Abstract
The use of bacteria has been considered as a suitable alternative for metalloids remediation. We isolated 84 tellurite-resistant bacteria, and characterized tellurite-resistant and tellurite-reducing bacterial strains from samples collected in Iran. We report here a halophilic Gram-positive strain can tolerate and accumulate equal to 26.39 mM (6598.66 µg/ml) concentrations of potassium tellurite from media. This strain were identified according to the 16S rRNA gene sequence as Staphylococcus xylosus. Here we show for the first time that S. xylosus can be efficiently remediate K2TeO3. Cell aggregation in the presence of tellurite was visually observed by colony color changes to black in media. Reduction of Te to Te0 determined with the spectrophotometric measurement method and sodium diethyldithiocarbamate trihydrate reagent (DDTC, A340nm). In order to provide high tellurite remediation, the optimum growth conditions of this bacterium were determined. The best terms are included 0.4 mM of oxyanion, 40 °C growth temperature, pH 6–8, 400 mM NaCl, and 50 RPM under aerobic conditions. Resistant to tellurite and a high level of tellurite reduction by S. xylosus might be interesting for further industrial applications.
Similar content being viewed by others
1 Introduction
Tellurium (Te0) as a rare metalloid is a member of the group 16 of the Periodic Table which its biological role hasn’t been determined yet [1, 2]. The tellurium oxyanion tellurite are well-known for their extremely toxicity for most bacteria and comparatively uncommon in the environment, they can be detectable at high concentrations specially near waste discharge fields as well as widespread in soil, silt, and wastewater that they have been considered serious environmental pollutants [3,4,5]. Utilization of tellurium, which has been used enormously in metallurgy, electronics, and applied chemical industries, is increasing highly harmful redox state of this elemental form, the oxyanion tellurite (TeO32−) [6, 7]. However, tellurium’s toxicity in human is not explored as in others but more than 4 mM (1 mg/ml) tellurite concentration is highly toxic to prokaryotic and eukaryotic cells [2, 8].
Environmentally, tellurite (TeO32−) is most abundant and its toxicity has been to a large extend associated to act as a strong oxidizing agent [4]. The production of reactive oxygen species (ROS) is another hypothesis [9]. Interestingly, production of ROS is enhanced by conditions such as drought, salt, and temperature stresses, as well as by the combination of these conditions [1, 10]. On the other hand, some investigation has been recommended thiol biochemistry and metabolism probably play a major role in tellurite toxicity and also tolerance of bacteria to this oxyanion [11, 12].
Hitherto, the tellurite toxicity’s molecular basis remains debatable. It has been suggested that generating non-functional proteins occurs in replacement S by Te in some amino acids [5, 13]. The tellurite resistance mechanisms in bacteria have been proposed as the non-enzymatic or enzymatic reduction of tellurite to amorphous elemental tellurium which results in immobilization and detoxification [1, 14]. Insoluble elemental tellurium found as extracellular or intracellular black inclusions in some bacterial-selective growth media [1, 15, 16].
Accumulation of Toxic oxyanions such as tellurite in near of waste discharge sites has expected to increase over water and soil contamination [7, 17]. Today, the technologies of microbial bioremediation of toxic compounds and wastewater purification are becoming more popular [18]. Although rare in the bacteria, tellurite resistance occurs quite naturally in Corynebacterium diphtheriae, Streptococcus faecalis, some of the strain of genus Staphylococcus and some species of aerobic phototrophic bacteria [19]. Alternatively halophilic and halotolerant microorganisms could be contemplated appropriate candidates for biotransformation and bioremediation of toxigenic metals due to their capability of growth in high concentration of ions [17]. Resistance to tellurite has been reported in both Gram-positive and Gram-negative bacteria as well as anaerobic and aerobic bacteria [2, 17, 20, 21].
Bacteria that are resistant to tellurite commonly decrease the toxicity and exchange it to elemental tellurium (Te0) which gather as black shade intracellular residue. Investigating the molecular mechanisms implying tellurite resistance mechanism is considerable interest in the application of bioremediation [6, 8, 22]. Since tellurite is toxic and environmentally important, determining tellurite-resistant bacteria, and moderately halophilic bacteria for bioremediation of polluted region with tellurite oxyanions is a very interesting issue for researchers [4, 17].
