Abstract
Presence of pesticides in drinking water is an issue of great concern in agricultural areas. In Argentina’s semiarid regions, where surface water sources are scarce and groundwater may be of poor quality, rainwater becomes important for safe water supply. The expansion of agriculture in these regions due to no till management has led to a high use of pesticides which jeopardize the safety of all water sources used for human consumption. The objective was to monitor the presence of pesticides in different water sources from two agricultural areas of Santiago del Estero. Samples belonged to cisterns in which rainwater is collected, wells and dams. The most contaminated sources were dams, followed by cisterns and wells. Applied doses and frequency of use played an important role in the presence of pesticides. Thus, the most frequent molecules were mainly herbicides; atrazine and metolachlor were the most abundant. Glyphosate and aminomethylphosphonic acid presented the highest concentrations. Almost all measured values were below the US Environmental Protection Agency limits, but 73% of the samples exceeded the limit of 0.5 μg L−1 established by the European Union for the sum of molecules although only 7.4% of individual molecules exceeded the limit of 0.1 μg L−1. However, risk assessment showed that pesticides from all sources presented a low potential risk to human health through drinking water exposure route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Presence of pesticides in drinking water is an issue of global concern. For this reason, environmental legislation in the world sets increasingly lower maximum residue limits (MRLs).Footnote 1 The European Union (EU) establishes a maximum concentration for the sum of pesticides of 0.5 µg L−1, where the concentration per individual molecule cannot exceed 0.1 µg L−1 [1]. In the USA, MRLs are established according to the toxicity of the active ingredient: MRL for atrazine is 3 µg L−1 while it is 700 µg L−1 for glyphosate [2]. In Argentina, the Argentine Food Code [3] determines MRLs for 26 organic products, but 90% of them are in disuse.
According to the World Health Organization (WHO), 2.1 billion people worldwide lack access to drinking water. In Argentina, two-thirds of the territory does not have access to safe water which means that 7 million people, particularly from rural areas, rely on alternative methods to provide themselves with drinking water. Rural populations in semiarid regions of Argentina have historically been supplied by dams fed by runoff water supplemented with groundwater [4]. In these environments, groundwater quality is usually regular to poor and rainwater becomes important for water supply since there are no nearby watercourses [5]. Nowadays, rainwater is collected from roofs of houses and public buildings and stored in tanks or cisterns built for this purpose. This system is increasingly promoted through state policies as a simple way for rural residents to access safe water.
Agricultural production is the main source of water pollution with nitrates, phosphates and pesticides. In Argentina, the pesticide market consists mainly of herbicides [6]; among them glyphosate, atrazine and 2,4-D are the most used. No till management (NT) is the predominant soil management system, taking up 91% of agricultural land [7] and requiring the use of herbicides as almost the only form of weed control. Thus, in the east of the province of Santiago del Estero during the 2016–2017 agricultural year 10 L ha−1 of glyphosate were used in soybean crops and 6–9 L ha−1 in corn, complemented with other herbicides such as atrazine, acetochlor and 2,4-D, at rates of 3.8, 2 and 1.2 L ha−1, respectively [8].
Precipitation is a major pathway for returning air pollutants to water bodies [9]. Volatilization linked to aerosol dispersion probably causes transport of pesticides into the atmosphere, resulting in quantifiable amounts in rainwater away from application sites [10]. Some studies indicated that herbicides were more prevalent in rainwater and had higher concentrations than insecticides and fungicides [11, 12]. Pesticides have also been detected in surface waters all over the world. In seven states of the USA, more than 90% of samples from different watersheds were contaminated with pesticides and the most frequently detected were aminomethylphosphonic acid (AMPA, glyphosate’s primary metabolite), glyphosate and atrazine [13]. Loos et al. [14] conducted an EU-wide study on the occurrence of organic pollutants in European rivers: Herbicides were found in low concentration ranges probably because the survey was conducted in autumn, which is an atypical application period for these compounds. Nevertheless, atrazine was detected in 68% of the samples with a maximum value of 0.08 µg L−1. In Argentina, De Gerónimo et al. [15] analyzed 29 pesticides in watercourses of basins from south of Buenos Aires, Tucumán and Misiones and determined that atrazine was present in all basins but the occurrence of other pesticides was related to the production systems of each region. In groundwater, Battaglin et al. [16] detected glyphosate in 5.8% and AMPA in 14.3% of samples from 807 sites throughout the USA. The pan-European study carried out by Loos et al. [17] determined that pesticides and their secondary metabolites were among the most relevant chemicals found in groundwater samples: atrazine and deethylatrazine (DEA) were among the most frequent compounds (56 and 55% of frequency, respectively).
The aim of this study is to analyze the presence of 30 pesticides and four secondary metabolites in water sources used mainly as drinking water in two agricultural areas of Santiago del Estero, Argentina.
