Abstract
A low-vol sampler was used to collect PM2.5 samples for half-year in three sampling sites, including industrial park (A1), school (A2), factory (A3) in Shihezi, China. The result showed that the high levels of Na+ and K+ were likely originate from burning Zhundong coal. In general, the atmospheric aerosol of A1, A2 and A3 was alkaline. Most of the OC (organic carbon)/EC (element carbon) values were greater than 2.2, which indicated that there was significant secondary pollution. In the carbon component analysis, the values of OC1, EC2 and EC3 were significantly lower than OC2, OC3, OC4 and EC1. In addition, OC2 accounts for the largest proportion of OC. Combined with the source analysis of water-soluble ions, carbon component and HYSPLIT Trajectory Model, the main sources of Shihezi were local coal-fired emissions, road dust and car exhaust, and pollutants transmitted from western cities. This study provides a basis for investigating the pollution sources of PM2.5 and the influence of Zhundong coal combustion in Shihezi in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fine particulate matter (PM2.5) pollution has become an inevitable problem with the rapid development of social economy [1]. PM2.5 was defined as particles with an aerodynamic diameter of less than 2.5 μm [2]. In general, PM2.5 has small size, large contact area, strong activity, long residence time and transport distance and thus can easily attach to toxic and harmful substances.
The main chemical components of PM2.5 are water-soluble ions, organic and elemental carbons (OC and EC) [3]. Water-soluble ions are the main components of fine particles [4]. According to previous studies, water-soluble ions has a direct impact on the atmospheric radiation balance and the formation of cloud condensation nuclei, which indirectly affect the regional climate and environment [5, 6]. Carbonaceous aerosols are an important component of atmospheric aerosols and have important implications for global climate change, radiative forcing, visibility, environmental quality, and human health [7, 8].
In the last decade, the composition and source of PM2.5 have been studied because of the severe PM2.5 pollution in northern China [9,10,11]. In Xinjiang, air quality was getting worse [12], especially in Shihezi. As an important industrial city in the North Xinjiang Economic Belt, Shihezi has been experiencing continuous deterioration of air quality in recent years. Therefore, it is necessary to carry out a detailed study on the main substances and their sources in the atmospheric PM2.5 of Shihezi.
2 Materials and methods
2.1 Sampling time and location
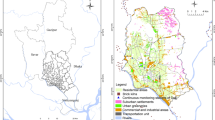
Three sampling sites were set in Shihezi where are located at the northwest of China, A1 (N44° 33′, E86° 04′), A2 (N44° 30′, E86° 06′), A3 (N44° 26′, E86° 10′),respectively (Table 1). The sampling sites were located in the economic belt of the northern slope of Tianshan Mountain, and in the south of the Jungle Basin. There are many breeze and calm winds in winter, and the terrain are low-lying areas, which is not conducive to the diffusion of fine particles in the air. Shihezi is also a man-made city where has special ecology, climatic environment and leapfrogging industry has made pollution increasingly serious. The sampling period of three sampling sites were from January 1, 2018 to June 30, 2018, for a total of 36 observation days, samples were taken every 5 days.
2.2 Sample collection
The present work combined the corresponding conditions and decided to use a low vol-rate sampler (PQ200 PM2.5 sampler, BGI Company, US). The sampling flow rate was set as 10 L min−1, and the sampling time was 23 h (from 23:00 to 22:00 in the next day). A QMA quartz membrane (47 mm, Whatman Company, the United Kingdom) was used. The filter was placed in a muffle furnace at 700 °C for 7 h before sampling to remove impure substances. The collected filter was placed in a sealed bag and stored in a freezer at − 18 °C. Disposable gloves were used for each sample to reduce human error in the experimental data.
2.3 Ion analysis
Four anions (F−, Cl−, NO3−, and SO42−) and five cations (Na+, NH4+, K+, Mg2+, and Ca2+) were determined in the extract of the sample (UP water extraction). A quarter of the quartz filter was cut, placed in an Erlenmeyer flask, and added with 30 mL of deionized water (18.2 MΩ resistivity) for ultrasonic extraction for 1 h. The Erlenmeyer flask was removed and shaken to remove bubbles. The flask was subjected to ultrasonic extraction again for 30 min and filtered with a 0.22 μm filter. The solution was stored in a refrigerator at 4 °C before analysis. F−, Cl−, NO3−, SO42− were measured by Dionex ion chromatograph (ICS-1100). Na+, K+, Mg2+, and Ca2+ were measured by ICP-MS (NexION 350X; PerkinElmer, USA). NH4+ was measured by two-channel ion chromatography.