The aim of this study was a successful attempt to isolation, characterization and identification the microorganisms capable of transforming toxic TeO32− into non-toxic elemental tellurium and to investigate their ability in tellurite removal from contaminated sites for potential biotechnological applications. These bacteria were retrieved from samples picked in dyeing and weaving industrial wastewater evacuated in extreme environment likely dry, heat and salty desert.
2 Materials and methods
2.1 Isolation, characterization and culture conditions of industrial wastewater tellurite-resistant bacteria
In total, 84 tellurite resistant bacteria were isolated from 15 environmental samples of wastewater, sediments around the factories, and residue waters in washing tankers were collected of Iran during the summer. Samples were enriched for tellurite resistant bacteria using Luria–Bertani culture media (Merck) [1]. Suspension of Isolated strains comprising nearly 1.5 × 108 CFU ml−1 was grown routinely in LB medium with different K2TeO3 concentrations of 0.4–36 mM (100–9000 µg ml−1), at 37 °C with agitation at 100 rpm for 1 day (24 h). All tests were performed at least in triplicate [23]. Pure cultures with the highest resistance to tellurite carried in Tryptic soy broth (TSB) media with 20% glycerol, allowing the bacteria stored in − 20 °C for 6 months.
After purification, the morphology of all isolated bacteria utilizing a low voltage electronic microscope, Gram-reaction, colony and cell morphology and motility were determined as demonstrated by Arenas et al. [1]. Growth curves as well as progression parameters such as optimal temperature and range, pH, Agitation and different NaCl concentrations range (0–20% w/v) (Merck) was determined for each isolate as described previously [1, 17, 23]. Other physiological and biochemical characterizations of selected tellurite-resistant bacteria likely Catalase, Oxidase, Voges–Proskauer (VP) test, Enzyme activity etc., were determined [24]. Further analyses were accomplished at the respective strain’s optimal growth parameters.
2.2 Identification of QWTm6 tellurite-resistant bacteria
For identification the accurate isolates, the linear amplification using DNA extraction kit (DNP Tm Kit, Cinnagene Inc., Iran) of their 16S rRNA gene was applied using the following the manufacturer’s recommended procedure and universal primers (8-27F-5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R-5′-GGTTACCTTGTTACGACTTC-3′ and 1541R-5′-AAGGAGGTGATCCAGCCGCA-3′) [25, 26]. The reaction mixture was incubated at 94 °C for 5 min (1 cycle) followed by 30 cycles of 30 s at 94 °C, annealing for 1 min at 57 °C (30 cycles), and extension for 1 min at 72 °C (30 cycles). Then, the reaction mixture was kept for 10 min at 72 °C, cooled to 4 °C, and PCR product purified by 1% (w/v) agarose gel and sequenced by Seqlab Laboratory (Germany) [27].
Phylogenetic trees of the amplified sequence of 16S rRNA of S. xulosys with closely related Staphylococcus were formed utilizing the neighbor-joining technique as executed in the CLC Sequence Viewer version 6.5.1. Software.
2.3 Determination of tellurium oxyanion tolerance and antimicrobial disk assay
Agar diffusion method was used to measure the resistance of QWTm6 strain to toxic oxyanion of tellurite [24]. After pouring molten nutrient agar in addition to various concentrations of K2TeO3 of 0.1 mM up to 36 mM (25–9000 µg ml−1) into plates, bacterial suspension (adjusted to 1.5 × 108 CFU ml−1) was inoculated on every plate and then incubated at 37 °C for 7 days with shaking.
Minimum inhibitory concentration (MIC) for tellurite was evaluated. Each plate was assembled in triplicates. Overnight cultures of elected strains were diluted with LB medium, and were spread on LB-agar (2%) plates [28]. Antibiotic disks were put in the middle of the plates, growth inhibition zones were measured after incubation 24 h at 37 °C [29].
2.4 Features impacting or influencing tellurite removal
Capability of tellurite removal by the strains were evaluated at varied pH values of 5–11, vigorous agitation (50–200 rpm), and temperatures ranging from 5 to 60 °C in basal medium supplemented with 0.5 mM potassium tellurite. To identify the efficacy of diverse concentration of sodium chloride (Merck) on tellurite removal, NaCl (50–450 mM) were included to the basal medium.