2 Materials and methods
2.1 Sampling area
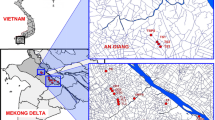
The East of Santiago del Estero is located within the Western Chaco District, and its climate is warm continental with rainfall concentrated in summer [18]. The average annual temperature is 19.6 °C; predominant winds are from the South and North quadrants [19]. The strongest winds blow in July, August and September, with hot and dry winds blowing from the North quadrants [20]. The sampled zones belong to the area of influence of the cities of Bandera and Sachayoj (Fig. 1); both correspond to semiarid agricultural-livestock areas with predominance of soybean and corn fields. Practically all the regions are managed under NT system. The average annual rainfall for the period 1949–2014 was 688 mm in Sachayoj and 822 mm in Bandera [21].
2.2 Sampling
Different water sources were selected: 26 cisterns collecting rainwater from roofs, 5 hand-dug wells (shallow water tables in Bandera), 2 boreholes (water table > 50 m, in Sachayoj) and 3 dams collecting runoff water (Table 1). Sampling points were selected based on their use: sites specifically used for human consumption (almost all the cisterns, wells B4 and B5 and dam B6) or consumed under certain conditions, such as periods of drought. Dams and wells were also included in the study because of their local relevance and the need to know whether they can really provide safe drinking water. Dams are used for urban supply and occasional consumption by the inhabitants or for animal consumption, while the water from wells is generally used for livestock consumption or household chores, except in times of water scarcity. Since the objective of this study is to evaluate contamination of drinking water, it was preferred to sample the tanks in which rainwater is collected and not rainwater itself, since their characteristics may differ due to the concentration or dilution of pesticides inside the cisterns. Four samples were taken per agricultural year coinciding with periods of pesticide application. Thus, sampling periods were spring fallow (September–October), pre-seeding and pre-emergence applications (December), application of insecticides (February) and post-harvest fallow (April–June). Sampling began in April 2014 and continued until June 2017. Figure 1 shows the location of sampling points, while Fig. 2 shows rainfall for the analyzed period. A total of 353 water samples, 159 from Sachayoj and 194 from Bandera, were analyzed to evaluate the presence of 34 molecules, including herbicides and secondary metabolites, insecticides and fungicides (see Table 2 and Online Resource 1).
2.3 Analytical procedure and instrumental analysis
Analytical procedures were carried out following the methodology described by De Gerónimo et al. [15] and Aparicio et al. [22]. Water samples were collected in polyethylene terephthalate bottles and stored in the dark at − 20 °C until analysis. Prior to analysis, they were thawed overnight at 4 °C and filtered through a 0.45 µm nylon membrane to separate water from suspended particulate matter.
Ultrahigh-performance liquid chromatography (UPLC) MS/MS analysis was performed using an Acquity UPLC system coupled to a Quattro Premier XE tandem quadrupole mass spectrometer (Waters, Mildford, MA). For chromatographic separation, an Acquity UPLC BEH C18 column (1.7 µm, 100 × 2.1 mm, Waters) fitted with an Acquity VanGuard BEH C18 pre-column (1.7 µm, 5 × 2.1 mm, Waters) were used. The mobile phase consisted in water/methanol (95:5) modified with ammonium acetate 0.1 mM and formic acid 0.01% (phase A) and methanol modified with ammonium acetate 0.1 mM and formic acid 0.01% (phase B) in gradients from 10 to 100% of phase B. Drying and nebulizing gas was nitrogen from a nitrogen generator from pressurized air in a N2 LC–MS. The collision gas was argon 99.99% with a pressure of 6.3 × 10−3 mbar in the T-Wave cell. Masslynx™ 4.1 (Waters) was used to process all data. MS/MS conditions for analyzed compounds are shown in Online Resource 2.
2.4 Human health risk assessment
To evaluate the risk of water consumption from all sources, the hazard quotient (HQ) was calculated using the following formula [23]:
where the chronic daily intake (CDI) represents the estimated amount of ingested pesticide per kilogram of body weight and RfD is the reference dose of the contaminant (µg kg−1 day−1) via the oral exposure route. The CDI was calculated by the equation [24]:
where C is the measured concentration of each pesticide in water (µg L−1); IR is the water ingestion rate (1 L day−1 for children; 2 L day−1 for adults); EF is the exposure frequency (365 days year−1); ED is the exposure duration (6 years for children and 70 for adults); BW is the body weight of the exposed person (15 kg for children; 60 kg for adults); AT is the average lifespan (2190 days for children; 25,550 days for adults). Cumulative risk assessment (HQs) was calculated using an additive approach by summing the individual HQ posed by each pesticide [25]. The sum of hazard quotients HQ for individual pesticide was:
2.5 Statistical analysis
Analysis of variance (ANOVA) was performed using a mixed linear model with the PROC MIXED procedure [26]. The “sampling date” variable was considered as a repeated measure, the ID as “subject” and source and location were “groups.”
3 Results and discussion
3.1 General observations
All compounds were detected in at least two samples and twenty of them showed a frequency higher than 10%: the most frequent compounds were mainly herbicides and their secondary metabolites (Table 2), which is consistent with other reports [12, 25, 27]. One groundwater sample was free of contaminants. Next, we will analyze the most important compounds found in this study.