2.4 Carbon component analysis
At present, there are many methods for analyzing OC and EC in aerosols at home and abroad, which can be mainly divided into thermal decomposition method and thermal decomposition-optical analysis method. Optical analysis is the most widely used and well-recognized, mainly by thermal transmission (TOT) and thermal reflection (TOR) [13, 14].
The measurement of filter was carried out using advanced Desert Research Institute’s (DRI) Thermal/Optical Carbon Analyzer operated on IMPROVE_A (Interagency Monitoring of Protected Visual Environments_ A) protocol to analyze OC and EC [15]. The analytical method is IMPROVE A.
2.5 Quality control and assurance
Five experimental blanks and water sample blanks per experiment were set up to remove experimental error and ensure the reliability of the data. All the vessels in the experimental process were washed three times with deionized water. The target recovery for all elements was in the range of 100% ± 20%. The correlation coefficients of the standard curves were greater than 0.999. The limit of detection (LOD) is the concentration of the signal-to-noise ratio (S/N) of 3. In order to ensure the reliability of the results, a calibration procedure was run before and after the sample was taken every day. Repeated analysis was performed for every 10 samples. The standard deviation of the repeated analysis for water-soluble ions and carbon component was 0.54–4.97%, 2.90–12.80%. For OC and EC, the LOD of OC, EC were 0.54 μg cm−2, 0.06 μg cm−2,respectively. For water-soluble ions, the LOD ranged from 0.002 to 0.030 μg m−3.
3 Results and discussion
3.1 Concentration of PM2.5
The 24-h average PM2.5 concentration in A1 varied from 4.32 μg m−3 (15 June 2018) to 573.80 μg m−3 (11 January 2018). The total mean and standard deviation were 108.09 ± 144.60 μg m−3. The 24-h average PM2.5 mass concentration in A2 ranged from 5.65 μg m−3 (30 June 2018) to 558.17 μg m−3 (11 January 2018). The total mean and standard deviation were 79.95 ± 84.22 μg m−3. The 24-h average PM2.5 mass concentration in A3 ranged from 5.27 μg m−3 (20 May 2018) to 492.16 μg m−3 (11 January 2018) (Fig. 1). The total mean and standard deviation were 102.00 ± 114.65 μg m−3. The concentration in A1 and A3 was lower than those in Raipur (150.90 μg m−3), and similar to Beijing (102.45 μg m−3). A2 was lowest in three sampling siate, but higher than those in Iksan (37.30 μg m−3) and Guangzhou (57.75 μg m−3) [16,17,18,19]. According to the latest China Ambient Air Quality Standard GB3095-2012, the concentration of PM2.5 in A1, A2, A3 has exceeded the daily average secondary standard of 75.00 μg m−3. The discovery caused serious health problems for local residents.
The highest monthly average PM2.5 concentration was detected in January in A2 (197.96 μg m−3), and in February in A1 (343.26 μg m−3), A3 (277.50 μg m−3). The lowest concentration was detected in June in A1 (7.88 μg m−3), A2 (10.57 μg m−3), and in May in A3 (16.96 μg m−3). From the Fig. 1, the values of January and February were higher than other months can be seen, which was a special phenomenon may be related to district heating and climate.
3.2 The characteristics of water-soluble ions
3.2.1 Concentration of water-soluble ions
In general, SO42−, NO3−, and NH4+ were the major components of water-soluble ions,similar to the researchs in other cities [20,21,22]. As shown in Fig. 2, the total concentration of SO42−, NO3−and NH4+ accounted for 81.57%, 75.27%, 81.60% of all water-soluble ions in A1, A2 and A3, respectively. The proportion of A1 and A3 in this set of data was basically the same, the fluctuation trend of water-soluble ions at the three sampling sites was expressed in Fig. 2. This indicated that the sources of contamination of water-soluble ions at the two sampling sites (A1and A3) may be similar. In A2, The concentration of Ca2+ was significantly higher than the other two sampling sites. This may be related to the emission of building dust. Ca2+ was the lowest water-soluble ions in A1 and A3, accounting for 0.62% and 0.77%, respectively. And the concentration of Mg2+ was low in three sampling sites.