To estimate the effect of initial tellurium concentration, cultures that incubated for 1 day were diluted 1:100 with new LB medium and at the same time grown with shaking (140 rpm), 37 °C. Then, K2TeO3 (0.1, 0.2, 0.3, 0.4, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1 mg ml−1) was added and aliquots were picked at various time periods over 144 h and for 10 min centrifuged at 10,000 rpm. The method for quantification of other remaining extracellular tellurite with the aid of Supernatants was colorimetric DDTC-method (340 nm) demonstrated by Turner et al. [17, 30]. All experiments were done in triplicate.
2.5 Statistical analysis
The normality of data on Tellurite Concentration (TC) was tested using Kolmogorov–Smirnov in SPSS software (IBM, version 20) and the result showed that data were not normally distributed (P = 0.000). Therefore, the significant difference in tellurite concentration among 9 groups was tested using One-Way ANOVA with SNK post-hoc test at the significance level of 0.0 (Table S1). The variation of TC as dependent variable over time was tested using the linear regression. Additionally, strongly autocorrelated time series analysis was performed for predicting tellurite bioreduction for the next 14 days.
3 Results and discussion
3.1 Isolation and identification of QWTm6 tellurite-resistant bacteria
Among 84 tellurite-resistant bacteria isolated from various environmental source, QWTm6 which separated from dyeing textile industrial wastewater near salt desert located in Qom, Iran, showed compatible growth in LB-agar in the presence of K2TeO3. This strain because of its high levels of tellurite resistance and reduction the toxicant, was selected for further analysis to estimate its capacity to resist and reduce tellurite under different temperatures and initial tellurite concentrations (Table 1). The minimum inhibitory concentration of QWTm6 was determined. Based on MICs, QWTm6 strain tolerated relatively high concentrations of tellurite, 26 mM. QWTm6 is a Gram-positive coccus whose optimal growth temperature was 37 °C. Basic morphological traits in an optimal growth situation followed by tellurite tolerance of the QWTm6 strain and optimal growth temperature are shown in Table 1.
Biochemical and physiological characterizations of the QWTm6 strain was accomplished (Table S2). Analyzing the capability of growth in the presence of various NaCl concentrations QWTm6 grew in up to 20% NaCl. Table S3 concludes some antibiotic resistance characteristics of the QWTm6 strain.
After determination and comparison of the 16S rRNA gene sequence of the QWtm6 strain with those existed in the NCBI database, phylogenetic trees were built. The results represented that QWtm6 strain was similar to Staphylococcus genus (Fig. 1).
For studying tellurite-uptake in tellurite-resistant bacteria, S. xylosus strain QWTm6 was inoculated into fresh LB medium. When the bacteria OD600 were ~ 0.8, the culture was adjusted with various tellurite concentrations (Fig. 2) and the present tellurite in the supernatants were evaluated as described above [30]. Figure 2 exhibits that in 24 h ~ 62% of the toxic oxyanion was eliminated from the culture medium by S. xylosus strain QWTm6.
3.2 Determination of optimal growth condition of QWTm6 tellurite-resistant bacteria
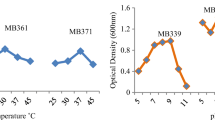
Optimal conditions for isolates including incubation temperature, pH, Agitation and salt concentrations were determined (Fig. 3a–d). These conditions had remarkable impact on potassium tellurite removal and the maximum removal in QWTm6 strain took place at pH value of 8.0, 40 °C, and 50 rpm.
a Effect of pH values on tellurite removal by QWTm6 strain in LB broth medium containing 0.4 mM potassium tellurite after 24 h (T = 37 °C, rpm = 50). b Temperature effect on tellurite removal by QWTm6 strain in LB broth medium containing 0.4 mM potassium tellurite after 24 h (pH 7, rpm = 50). c Shaking incubator effect on tellurite removal by QWTm6 strain in LB broth medium containing 0.4 mM potassium tellurite after 24 h (T = 37 °C, pH 7). d Effect of various salt concentration (mM) on tellurite removal by S. xylosus strain QWTm6 in basal medium containing 0.5 mM potassium tellurite (pH 7.5)
The maximum removal efficiency in QWTm6 strain at pH value of 8 was 97%. At pHs less than 6 and more than 9, the quantity of potassium tellurite removal was significantly reduced (Fig. 3a). Te removal in temperature of 40 °C, and shaking at 50 rpm were, 46% and 97%, respectively (Fig. 3b, c). Temperature decreasing and increasing the agitation reduced the elimination of Te from the culture media compared to the optimal growth conditions.