3.2 Glyphosate and its metabolite AMPA
Glyphosate is the most widely used compound in number of applications per year and doses and this is reflected in the high concentrations we found, as previously stated by other authors [27,28,29]. Its intensive use extends throughout the agricultural year and this caused a diffuse occurrence (Fig. 3a), as reported by Quaghebeur et al. [30]. However, detection frequency fell from 77% in the 2014/15 season to 32% in 2015/16 and 54% in 2016/17. This variation occurred more noticeably in cisterns and wells and could be due to several factors: The year 2014 was very rainy in Sachayoj (1381 mm) and the 2014/15 season was rainier than the second and third ones in Bandera (Fig. 2), which could have generated a greater movement of glyphosate toward the different water sources during the first sampling season [28, 31]. Additionally, during the second and third sampling year a larger area of wheat was sown: the increase was greater than 100% between the first season and the next two. This crop implies a lower use of herbicides than winter fallow and reduces the environmental movement of pesticides by reducing erosion since it generates coverage in the season of stronger winds and decreases wind erosion, which is responsible for displacing soil particles enriched with glyphosate and AMPA [32]. Finally, due to the emergence of resistant weeds and spot spray systems, there is a downward trend of applied doses although the number of applications per year remains constant. Half-life values of glyphosate in soils range 1 to 68 days while those of AMPA range 39–331 days [33], showing that AMPA is more persistent. Sorption is considered to decrease glyphosate and AMPA degradation since they are both small molecules with polar functional groups, and they are strongly sorbed by soil minerals [34, 35]. Freundlich adsorption coefficient (Kf) ranged 9.4–700 L kg−1 and Freundlich constant normalized to organic carbon (Kfoc) ranged 1.600–60.000 for glyphosate while Kf ranged 10.0–1570 L kg−1 and Kfoc ranged 1.119–11.100 L kg−1 for AMPA [33], proving that adsorption is the dominant process in the behavior of these compounds. According to several authors [36, 37], organic carbon content is not a major factor in glyphosate adsorption due to the high polarity of the molecule, which minimizes the contribution of the Kfoc index as a descriptor of this herbicide retention. The persistence of both compounds would enable their movement in the environment through soil erosion [38, 39].
Since the limit of quantification (LQ) is 0.10 µg L−1 for glyphosate and 0.13 µg L−1 for AMPA quantifiable values of both compounds were above the EU limit of 0.1 µg L−1. Concentrations of glyphosate were highly variable; therefore, no significant differences were found between sources, locations or sampling dates (p > 0.05). On the contrary, significant differences were found for AMPA between sources (dams > cisterns and wells, p < 0.0001), locations (Sachayoj > Bandera, p = 0.006) and dates (p < 0.0001), since for both locations the concentrations of AMPA in dams were higher during the last agricultural year. The results of both compounds were grouped by water source to analyze their possible origin.
In cisterns, maximum concentrations were 35 µg L−1 for glyphosate (mean 1.20 ± 3.93 µg L−1) and 1.90 µg L−1 for AMPA (mean 0.52 ± 0.28 µg L−1). Presence of AMPA (Fig. 3b) suggests that drift is not the only transport mechanism: glyphosate is degraded by soil microorganisms [40] and AMPA is the main product of its partial degradation [41]. Occurrence of AMPA implies contact of glyphosate with soil, its partial decomposition and transport of AMPA to the atmosphere through soil erosion.
Due to its very low vapor pressure, glyphosate has two ways of reaching water sources: spray drift from fields and wind erosion of soil particles enriched with it [28]. In semiarid areas, wind erosion plays an important role since the risk of transporting glyphosate and AMPA with suspended dust is very high and they hardly decompose under dry soil conditions [38]. Aparicio et al. [32] analyzed sediments collected in the province of Chaco, Argentina, and found 0.66–313 µg kg−1 of glyphosate and 1.3–83 µg kg−1 of AMPA, confirming that wind-eroded material can contribute to water pollution. Furthermore, glyphosate reaching the atmosphere through drift or wind erosion can be washed away by rain. US studies found glyphosate and AMPA frequencies greater than 50% in rainwater samples from agricultural areas and determined that intense rainfall is more efficient in removing glyphosate from the atmosphere [16, 28], which may explain our higher frequency during the first season. In Argentina, Alonso et al. [29] found a high frequency of detection of glyphosate (> 80%) in rainwater and pointed out that its atmospheric deposition through rain in surface water bodies, soils and urban sites constitutes a relevant source of population exposure to this pollutant. Lamprea and Ruban [42] consider atmospheric deposition to be possibly the main contributor to glyphosate and AMPA runoff on rooftops.
Presence of bacterial communities in cisterns and persistence of glyphosate in dark conditions should be considered for future studies since Mercurio et al. [43] determined that microorganisms present in seawater were able to degrade glyphosate and the herbicide persistence in darkness was much greater than in dim light (267 vs. 47 days). According to Mallat and Barcelló [44], the complexity of the water matrix can change the rate of glyphosate degradation and the main factors affecting this process are a combination of microbial activity, temperature and photolysis.