A special finding in this study was the concentration of K+ and Na+ at three sampling sites where were significantly higher than other cities in China (Table 2). K+ and Na+ reached the highest value in January and February, when heating began. This was a very common phenomenon in northern China. In this study, the combustion of coal (Zhundong Coal) in Xinjiang was used to explain this phenomenon. Zhundong coalfield was one of the discovered super-large coal fields in Xinjiang Autonomous Region [23]. Zhundong Coalfield’s estimated reserves reach 390 billion tons, and the transportation distance between Zhundong Coalfield and Shihezi is about 300 km. Zhundong Coal (ZDC) has attracted much attention due to its high content of alkali, especially potassium oxide and sodium oxide [24].
In Shihezi, the Zhundong Coal usually came from Xinjiang Shenhua, Zhundong Wucaiwan, Zhonglian, Tianchi Energy and so on,which was heavily used for industrial production.
Therefore, the high levels of K+ and Na+ in PM2.5 were likely to originate from burning Zhundong coal, such as the burning of Zhundong Coal in heating companies, small boilers and other factories.
3.2.2 The monthly change of water-soluble ions
In this study, samples were collected for half year, which was divided into heating season (January to mid-March) and non-heating season (Mid-March to June) according to the heating time. In A1, A2, A3, the highest monthly concentration of water-soluble ions were detected in January (187.14 μg m−3), February (102.11 μg m−3), January (148.36 μg m−3), respectively (Fig. 3).
In three sampling sites, the value of water-soluble ions follows the trend of heating season > non-heating season. This finding was similar to most northern cities in China that have the highest value in winter possibly due to central heating [27] [28]. Monthly changes in water-soluble ions in three sampling sites were the same as PM2.5. In A1 and A3, the levels of Cl− and K+ increased rapidly in June may be due to the burning of local biomass [29]. High concentration of SO42− and NO3− were found in June, which indicated that significant vehicle exhaust and industrial emissions still existed during non-heating seasons. The high concentration of NH4+ in the heating season may be caused by the discharge of domestic waste from surrounding farms or rural areas [30].
Similar to 3.2.1, the high concentrations of K+ and Na+ in the heating season again indicate that they are related to the combustion of Zhundong coal, and that air pollution has a direct relationship with coal burning in the heating season.
3.2.3 Chemical characteristics of water-soluble ions
Aerosol acidity is important for atmospheric treatmentacidity and alkalinity reflect atmospheric pollution in a city or region [31]. Calculation formula follows by (1), (2):
If anion/cation less than 1, then atmospheric aerosol in this region is alkaline; if it is more than 1, then it is acidic.
From Table 3, the acidity and alkalinity of the aerosols at the three sampling sites can be found. First of all, overall averages were all lower than 1, which indicated that the aerosols at the three sampling sites were alkaline. Secondly, the aerosols in the heating season were basically acidic. This may be due to a large amount of sulfides, especially sulfur dioxide, produced by heating coal in winter. After this series of conversions, the sulfate level in the atmosphere gradually increased and reached the highest in winter [32]. In non-heating season, the levels of NO3− and SO4 2− were reduced due to elevated temperatures, resulting in decreased anions levels [33]. However, the levels of Na+, Mg2+, and K+ increased slightly which were likely caused by sandstorm in Xinjiang [34].
3.3 The characteristics of carbon component
3.3.1 The concentration of carbon component
Comparing the values of three sampling sites from Fig. 4, the trend of TC, OC and EC in three sampling sites was basically the same. Values of TC, OC and EC during heating season were significantly larger than non-heating season. Overall, the OC concentration was relatively high and the EC was relatively low. What’s more, Fig. 4 indicated that carbonaceous aerosol was one of the important components in PM2.5, in which OC was the main contribution and accounted for 19.49% of PM2.5 in Shihezi, which was slightly smaller than of Beijing (30%) [35]. But the high value of TC can be found, this may be due to a certain experimental error between the filter during the acquisition and the calculated value of PM2.5.
3.3.2 Analysis of secondary organic carbon (SOC)
The recent rise in OC/EC and SOC/OC indicates that secondary carbonaceous species are increasingly polluted [36]. In 1995, Turpin and Huntzicher proposed the following formula to calculate the SOC follow by (3).
where SOC is secondary organic carbon, OC is total organic carbon, and (OC/EC) min is the minimum value of all OC/EC [37].