To define the effects of different salt concentrations on the potassium tellurite removal capacity of the strains, NaCl was added to the medium in the concentrations of 250–450 mM. Maximum potassium tellurite elimination in QWTm6 strain was seen in the presence of 400 mM NaCl (Fig. 3d). When the concentrations of NaCl raised in culture media, the removal of potassium tellurite was attained, however, increasing the concentrations of salt from 400 mM caused decline in the potassium tellurite removal by the QWTm6 strain.
Using linear regression analysis, it was found that Tellurite concentration in determination of different concentrations effect of tellurite on their removal by strain QWTm6 experiments is equal to “0.844 mM—0.001 Time” (mM: Tellurite molarity, R2 = 0.61, P = 0.051). This model represents the linear relationship between the independent and dependent variables alternations at time. Time series forecasting based on a model fitted to present and past observations shows no difference between observed and expected data according other investigations [31, 32]. In general, we see a decreasing trend in the TC (tellurite concentration) pattern, and accordingly, best bioreduction occurs in 0.4 mM tellurite and in this concentration the tellurite content will be zero at the end of an 8-day bacterial exposure (Fig. 4).
According to morphological and biochemical assays Strain QWTm6 belong to genera Staphylococcus and relying on 16S rRNA nucleotide gene sequences, QWTm6 strain was assigned to the genera S. xylosus (Table 1, Fig. 1). MIC assays in liquid media supported tellurite-tolerance outcomes. Strain QWTm6 was exhibited high MICs for tellurium (MIC 26 mM equal to 6599 μg ml−1). Considering of high tellurite MICs, Isolated Staphylococcus strain QWTm6 possesses best tellurite-reducing and the highest tellurite-resistance ability which is not yet reported among the bacteria and the genera Staphylococcus. Arenas et al. isolated some Staphylococcus bacteria which best MiCs among them was 525 µg ml−1 [1]. In fact, QWTm6 strain being able to thrive at concentrations ∼ tenfold higher than their isolated bacteria or ∼ sevenfold compare to Shakibaie, et al. results [33].
Generally, Gram-positive bacteria likely Staphylococcus display higher levels of tellurite resistance than Gram-negative microorganisms [13] but No tellurite-resistant S. xylosus were reported in earlier studies. Compared to sensitive bacteria like E. coli with 1 mg ml−1 tellurite MIC [34], high level tellurite resistance (MICs > 500 mg ml−1) was distinguished for 61.90% of the isolates (52 out of 84 isolates).
For determination of tellurite uptake using diethyldithiocarbamate (DDTC) tellurite method, it was observed that QWTm6 strain can reduce tellurite to Te0 for just the first 12 h of a 24 h culture so it has high effectiveness in tellurite detoxification. Additionally, almost ~ 90% of the tellurite originally stock in the culture medium was eliminated by QWTm6. In this method, the tellurite concentration in the culture supernatant at different intervening periods was measured using DDTC (Merck, Germany) reagent [30]. Our result is similar to other investigations which tellurite uptake is very quickly [35]. Instead, some research has shown that removing of tellurium into the bacterium is very slowly [1].
As potassium and sodium are two essential requirement for the activity of enzymes and mostly pumps in halophiles, it appears that enhancement of toxic metal tolerance and removal happen due to these elements [24]. QWTm6 strain removes Te oxyanion in the lack of any salts and over a range of moderate NaCl concentrations (up to 450 mM, Fig. 3d). In these circumstances, the capability of this halotolerant microorganism to eliminate tellurite in the existence of a wide variety of salt concentrations, pH and temperature makes it a worthy candidate for biotransformation and bioremediation of toxic metals and metalloids [2, 24, 36]. According to investigations, thermal and salt areas, has provided cultures of bacteria display very high-level resistance to tellurite which was consistent with the results of our experiments [9].
Prominently, QWTm6 strain exhibited a strong black color and black colony in broth and solid media, respectively. This appearance can occur when Te accumulates as black intracellular residue which previously have been shown in a variety of investigations and seems to be one of the main ways to tellurite detoxification in microorganisms. Blackening of culture indicate the presence of elemental Te accumulation in cell as well as bioreduction [7, 12, 22].