Concentrations in dams were up to 13.2 µg L−1 of glyphosate and 5.1 µg L−1 of AMPA (mean 1.70 ± 2.82 and 1.19 ± 1.24 µg L−1, respectively). Frequencies reported by other authors in watercourses of agricultural regions were generally lower but concentrations were several times higher [31, 45, 46]. Okada et al. [47] monitored a stream southeast of the province of Buenos Aires (Argentina) and found glyphosate and AMPA in 28% and 50% of the samples, with a maximum concentration of 8.2 μg L−1 and 3.7 μg L−1, respectively. On the other hand, Mac Loughlin et al. [48] detected glyphosate and AMPA in a water body passing through a horticultural region in the Carnaval basin at concentrations up to 17.0 µg L−1 for glyphosate and 4.5 µg L−1 for AMPA, with a non-distinguishable behavior between seasons like our case. Likewise, Battaglin et al. [16] detected glyphosate in 33.7% and AMPA in 29.8% of samples belonging to lakes, ponds or wetlands, with maximum concentrations of 301 µg L−1 and 41 µg L−1, respectively. All the dams in our study receive runoff water from surrounding fields, so that would be the most important source of pollution [49], not ruling out atmospheric deposition and groundwater discharge in the case of the Bandera as other possible pollution pathways [50].
Groundwater concentrations reached 10.6 µg L−1 for glyphosate in Bandera (mean 1.1 ± 2.29 µg L−1) and 0.9 µg L−1 in Sachayoj (mean 0.6 ± 0.36 µg L−1) while AMPA concentrations were up to 1.9 µg L−1 (mean 0.49 ± 0.36 µg L−1) and 0.5 µg L−1 (mean 0.35 ± 0.21 µg L−1), respectively. The greater presence of both molecules in shallow groundwater of Bandera (although difference was not significant for glyphosate) could be attributed mainly to leaching through soil profile. In fine-textured well-structured soils, such as the ones from Bandera, preferential flow would constitute an important pathway [51]. In groundwater from southeast of Buenos Aires, Okada et al. [47] detected glyphosate and AMPA in 24% and 33% of the samples, with maximum levels of 8.5 μg L−1 and 1.9 μg L−1, respectively. The lower frequencies and concentrations found in boreholes coincide with other studies [16, 31], although Primost et al. [46] did not detect these compounds in aquifers of the province of Entre Ríos with depths similar to Sachayoj, and Okada et al. [47] did not find an association between depth and presence of glyphosate or AMPA. Lutri et al. [52] detected glyphosate (1.2–2.0 µg L−1) and AMPA (1.5–3.1 µg L−1) in 15.8% of groundwater samples, pointing out that their detection was related to areas with shallow water table (< 4 m), low hydraulic conductivity (1.5 m d−1), low hydraulic gradient (0.16%) and very low flow rate (0.024 m d−1). Their presence in an unconfined aquifer shows that their use under the predominant agricultural model exceeds the degradation potential of the soil system, causing groundwater contamination.
Higher concentrations and frequency of AMPA would be explained by its greater persistence in the environment, as mentioned above [33, 40]. To understand the fate and transport of pesticides, the relation between pesticide metabolites and the parent compound is often used to indicate the closeness of sampling to application source, either in time or space [16]. The %AMPA would be calculated as follows:
where [AMPA] and [glyphosate] are their concentrations in water. Battaglin et al. [16] found the highest values for this ratio in groundwater samples (median 100%) and the lowest values in rainwater (median 20%). In our case, the %AMPA was very variable in all sources but it tended to be a higher in cisterns (mean 66% ± 32%) and dams (61% ± 31%) than in wells (52% ± 43%) due to accumulation of AMPA and/or glyphosate degradation to AMPA in these sources. This would indicate that these compounds came from ancient sprays or distant sites, possibly mobilized by surface runoff or wind erosion, confirming that spray drift would not always be the main source of water pollution.
3.3 Atrazine and its metabolites hydroxyatrazine (HOA), deethylatrazine (DEA) and deisopropylatrazine (DIA)
The widespread use of atrazine throughout the season determines a diffuse occurrence, as described for glyphosate and by Quaghebeur et al. [30], although the lower doses used in comparison with those of glyphosate are reflected in the concentrations we found. Other factors affecting its presence in water samples are its low adsorption to soil [53] and its persistence and ease of movement in the environment by drift, leaching or runoff [54], behavior similar to its secondary metabolites [55]. Thus, Solomon et al. [56] collected half-life values of atrazine in water of 41–237 days and some authors reported residues of atrazine and its metabolites in surface and groundwater several years after use [51, 57]. This causes a higher frequency compared to glyphosate and AMPA (see Table 2). Other studies also showed high frequencies, even similar to ours [12, 14, 17]. The environmental behavior of this molecule is one of the reasons for its prohibition in the European Union [58].
33% of quantified values of atrazine were below the EU limit of 0.1 µg L−1. HOA exhibited a similar percentage, while more than 90% of the quantifiable DIA and DEA values were below that limit. The concentration of HOA was on average 13 times higher than DEA and DIA and 2.4 times higher than atrazine. The %HOA ratio was calculated in a similar way to the %AMPA ratio and presented a wide variability in all sources but it was higher for dams (68% ± 21%) than groundwater (49% ± 32%) and cisterns (47% ± 20%). This may be due to degradation of atrazine during transport or in the water source [59] and because HOA has the longest half-life of the triazines in this study [33]. The higher concentration of HOA than DIA and DEA in all sources indicates that degradation would be a chemical process since the latter two are products of atrazine biodegradation [60].