Turpin and Huntzicher studied air samples in the Los Angeles Basin and concluded that an OC/EC ratio of 2.2 could be used as a threshold for secondary pollution [38].
From Table 4, most of the OC/EC values were greater than 2.2, indicating significant secondary pollution. This situation may be due to two reasons. First, a large amount of carbon-containing aerosols were emitted during the coal burning in winter. Secondly, the height and temperature of the atmospheric mixed layer were relatively low in winter, and the wind was relatively small, which was not conducive to the spread of OC, so SOC was formed by more collisions in a small space.
In addition, the average value of SOC in A1, A2 and A3 were 5.17, 6.85, 3.68, and accounting for 24.90%, 28.70%, 17.44% of TOC, respectively. The value of SOC in A3 was lower than A1 and A2 where the concentration of OC in the exhaust of motor vehicles was large. The SOC of the three sampling sites accounted for about 22.84–33.20% of the OC, the ratio was slightly lower than Beijing and Sanya [39, 40].
3.3.3 Analysis of carbon components
The carbon components were mainly divided into OC (OC1, OC2, OC3, OC4) and EC (EC1, EC2, EC3), and different components also represent different sources of pollution. According to previous research, OC1 mainly comes from biomass combustion, OC2 mainly comes from coal combustion, OC3 and OC4 mainly comes from road dust, and EC1 mainly comes from automobile exhaust, EC2 and EC3 mainly comes from diesel emissions [41,42,43,44].
According to Fig. 5, the proportion of carbon components of A1, A2 and A3 was similar, and the concentration levels were basically consistent. The characteristics presented were M-type, OC1 and EC2, EC3 was significantly lower than OC2, OC3, OC4 and EC1. The sources of the three sampling sites were mainly coal-fired emissions, road dust and automobile exhaust. The OC2 of three sampling sites was the largest proportion of OC, so the most important pollution in the three sampling sites was coal-fired emissions [45].
The proportion of OC1 was less than 5%, indicated that the proportion of biomass burning was small. Compared with other results (Fig. 3), OC1 and NH4+ were found in A1, which may be caused by burning straw in some rural areas around the north of city. EC1 was higher than EC2 and EC3, which shows that one of the main pollutants was not diesel emissions, but automobile exhaust.
3.4 Hysplit trajectory mode
The HYSPLIT Trajectory Model developed by the National Oceanic and Atmo-
spheric Research Center (NOAA) is a common source analysis model. This model is mainly used to explain the transport trajectory and diffusion pattern of atmospheric pollutants in the atmosphere [46].
The simulation time is 72 h, the starting height is 500 meters, and the starting point coordinates were set in the city center (N44° 27′,E85° 94′). The simulation time is from January 1, 2018 to June 30, 2018. A backward trajectory was generated every 6 h, 720 backward trajectories were generated and then trajectory clustering was performed to obtain a total of four trajectories (Fig. 6).
The first type of trajectory was the most important trajectory, accounting for about 61%. It was observed every month that the PM2.5 concentration was higher than the standard mass concentration, which indicated that the atmosphere of Shihezi was likely to be affected by the transmission of air pollution in the area where the track passes. Followed by the second type of trajectory, although the proportion was the lowest, but it has the highest PM2.5 concentration, so this trajectory may also increase air pollution in Shihezi, and it most likely came from the desert and may caused atmospheric dust pollution, which was consistent with the analysis results in 3.2.3. The third and fourth types of trajectories were from faraway foreign regions, the transmission distance was long, and the PM2.5 concentration was lower than the standard value. It can be clearly found that the airflow in Shihezi mainly came from the west, northwest of Shihezi, and the area was the northern Xinjiang economic belt where has lots of industrial cities, such as Karamay, Kuitun, Urumqi and so on. Therefore, Shihezi may also be affected by the transmission of atmospheric pollutants from western cities (Table 5).