According to investigations, existence of heavy metals could induce and enhance bacterial antibiotic resistance [37]. These reports highlight increasing risks to public health and environmental contamination which would be the most important result of the experiments [37]. Furthermore, co-existence of heavy metal and antibiotic can change their each impact on the expanse of pollution, in addition to biological removal of pollutants, which can affect bioremediation and bioreduction processes [29]. On the other hand, antibiotic-resistant bacteria, known as ‘bio-indicator’, have received much attention for the evaluation and detection of environmental pollution [18]. We have derived a Regression Equation for the evaluation of a TC reduction change point in a linear regression. Through analyzing our bioremediation data, we have elucidated an Equation to be an effective tool in estimating enhancement of tellurite removal from wastewater during the time of bacterial exposure.
4 Conclusion
Considering the dangers of utilizing metals and metalloids in industries and their release into the environment, the use of bacteria has been proposed as an appropriate and performance choice for potassium tellurite bioremediation.
The capacity of the moderately halophilic QWTm6 strain belonging to Staphylococcus genus, to grow aerobically in the presence of high concentrations of the toxic oxyanion tellurite and to reduce it into elemental tellurium (Te0) was determined. The estimated MIC value (26.39 mM or 6598.66 µg/ml) of TeO32− oxyanions for aerobic growth of QWTm6 strain highlighted its feature to tolerate high concentration of this toxic oxyanion, as compared to other Gram-positive bacteria previously described as tellurite tolerant and/or resistant microorganisms. Tellurite bioassays indicate that the bacteria were about 2 to 3-times more resistant to tellurite than the best literature reports for the same genus [17] or other Gram-positive bacteria [38].
The result clearly demonstrates that by use of S. xylosus QWTm6 which isolated from an extreme environmental conditions such as high temperature and salt desert (Qom salt lake, Iran), we can eliminate ~ 62% of the toxic oxyanion/24 h from the culture medium. S. xylosus QWTm6 bacteria could perform tellurite reduction under salinity conditions upper than % 20.
As the S. xylosus is a safe bacterium for commercial application and its pathogenicity in human and veterinary medicine is scarce [39], the present study demonstrated that aerobically grown QWTm6 strain can be utilized as a good candidate for “green technology” in bioremediation of highly polluted sewage instead of conventional clean-up technologies.
References
Arenas FA, Pugin B, Henríquez NA, Arenas-Salinas MA, Díaz-Vásquez WA, Pozo MF, Muñoz CM, Chasteen TG, Pérez-Donoso JM, Vásquez CC (2014) Isolation, identification and characterization of highly tellurite-resistant, tellurite-reducing bacteria from Antarctica. Polar Sci 8(1):40–52. https://doi.org/10.1016/j.polar.2014.01.001
Presentato A, Turner RJ, Vásquez CC, Yurkov V, Zannoni D (2019) Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ Chem 16:266–288. https://doi.org/10.1071/EN18238
Rathgeber C, Yurkova N, Stackebrandt E, Beatty JT, Yurkov V (2002) Isolation of tellurite-and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Appl Environ Microbiol 68(9):4613–4622. https://doi.org/10.1128/AEM.68.9.4613-4622.2002
Chasteen TG, Fuentes DE, Tantaleán JC, Vásquez CC (2009) Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev 33(4):820–832. https://doi.org/10.1111/j.1574-6976.2009.00177.x
Taylor DE (1999) Bacterial tellurite resistance. Trends Microbiol 7(3):111–115. https://doi.org/10.1016/s0966-842x(99)01454-7
Turner RJ, Borghese R, Zannoni D (2012) Microbial processing of tellurium as a tool in biotechnology. Biotechnol Adv 30(5):954–963. https://doi.org/10.1016/j.biotechadv.2011.08.018