Atrazine concentrations differed between water sources (dams > cisterns > wells, p = 0.012) and sampling dates (the last agricultural year showed higher concentrations, p = 0.019) but not between locations (p = 0.898). HOA had a similar behavior, with differences between water sources (dams > cisterns and wells, p < 0.0001) and sampling dates, since dams exhibited higher values during the last agricultural year, especially in Sachayoj (p < 0.0001). Figure 4 shows the concentrations of atrazine and HOA in the different water sources throughout the sampling period.
Maximum concentrations of atrazine in cisterns were 7.92 µg L−1 (mean 0.24 ± 0.56 µg L−1) and 3.83 µg L−1 for HOA (mean 0.24 ± 0.48 µg L−1). Mechanisms for reaching these water sources would include spray drift, transport associated with soil particles and volatilization from the surface of treated soils [61]. Although atrazine presents a vapor pressure and a Henry’s constant barely greater than glyphosate and therefore a low volatility [33], Goolsby et al. [62] considered that its greater persistence in soil would allow volatilization to be an important process and explain its long detection period. These authors found values of 0.11–0.40 µg L−1 in rainwater from some states of the USA. In our country, Alonso et al. [29] detected atrazine in 80% of rain samples from several sites in the Pampean Region with concentrations of 0.10–26.9 µg L−1. Transport of atrazine by wind erosion of soil particles to which it is adsorbed can also move it several kilometres from where it was applied [11, 61, 63].
The highest values of atrazine and HOA corresponded to dams, showing the importance of runoff as the main transport route [56]. Quantifiable atrazine concentrations were up to 2.45 µg L−1 (mean 0.65 ± 0.57 µg L−1) and HOA maximum concentrations were 13.81 µg L−1 (mean 1.82 ± 2.56 µg L−1). Decrease in surface runoff caused by NT is not always accompanied by a lower loss of pesticides: Mickelson et al. [64] determined that a lower volume of runoff generated by a maize crop under NT was compensated by higher concentrations of atrazine in runoff water, resulting in a greater herbicide loss. Besides, the lack of flow in dams determines that, although the amount of active ingredient entering is similar to a watercourse, dilutions will be lower and water evaporation may even increase concentrations [56].
In groundwater, atrazine concentrations reached up to 0.8 µg L−1 (mean 0.11 ± 0.16 µg L−1) and those of HOA up to 2.53 µg L−1 (mean 0.28 ± 0.49 µg L−1). Preferential flow would play an important role in vertical movement of atrazine in soil, as demonstrated by Hang et al. [65]: when comparing two soils, the one with the higher retention capacity due to its more clayey texture presented, however, the highest herbicide losses through leaching. Vryzas et al. [66] concluded that adsorption and dissipation were not sufficient to decrease concentrations of atrazine in soil water when rain events occurred shortly after its application and most of the leaching would take place within the first month after application. Finally, atrazine is more persistent in groundwater than in soil due to the lack of degrading microorganisms, low organic carbon content which is determinant for their growth and low oxygen content in groundwater [60].
3.4 Metolachlor and acetochlor
Metolachlor concentrations showed significant differences between water sources (dams > cisterns and wells, p = 0.0008) and sampling dates (p = 0.016): It exhibited concentration peaks (in December in the case of cisterns and in February for dams) since it is applied exclusively in pre-emergence of summer crops coinciding with December samplings (Fig. 5). Other authors reported similar behaviors in surface and rainwater [12, 67, 68]. Concentrations of metolachlor were well below those of glyphosate and atrazine due to its lower use (treated area and field doses): 84% of concentrations were underneath the EU limit of 0.1 µg L−1.
In cisterns, maximum quantifiable concentrations were 0.291 µg L−1 (mean 0.05 ± 0.06 µg L−1) with 91% of detection. A similar frequency was found by Majewski et al. [11] in rainwater from sites in the central-eastern USA and it was detected at a control site away from agricultural areas, indicating that atmospheric transport would play an important role. Years later, detections in rainwater from the same area decreased due to a reduction in the use of the product [27]. Potter and Coffin [68] concluded that the high volatility of metolachlor would produce wet deposition and estimated it would represent approximately 1% of the applied product, five-fold greater than that mobilized by surface runoff. Vogel et al. [12] detected a mean frequency of 83% in rainwater from four basins of the USA. They concluded that as metolachlor vapor pressure is two orders of magnitude higher than that of atrazine, a greater amount of metolachlor would volatilize into the atmosphere; however, it would be susceptible to rapid degradation and therefore more ephemeral, which would explain the peak of concentration in cisterns.
Dams had the highest occurrence of metolachlor: it was absent in only one sample and quantifiable samples had mean and maximum values (0.13 ± 0.14 µg L−1 and 0.495 µg L−1, respectively) that doubled the other sources. This highlights the importance of surface runoff. Like atrazine, it was found that lower runoff volumes in plots under NT were compensated by higher concentrations resulting in greater herbicide losses [64]. Other surface water studies found similar frequencies but different concentrations. Fairbairn et al. [69] found metolachlor in 88% of samples from the Zumbro river basin (US), with a mean concentration of 10 ng L−1. Zablotowicz et al. [67] found very disparate frequencies of metolachlor in oxbow lakes of the Mississippi river delta and concentrations up to 14.9 µg L−1.
