://doi.org/10.1007/BF00886018
Melngailis I, Harman TC (1970) Single-crystal lead-tin chalcogenides. Semicond Semimet 5:111–174. https://doi.org/10.1016/S0080-8784(08)62815-X
Mills KC (1974) Thermodynamic data for inorganic sulphide selenides and tellurides. Butterworth, London, pp 1–874
Simpson DR (1964) The binary PbS-PbSe. Econ Geol 59:150. https://doi.org/10.2113/gsecongeo.59.1.150
Straus AJ, Harman TC (1973) Pseudobinary phase diagram and existence regions for PbS1-xSex. J Electron Mater 2:71–85. https://doi.org/10.1007/BF02658104
Dutrizac JE (1980) The Fe1-xS-PbS-ZnS phase system. Can J Chem 58:739–743
Langer DW, Vesely CJ (1970) Electronic core levels of zinc chalcogenides. Phys Rev B 2:4885. https://doi.org/10.1103/PhysRevB.2.4885
Islam R, Rao DR (1996) X-ray photoelectron spectroscopy of Zn1−xCdxSe thin films. J Electron Spectrosc Relat Phenom 81:69. https://doi.org/10.1016/0368-2048(95)02551-0
Weser U, Sokolovski G, Pilz W (1977) Reaction of selenite with biochemically active thiols: An X-ray photoelectron spectroscopic study. J Electron Spectrosc Relat Phenom 10:429. https://doi.org/10.1016/0368-2048(77)85039-1
Pederspm LR (1982) J Two-dimensional chemical-state plot for lead using XPS. Spectrosc Relat Phenom 28:203. https://doi.org/10.1016/0368-2048(82)85043-3
Shalvoy RB, Fisher GB, Stiles PJ (1977) Bond ionicity and structural stability of some average-valence-five materials studied by x-ray photoemission. Phys Rev B 15:1680. https://doi.org/10.1103/PhysRevB.15.1680
Colin R, Drowart J (1962) Thermodynamic study of tin sulfide and lead sulfide using a mass spectrometer. J Chem Phys 37:1120–1125. https://doi.org/10.1063/1.1733223
Larach S, Shrader RE, Stocker CF (1957) Anomalous variation of band gap with composition in zinc sulfo-and seleno-tellurides. Phys Rev 108:587. https://doi.org/10.1103/PhysRev.108.587
Rockwell B, Chandrasekhar R, Chandrasekhar M, Kobayashi M, Gunshor L (1991) Pressure tuning of strains in semiconductor heterostructures: (ZnSe epilayer)/(GaAs epilayer). Phys Rev B 44:11307. https://doi.org/10.1103/PhysRevB.44.11307
Kontos AG, Anastassakisa E (1999) Strain profiles in overcritical (001) ZnSe/GaAs heteroepitaxial layers. J Appl Phys 86:412–417. https://doi.org/10.1063/1.370745
Acknowledgements
The author gratefully acknowledges the XPS analysis of Ms. K. Ishikawa [Foundation for Promotion of Materials Science and Technology of Japan (MST), Tokyo, Japan] and STEM observation of Mr. K. Hoshino (MST). The author is also grateful to Ms. S. Sato (Research Institute for Electromagnetic Materials, Tomiya, Japan) for assisting in the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abe, S. Phase transformation from PbS to PbSe:S in hot-wall deposition of nanocomposite thin film with evaporation sources of PbS and ZnSe. SN Appl. Sci. 3, 212 (2021). https://doi.org/10.1007/s42452-021-04236-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04236-w