4 Conclusion
During the sampling period, the average PM2.5 mass concentrations at the three sampling sites in Shihezi were 108.09 ± 144.60 μg m−3, 79.95 ± 84.22 μg m−3 and 102.00 ± 114.65 μg m−3, respectively. In Shihezi, the time-varying sequence of water-soluble ions was heating season > non-heating season. In general, SO42−, NO3−, and NH4+ were the major components of water-soluble ions. By analyzing the sources, high levels of Na+ and K+ may came from burning Zhundong coal. The carbonaceous aerosol was an important component in atmospheric fine particles, of which OC was the main contribution. Similarly, the temporal change order of OC and EC of Shihezi was heating season > non-heating season. The OC/EC values at three sampling sites were greater than 2.2 can be found, indicating the presence of significant secondary pollution. The carbon component concentrations in A1, A2, and A3 were similar and were characterized by M-type. The ratios of OC1, EC2 and EC3 were significantly lower than OC2, OC3, OC4 and EC1. Combining with the analysis results of water-soluble ions, it was shown that the emission sources of Shihezi were mainly local coal emissions and automobile exhaust. According to the HYSPLIT Trajectory Model, Shihezi is also affected by the transmission of atmospheric pollutants from western cities.
This study deepened the theoretical research of PM2.5 pollution in Shihezi, analyzed the local pollution sources and the spatial and temporal distribution, and provided a strong basis for local governments to control PM2.5 pollution.
References
Slezakova K, Pereira MC, Reis MA, Alvim-Ferraz MC (2007) Influence of trafficemissions on the composition of atmospheric particles of different size-part 1: concentrations and elemental characterization. J Atmos Chem 58(1):55–68. https://doi.org/10.1007/s10874-007-9078-6
Querol X, Andrés A, Rodriguez S (2001) PM10 and PM2.5 source apportionment in the Barcelona Metropolitan Area, Catalonia, Spain. Spain. Atmos Environ 35(36):6407–6419. https://doi.org/10.1016/S1352-2310(01)00361-2
Chow JC, Lowenthal DH, Chen LW (2015) Mass reconstruction methods for PM2.5: a review. Air Quai Atmos Hlth 8(3):243–263. https://doi.org/10.1007/s11869-015-0338-3
Hewitt CN (2001) The atmospheric chemistry of sulphur and nitrogen in power station plumes. Atmos Environ 35:1155–1170. https://doi.org/10.1016/S1352-2310(00)00463-5
Engler C, Lihavainen H, Komppula M (2007) Continuous measurements of aerosol properties at the Baltic Sea. Tellus 59(4):728–741. https://doi.org/10.1111/j.1600-0889.2007.00285.x
Bellouin N, Boucher O, Haywood J (2005) Global estimate of aerosol direct radiative forcing from satellite measurements. Nature 438(7071):1138–1141. https://doi.org/10.1038/nature04348
Larson S, Cass G, Andrewgray H (1989) Atmospheric carbon particles and the Los Angeles visibility problem. Aerosol Sci Technol 10(1):118–130. https://doi.org/10.1080/02786828908959227
Schwartz J, Dockery D, Neas L (1996) Is daily mortality associated specifically with fine particles? J Air Waste Manage Assoc 46(10):927–939. https://doi.org/10.1080/10473289.1996.10467528
He LY, Huang XF, Xue L, Hu M, Lin Y, Zheng J (2011) Submicron aerosol analysis and organic source apportionment in an urban atmosphere in pearl river delta of china using high-resolution aerosol mass spectrometry. J Geophys Res Atmos. https://doi.org/10.1029/2010JD014566
Huang RJ, Zhang Y, Bozzetti C, Ho KF, Cao JJ, Han Y, Daellenbach KR, Slowik JG, Platt SM, Canonaco F (2014) High secondary aerosol contribution to particulate pollution during haze events in China. Nature 514:218–222. https://doi.org/10.1038/nature13774
Wang L, Wei Z, Yang J, Zhang Y, Zhang F, Su J, Meng C, Zhang Q (2014) The 2013 severe haze over southern Hebei, China: model evaluation, source apportionment, and policy implications. Atmos Chem Phys 14:3151–3173. https://doi.org/10.5094/APR.2014.085