Wells had the lowest frequency (86%) and concentrations: mean and maximum values were 0.04 ± 0.05 µg L−1 and 0.224 µg L−1, respectively. European studies [17] showed frequencies of 20% in groundwater. However, in north-eastern Greece frequency of metolachlor was 63% with a mean of 0.33 µg L−1 [70] and in France metolachlor was detected in all samples with a mean concentration of 0.25 ± 0.32 µg L−1 [71]. Similarly, in a field leaching test conducted in Greece [66] it was detected in 99% of soil water samples, showing a high persistence (values of 10 µg L−1 were found 18 months after application). Macroporosity and adsorption are the predominant factors governing leaching of metolachlor [72].
Acetochlor behaved in a similar way to metolachlor (higher concentrations in December) since it is also used as a pre-emergent herbicide in maize and soybean crops, although it had a lower frequency due to the preference of metolachlor for its better herbicidal effect. In this case, apart from differing between water sources (dams > cisterns and wells, p = 0.0009) and sampling dates (p = 0.0068), there were also significant differences between locations (Sachayoj > Bandera, p = 0.037).
Frequency of detection in cisterns was 75%, with maximum concentrations of 1.70 µg L−1 (mean 0.05 ± 0.15 µg L−1). Majewski et al. [11] also found a high frequency of acetochlor in rainwater from agricultural sites. However, it was not detected at the control site; therefore, its atmospheric life would be short and it would be transported over limited distances. Besides, it was detected in gas and particle phase of air samples from those sites [63], demonstrating that volatilization and wind erosion would contribute to contamination of cisterns.
Dams showed a similar frequency to cisterns, but higher mean and maximum concentrations: 0.18 ± 0.42 µg L−1 and 1.81 µg L−1, respectively. Lerch and Blanchard [73] also detected high frequency of acetochlor in surface waters of Missouri and Iowa (US). Ferenczi et al. [74] determined that acetochlor was transported mainly in its dissolved form by surface runoff and loss represented 1% of the applied product, with a mean concentration of 48 µg L−1 (range 7–81 µg L−1).
Groundwater exhibited the lowest values and frequency: 0.04 ± 0.03 µg L−1 and 0.10 µg L−1 of mean and maximum concentration, respectively, and 47% of detection, which is similar to other studies [75, 76]. In gravity lysimeters, Caprile et al. [51] detected acetochlor 7 years after its last application; the formation of non-extractable residues would constitute a significant reservoir that could result in a long time of permanence in the soil and a source of acetochlor to water tables as a result of its desorption.
3.5 Other pesticides: tebuconazole and imidacloprid
Top ten pesticides included tebuconazole and imidacloprid, the only ones that are not herbicides. Tebuconazole is a fungicide used to control diseases in different extensive crops. All sources showed concentrations < 0.1 µg L−1, except for two samples from dams. The number of cases < LQ was also important (35% for cisterns, 17% in dams and 58% in wells). Quantifiable concentrations presented significant differences between sources (dams > cisterns and wells, p = 0.0004) and sampling dates (some sampling dates exhibited higher concentrations but varying among sources, p < 0.0001).
Detection frequency in cisterns was 68%, with mean and maximum concentrations of 0.01 ± 0.01 µg L−1 and 0.06 µg L−1, respectively. Hüskes and Levsen [10] detected tebuconazole mainly in rain samples in Germany that coincided with the application of the product, in concentrations ranging 0.003–0.32 µg L−1. Potter and Coffin [68] detected a low frequency (11%) in rainwater from south-eastern USA but concentrations were up to 2 µg L−1.
Dams had the highest concentrations (mean 0.02 ± 0.03 µg L−1, maximum 0.12 µg L−1) and detection frequency (92%), highlighting the importance of runoff as a source of pollution. According to Potter et al. [77], runoff caused losses of 3.6–9.8% of the product, depending on the tillage system. De Gerónimo et al. [15] detected tebuconazole in surface waters of a basin from southeast of Buenos Aires, with 91% of detection frequency and mean concentration of 0.033 µg L−1. Glinski et al. [78] found it was the second most frequent pesticide in ponds and streams from an agricultural area in the USA, with a frequency of 62% and a maximum concentration of 0.48 µg L−1.
Wells exhibited the lowest detection frequency (43%) and concentrations (mean 0.007 ± 0.008 µg L−1, maximum 0.03 µg L−1). In contrast, Herrero-Hernández et al. [75] detected tebuconazole in 75% of groundwater samples from La Rioja, Spain (maximum concentration: 3.24 µg L−1). Differences between studies are due to a higher use of fungicides in vineyards than in crops such as soybean and corn.
Imidacloprid is an insecticide used for pest control mainly in soybean cultivation and as a seed therapist. It was generally found in concentrations < 0.1 µg L−1 and frequency was similar in all sources. Like glyphosate, it showed no significant differences between locations, sources or sampling dates.