Choi W, Ha Y, Gu Y, Lee C, Park J, Jang G, Shin C, Cho S (2019) Microbial tellurite reduction and production of elemental tellurium nanoparticles by novel bacteria isolated from wastewater. J Ind Eng Chem 78:246–256. https://doi.org/10.1016/j.jiec.2019.06.006
Ba LA, Döring M, Jamier V, Jacob C (2010) Tellurium: an element with great biological potency and potential. Org Biomol Chem 8(19):4203–4216. https://doi.org/10.1039/C0OB00086H
Maltman C, Yurkov V (2019) Extreme environments and high-level bacterial tellurite resistance. Microorganisms 7(12):601. https://doi.org/10.3390/microorganisms7120601
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Tremaroli V, Workentine ML, Weljie AM, Vogel HJ, Ceri H, Viti C, Tatti E, Zhang P, Hynes AP, Turner RJ (2009) Metabolomic investigation of the bacterial response to a metal challenge. Appl Environ Microbiol 75(3):719–728. https://doi.org/10.1128/AEM.01771-08
Piacenza E, Presentato A, Zonaro E, Lampis S, Vallini G, Turner RJ (2018) Microbial-based bioremediation of selenium and tellurium compounds. In: Derco J (ed) Biosorption’. IntechOpen, London, pp 117–147. https://doi.org/10.5772/intechopen.72096
Pugin B, Cornejo FA, García JA, Díaz-Vásquez WA, Arenas FA, Vásquez CC (2014) Thiol-mediated reduction of Staphylococcus aureus tellurite resistance. Adv Microbiol. https://doi.org/10.4236/aim.2014.44024
Ramos-Ruiz A, Sesma-Martin J, Sierra-Alvarez R, Field JA (2017) Continuous reduction of tellurite to recoverable tellurium nanoparticles using an upflow anaerobic sludge bed (UASB) reactor. Water Res 108:189–196. https://doi.org/10.1016/j.watres.2016.10.074
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156(3):609–643. https://doi.org/10.1099/mic.0.037143-0
Kim D-H, Kim M-G, Jiang S, Lee J-H, Hur H-G (2013) Promoted reduction of tellurite and formation of extracellular tellurium nanorods by concerted reaction between iron and Shewanella oneidensis MR-1. Environ Sci Technol 47(15):8709–8715. https://doi.org/10.1021/es401302w
Amoozegar MA, Ashengroph M, Malekzadeh F, Razavi MR, Naddaf S, Kabiri M (2008) Isolation and initial characterization of the tellurite reducing moderately halophilic bacterium, Salinicoccus sp. strain QW6. Microbiol Res 163(4):456–465. https://doi.org/10.1016/j.micres.2006.07.010
Joshi SJ (2016) Microbial biotechnology and environmental bioremediation: challenges and prospects. Open Biotechnol J 10:287–288. https://doi.org/10.2174/1874070701610010287
Alavi S, Amoozegar MA, Khajeh K (2014) Enzyme (s) responsible for tellurite reducing activity in a moderately halophilic bacterium, Salinicoccus iranensis strain QW6. Extremophiles 18(6):953–961. https://doi.org/10.1007/s00792-014-0665-6
Klonowska A, Heulin T, Vermeglio A (2005) Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol 71(9):5607–5609. https://doi.org/10.1128/AEM.71.9.5607-5609.2005
Bajaj M, Winter J (2014) Se (IV) triggers faster Te (IV) reduction by soil isolates of heterotrophic aerobic bacteria: formation of extracellular SeTe nanospheres. Microb Cell Fact 13(1):168. https://doi.org/10.1186/s12934-014-0168-2
Soudi MR, Ghazvini PTM, Khajeh K, Gharavi S (2009) Bioprocessing of seleno-oxyanions and tellurite in a novel Bacillus sp. strain STG-83: a solution to removal of toxic oxyanions in presence of nitrate. J Hazard Mater 165(1):71–77. https://doi.org/10.1016/j.jhazmat.2008.09.065
Fuentes DE, Fuentes EL, Castro ME, Pérez JM, Araya MA, Chasteen TG, Pichuantes SE, Vásquez CC (2007) Cysteine metabolism-related genes and bacterial resistance to potassium tellurite. J Bacteriol 189(24):8953–8960. https://doi.org/10.1128/JB.01252-07
Amoozegar MA, Khoshnoodi M, Didari M, Hamedi J, Ventosa A, Baldwin SA (2012) Tellurite removal by a tellurium-tolerant halophilic bacterial strain, Thermoactinomyces sp. QS-2006. Ann Microbiol 62(3):1031–1037. https://doi.org/10.1007/s13213-011-0343-1
Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans AD (1997) Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143(9):2983–2989. https://doi.org/10.1099/00221287-143-9-2983