Chen J, Lu J, Ning J (2019) Pollution characteristics, sources, and risk assessment of heavy metals and perfluorinated compounds in PM2.5 in the major industrial city of northern Xinjiang. China. Air Qual Atmos Health. https://doi.org/10.1007/s11869-019-00706-8
Fan X, Brook JR, Mabury SA (2004) Measurement of organic and elemental carbon associated with PM2.5, during Pacific 2001 study using an integrated organic gas and particle sampler. Atmos Environ 38(34):5801–5810. https://doi.org/10.1016/j.atmosenv.2004.01.052
Begum BA, Hopke PK (2013) Identification of haze-creating sources from fine particulate matter in Dhaka aerosol using carbon fractions. J Air Waste Manage Assoc 63(9):1046–1057. https://doi.org/10.1080/10962247.2013.784716
Ali K, Panicker AS, Beig G, Srinivas R, Acharja P (2016) Carbonaceous aerosols over pune and hyderabad (india) and influence of meteorological factors. J Atmos Chem 73:1–27. https://doi.org/10.1007/s10874-015-9314-4
Deshmukh DK, Deb MK, Tsai YI, Mkoma SL (2010) Atmospheric ionic species in PM2.5 and pm1aerosols in the ambient air of eastern central india. J Atmos Chem 66(1–2):81–100. https://doi.org/10.1007/s10874-011-9194-1
Yang G, Hong BJ (2018) Microscopic morphology and seasonal variation of health effect arising from heavy metals in PM2.5 and PM10: 1-year measurement in a densely populated area of urban Beijing. Atmos Res 212:213–226. https://doi.org/10.1016/j.atmosres.2018.04.027
Kang GU, Lee JH (2005) Comparison of PM2.5 and PM10 in a Suburban Area in Korea During April. Water Air Soil Poll 5(3):71–87. https://doi.org/10.1007/s11267-005-0727-y
Chen W, Wang X, Zhou S (2016) Chemical composition of PM2.5 and its impact on visibility in Guangzhou, Southern China. Aerosol Air Qual Res 16(10):2349–2361. https://doi.org/10.4209/aaqr.2016.02.0059
Budhavant KB, Rao PSP, Safai PD, Gawhane RD, Raju MP (2010) Chemistry of rainwater and aerosols over bay of bengal during ctcz program. J Atmos Chem 65(2–3):171–183. https://doi.org/10.1007/s10874-011-9187-0
Han YJ, Kim SR, Jung JH (2011) Long-term measurements of atmospheric PM2.5 and its chemical composition in rural korea. J Atmos Chem 68(4):281–298. https://doi.org/10.1007/s10874-012-9225-6
Hu M, Wu Z, Slanina J (2008) Acidic gases, ammonia and water-soluble ions in PM2.5 at a coastal site in the Pearl River Delta, China. Atmos Environ 42(25):6310–6320. https://doi.org/10.1016/j.atmosenv.2008.02.015
Li G, Wang C, Yan Y, Jin X, Liu Y, Che D (2016) Release and transformation of sodium during combustion of zhundong coals. J Eenrgy Inst 89(1):48–56. https://doi.org/10.1016/j.joei.2015.01.011
Li X, Bai ZQ, Li W (2018) Chemical transformation of sodium species during direct liquefaction of a sodium-rich zhundong coal under different atmospheres and CO2, gasification of the direct coal liquefaction residue. Fuel 213:144–149. https://doi.org/10.1016/j.fuel.2017.10.119
Zhao PS, Dong F, He D (2013) Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China. Atmos Chem Phys 13:4631–4644. https://doi.org/10.5194/acp-13-4631-2013
Wang Y, Zhuang G, Zhang X (2006) The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos Environ 40(16):2935–2952. https://doi.org/10.1016/j.atmosenv.2005.12.051
Sagar GA, Singh NR, Santosh S, Kumar SA, Suresh T, Singh BD (2018) Chemical characteristics of atmospheric aerosol at alaknanda valley (srinagar) in the central himalaya region, india. Int J Environ Res 12(5):681–691. https://doi.org/10.1007/s41742-018-0125-8
Han X, Guo Q, Liu C (2016) Effect of the pollution control measures on PM2.5 during the 2015 China Victory Day Parade: implication from water-soluble ions and sulfur isotope. Environ Pollut 218:230–241. https://doi.org/10.1016/j.envpol.2016.06.038
Chueinta W, Hopke PK, Paatero P (2000) Investigation of source of atmospheric aerosol at urban and suburban residential areas in Thailand by positive matrix factorization. Atmos Environ 34:3319–3329. https://doi.org/10.1016/S1352-2310(99)00433-1
Zhang XY, Cao JJ, Li LM, Arimoto R, Cheng Y, Huebert B, Wang D (2002) Characterization of atmospheric aerosol over Xi’an in the southmargin of the Loess Plateau. China Atmos Environ 36(26):4189–4199. https://doi.org/10.1016/s1352-2310(02)00347-3