Cisterns presented the lowest mean (0.03 ± 0.05 µg L−1) and maximum concentration (0.41 µg L−1). Its high persistence in soil [33] would turn wind erosion into a source of pollution of cisterns, but no records were found about rain to establish the importance of this mechanism.
Dams exhibited slightly higher mean (0.05 ± 0.10 µg L−1) and maximum concentrations (0.46 µg L−1) than cisterns. Battaglin et al. [50] detected imidacloprid almost in all vernal pools and streams, although concentrations were mostly below the laboratory reporting limit. Hladik et al. [79] also found this insecticide in US rivers, with a frequency of 53% and concentrations up to 0.15 µg L−1.
Wells had a slightly lower frequency than the other sources (42%), but mean and maximum concentrations were higher: 0.08 ± 0.18 µg L−1 and 0.80 µg L−1, respectively. Herrero-Hernández et al. [76] detected imidacloprid in less than 20% of groundwater samples but sometimes exceeding the EU limit. The Groundwater Ubiquity Score (GUS) of imidacloprid is 3.74 [33]; therefore, it has a high possibility of leaching and this would explain its presence in groundwater.
3.6 Sum of pesticides and health risk assessment
Up to this point, the main molecules were considered; in this section, we will analyze the set of pesticides and the risks to human health. Only 7.4% of all values exceeded the limit of 0.1 µg L−1 per individual molecule set by the EU. However, 73% of the samples exceeded the tolerance of 0.5 µg L−1 established for the sum of organic contaminants. Percentages varied depending on the source and sum of molecules differed mainly due to changes in concentration and frequency of the main pesticides. Thus, cisterns presented a 7% frequency of concentrations greater than 0.1 µg L−1, but the sum exceeded 0.5 µg L−1 in 76% of the samples. All samples from dams presented sums higher than 0.5 µg L−1, confirming they were the most polluted source, with 12% of values above 0.1 µg L−1. Conversely, wells had the lowest contamination since concentrations greater than 0.1 µg L−1 and sums greater than 0.5 µg L−1 were 4% and 51%, respectively. Besides, significant differences were found in the mean sum of molecules between sources (dams > cisterns > wells, p = 0.0004) and dates (p = 0.0003), but not between locations. Similarly, more than 50% of surface and groundwater from La Rioja, Spain, exceeded the sum of 0.5 µg L−1 [76]. In a pan-European study on the presence of organic pollutants in groundwater [17], 29% of the samples had at least one pesticide exceeding the limit of 0.1 µg L−1 and 10% exceeded the tolerance of 0.5 µg L−1. Compared to samples from rivers in Europe [14], groundwater was less contaminated.
Another remarkable issue was the contribution of the most used pesticides and their metabolites: glyphosate, atrazine, AMPA and HOA. Their high concentration and/or frequency reflected in their participation in water pollution (Fig. 6). They contributed to 80% of the total sum of pesticides; 14% of that percentage corresponded to glyphosate, 22% to AMPA, 20% to atrazine and 24% to HOA. They represented at least 50% of the sum of molecules in 328 out of 353 samples. However, percentages were very variable and their contribution varied from 0% (samples in which they were not detected) to 100% (some well samples that had atrazine/HOA as the only quantifiable molecule).
Table 3 shows the RfD for all compounds. The RfD defines the maximum dose which, according to all known facts at the time, will result in no harm to human health when consuming it for a lifetime. When the CDI exceeds the value of the RfD (HQ > 1), it means that water consumption can have an adverse effect on human health. In our study, HQ values for adults and children were less than 1 (Table 3), suggesting that these levels of pesticides are unlikely to pose any adverse health effects. Similar results have been reported in previous studies [23,24,25]. The maximum estimated value of HQ was 3.35 E-2 for children and 1.67 E-2 for adults. Since HQ was well below 1 for all pesticides, sampling dates and sites, accumulated risk (HQs) was also minimal and far from 1 both in children and adults.
According to Hernández et al. [80], human exposure to mixtures of pesticides in low doses can occur from environmental or nutritional sources and can have a long-term negative impact on health, related to the increase in chronic and degenerative diseases, developmental neurotoxicity and cancer. In addition, for some pesticide mixtures, health effects may be higher or lower than expected from the simple addition of the effects of the individual components, which raises concern about their possible impact on health. These authors conclude that, from a regulatory view, it is important to advance in understanding the hazard assessment of pesticide mixtures at realistic doses and to model their cumulative effects in humans and the environment. More research is needed to identify the lower thresholds for real pesticide mixtures to prevent their impact on human and environmental health.
3.7 Water treatment and pollution prevention
Several methods can be used to remove pesticides from water but their efficiency depends on the characteristics of the pesticides and they are not always applicable at household level. Compounds like atrazine and its metabolites (molecules with low solubility in water) are easily adsorbed by the powdered activated carbon (PAC), while glyphosate and AMPA show low adsorption onto PAC due to their high polarity [81, 82]. On the other hand, ozone oxidation produces complete degradation of glyphosate and AMPA, but not for atrazine, HOA, DIA or DEA [81, 83]. Therefore, total elimination would be achieved with a combination of both methods [81]. Sand filtration is moderately effective in glyphosate and AMPA elimination, but removal is highly dependent on conditions and therefore variable [82]. According to Brosillon et al. [84], chlorination can provide full degradation of glyphosate. Low-pressure direct photolysis using high UV fluences degrades substances such as atrazine [85], but is not effective for glyphosate and AMPA. Membrane filtration (nanofiltration and reverse osmosis) proved to be highly effective for the removal of several pesticides, including glyphosate, atrazine and AMPA [82, 86].