Rajendhran J, Gunasekaran P (2011) Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiol Res 166(2):99–110. https://doi.org/10.1016/j.micres.2010.02.003
Ibeyaima A, Rana J, Dwivedi A, Gupta S, Sharma SK, Saini N, Sarethy IP (2016) Characterization of Yuhushiella sp. TD-032 from the Thar Desert and its antimicrobial activity. J Adv Pharm Technol Res 7(2):32–36. https://doi.org/10.4103/2231-4040.177201
Bonificio WD, Clarke DR (2014) Bacterial recovery and recycling of tellurium from tellurium-containing compounds by Pseudoalteromonas sp. EPR 3. J Appl Microbiol 117(5):1293–1304. https://doi.org/10.1111/jam.12629
Zhou Y, Xu Y-B, Xu J-X, Zhang X-H, Xu S-H, Du Q-P (2015) Combined toxic effects of heavy metals and antibiotics on a Pseudomonas fluorescens strain ZY2 isolated from swine wastewater. Int J Mol Sci 16(2):2839–2850. https://doi.org/10.3390/ijms16022839
Turner RJ, Weiner JH, Taylor DE (1992) Use of diethyldithiocarbamate for quantitative determination of tellurite uptake by bacteria. Anal Biochem 204(2):292–295. https://doi.org/10.1016/0003-2697(92)90240-8
Chen D, Fries M, Lyon JM (2003) A statistical method of detecting bioremediation. J Data Sci 1(1):27–41
Huang G, Huang Y, Wang G, Xiao H (2006) Development of a forecasting system for supporting remediation design and process control based on NAPL-biodegradation simulation and stepwise-cluster analysis. Water Resour Res. https://doi.org/10.1029/2005WR004006
Shakibaie M, Adeli-Sardou M, Mohammadi-Khorsand T, ZeydabadiNejad M, Amirafzali E, Amirpour-Rostami S, Ameri A, Forootanfar H (2017) Antimicrobial and antioxidant activity of the biologically synthesized tellurium nanorods; a preliminary in vitro study. Iran J Biotechnol 15(4):268
Silkina A, Nelson GD, Bayliss CE, Pooley CL, Day JG (2017) Bioremediation efficacy—comparison of nutrient removal from an anaerobic digest waste-based medium by an algal consortium before and after cryopreservation. J Appl Phycol 29(3):1331–1341. https://doi.org/10.1007/s10811-017-1066-x
Maltman C, Yurkov V (2018) Bioremediation potential of bacteria able to reduce high levels of selenium and tellurium oxyanions. Arch Microbiol 200(10):1411–1417
Chien CC, Han CT (2009) Tellurite resistance and reduction by a Paenibacillus Sp isolated from heavy metal-contaminated sediment. Environ Toxicol Chem 28(8):1627–1632. https://doi.org/10.1897/08-521.1
Chen S, Li X, Sun G, Zhang Y, Su J, Ye J (2015) Heavy metal induced antibiotic resistance in bacterium LSJC7. Int J Mol Sci 16(10):23390–23404. https://doi.org/10.3390/ijms161023390
Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ (2016) Rhodococcus aetherivorans BCP1 as cell factory for the production of intracellular tellurium nanorods under aerobic conditions. Microb Cell Fact 15(1):204. https://doi.org/10.1186/s12934-016-0602-8
Gozalo AS, Hoffmann VJ, Brinster LR, Elkins WR, Ding L, Holland SM (2010) Spontaneous Staphylococcus xylosus infection in mice deficient in NADPH oxidase and comparison with other laboratory mouse strains. J Am Assoc Lab Anim Sci 49(4):480–486
Acknowledgements
Part of this article was presented at The 16th International and Iranian Congress of Microbiology, Tehran, Iran, 2015. The corresponding author is grateful for the underpinning support of colleagues at Qom Azad University, Iran.
Author information
Authors and Affiliations
Contributions
MSS designed and performed the research experiments, analyzed data, wrote the manuscript and Corresponding author, SH and SS Co-wrote the paper. MRZ supervised the research and MS advised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Consent for publication
All of the authors have read and approved to submit it to SN applied science.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soleimani Sasani, M., Heidarzadeh, S., Zolfaghari, M.R. et al. High potential of tellurite bioremediation by moderately halophilic Staphylococcus xylosus. SN Appl. Sci. 2, 1338 (2020). https://doi.org/10.1007/s42452-020-3149-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3149-6