Jia S, Sarkar S, Zhang Q (2018) Characterization of diurnal variations of PM2.5 acidity using an open thermodynamic system: a case study of Guangzhou, China. Chemosphere 202:677. https://doi.org/10.1016/j.chemosphere.2018.03.127
Wang Y, Jia C, Tao J (2016) Chemical characterization and source apportionment of PM2.5 in a semi-arid and petrochemical-industrialized city, Northwest China. Sci Total Environ 573:1031–1040. https://doi.org/10.1016/j.scitotenv.2016.08.179
Tseng CY, Lin SL, Mwangi JK (2016) Characteristics of atmospheric PM2.5 in a densely populated city with multi-emission sources. Aerosol Air Qual Res 16(9):2145–2158. https://doi.org/10.4209/aaqr.2016.06.0269
Ye WF, Ma ZY, Ha XZ (2018) Spatial-temporal patterns of PM2.5, concentrations for 338 Chinese cities. Sci Total Environ 631–632:524–533. https://doi.org/10.1016/j.scitotenv.2018.03.057
He K, Yang F, Ma Y (2001) The characteristics of PM2.5 in Beijing. China. Atmos Environ 35(29):4959–4970. https://doi.org/10.1016/S1352-2310(01)00301-6
Lang J, Zhang Y, Zhou Y (2017) Trends of PM2.5 and chemical composition in Beijing, 2000–2015. Aerosol Air Qual Res 17(2):412–425. https://doi.org/10.4209/aaqr.2016.07.0307
Turpin BJ, Huntzicker JJ (1995) Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos Environ 29(23):3527–3544. https://doi.org/10.1016/1352-2310(94)00276-Q
Turpin BJ, Huntzicker JJ (1991) Secondary formation of organic aerosol in the Los Angeles basin: a descriptive analysis of organic and elemental carbon concentrations. Atmos Environ 25(2):207–215. https://doi.org/10.1016/0960-1686(91)90291-E
Hu GY (2015) Chemical composition of PM2.5 based on 2-year measurements at an urban site in Beijing. Aerosol Air Qual Res 15(5):1748–1775. https://doi.org/10.4209/aaqr.2014.11.0284
Wang J, Ho SS, Cao J (2015) Characteristics and major sources of carbonaceous aerosols in PM2.5 from Sanya, China. Sci Total Environ 530–531:110–119. https://doi.org/10.1016/j.scitotenv.2015.05.005
Watson JG, Chow JC, Lowenthal DH (1994) Differences in the carbon composition of source profiles for diesel- and gasoline-powered vehicles. Atmos Environ 28(15):2493–2505. https://doi.org/10.1016/1352-2310(94)90400-6
Kim E, Hopke PK, Edgerton ES (2004) Improving source identification of Atlanta aerosol using temperature resolved carbon fractions in positive matrix factorization. Atmos Environ 38(20):3349–3362. https://doi.org/10.1016/j.atmosenv.2004.03.012
Kim E, Hopke PK, Paatero P (2003) Incorporation of parametric factors into multilinear receptor model studies of Atlanta aerosol. Atmos Environ 37(36):5009–5021. https://doi.org/10.1016/j.atmosenv.2003.08.035
Cao JJ (2006) Characterization of roadside fine particulate carbon and its eight fractions in Hong Kong. Aerosol Air Qual Res 6(2):106–122. https://doi.org/10.4209/aaqr.2006.06.0001
Wang Z, Zhang D, Liu B, Li Y, Chen T, Sun F (2016) Analysis of chemical characteristics of PM2.5 in beijing over a 1-year period. J Atmos Chem 73(4):407–425. https://doi.org/10.1007/s10874-016-9334-8
Draxler RR, Hess GD, Draxler RR (1997) Description of the hysplit 4 modeling system[C]. National Oceanic and Atmospheric Administration Technical Memorandum Erl Arl. https://doi.org/10.1016/S0140-6736(02)98733-7
Acknowledgements
This work was financed by the National Natural Science Foundation of China projects (NO. 21667026) and the Social Science Foundation of Xinjiang Production and Construction Corps (NO. 18YB13).
Author information
Authors and Affiliations
Contributions
JL conceived and designed the experiments. JC responsible for experimental operations, data analysis and article writing.
Corresponding authors
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Lu, J., Ning, J. et al. Characteristics and sources analysis of PM2.5 in a major industrial city of northern Xinjiang, China. SN Appl. Sci. 2, 737 (2020). https://doi.org/10.1007/s42452-020-2530-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2530-9