To prevent environmental pollution with pesticides, usual practices include control of weather conditions during and after spraying activities and spot spray systems. However, some of the studies cited above show it is necessary to implement soil conservation practices in order to reduce erosion and the movement of pesticides along with soil particles and/or water runoff. Although NT is currently the main cultivation system, researches demonstrate that soil continues to be eroded under this management system [87]. Contour tillage is the simplest soil erosion control measure, which reduces runoff and increases water infiltration compared to that which occurs with cultivation parallel to the slope [88]. Winter cover crops also reduce wind and water erosion, since they increase soil cover and water infiltration, and protect aggregates from the impacts of raindrops; besides, they can compete with weeds and reduce herbicide use [89]. Terraces are more complex land management practices and consist in earth embankments constructed across the slope to intercept surface runoff. They convey runoff to a stable outlet at a non-erosive velocity and shortened slope length [90]. The use of living windbreaks, which are rows of trees and shrubs, reduces wind erosion: in dry areas, suitably distributed windbreaks on 5% of the area can reduce wind speed by 30–50% and soil losses even by 80% [91]. Therefore, reforestation should be a land management practice promoted by governments. Finally, riparian vegetation strips (RVS) reduce surface runoff volume and retain sediments, pesticides and nutrients that are transported across them from adjacent crop fields and a significant factor affecting this process is the floristic composition of the RVS [92]. In this way, Yang et al. [39] propose setting protection areas located between farming lands and public rivers.
4 Conclusions
Three types of water sources were analyzed to determine the environmental fate of pesticides. They were found mainly in dams and cisterns, confirming the importance of runoff and atmospheric deposition in the movement of pesticides in the environment. Although environmental behavior was governed by the characteristics of the compounds, doses and frequency of use also played an important role in defining presence and concentration of a compound. Hence, the most frequent molecules were herbicides and the most important compounds were glyphosate, atrazine, AMPA and HOA. Nevertheless, occurrence of active ingredients with little or no use in the area, such as ametryn, suggests the need to carry out research on a larger scale to determine movement of pesticides at a macro-regional level.
Consumption of rainwater is a common practice in rural areas of semiarid region of Argentina and it is encouraged by different state policies tending to improve the quality of life of rural populations with no access to tap water. Although rainwater is safe according to the health risk assessment, population is exposed to regular pesticide consumption at levels that exceed the standards of the EU.
In different agricultural areas of Argentina, pesticides are distributed in all the environmental matrices thus contaminating sources of water used for human consumption. Current focus on agricultural practices in order to reduce water contamination usually refers to spraying systems and conditions, but it is necessary to include other practices that will have an impact on the reduction in water pollution.
Notes
MRLs are the maximum levels of pesticide residues that are legally permissible in or on food or animal feed, based on good agricultural practice and the lowest consumer exposure necessary to protect vulnerable consumers.
References
Council of the European Union (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Communities L330:32–54
USEPA (2018) Drinking water standards and health advisories table. https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf. Accessed 18 Oct 2018
ANMAT (2012) Argentine food code—chapter XII: water drinks, water and sparkling water. http://www.anmat.gov.ar/alimentos/codigoa/CAPITULO_XII.pdf. Accessed 8 Aug 2018 (in Spanish)
Basán Nickisch M (2009) Chapter 2: multipurpose water systems for semi-arid and arid regions in rainfed areas. In: Barreda M, Ledesma S (eds) Access to water: stories and reflections from some experiences of organization in the territories. Buenos Aires, Argentina, pp 19–26 (In Spanish)
Basán Nickisch M (2016) Water quality for multiple uses. In: Zamora Gómez JP, Prieto Garra D (eds) Quality water with equity: experiences, debates and challenges on access, treatment and use of water for family farming in Latin America. Buenos Aires, Argentina, pp 63–65 (In Spanish)
Kleffmann Group Argentina (2012) Argentine market of phytosanitary products 2012. Chamber of agricultural health and fertilizers. http://www.casafe.org/pdf/2015/ESTADISTICAS/Informe-Mercado-Fitosanitario-2012.pdf. Accessed 12 May 2016 (in Spanish)
Aapresid (2018) Evolution of no till management in Argentina—Season 2016–2017. http://www.aapresid.org.ar/wp-content/uploads/2018/03/Estimacio%CC%81n-de-superficien-en-SD.pdf. Accessed 26 June 2018 (in Spanish)
Grain Exchange Buenos Aires (2017) Survey of applied agricultural technology Season 2016/2017. http://www.bolsadecereales.com/retaa-informes-anuales. Accessed 10 Oct 2018 (in Spanish)
Carrera G, Fernandez P, Vilanova RM, Grimalt JO (2001) Persistent organic pollutants in snow from European high mountain areas. Atmos Environ 35:245–254
Hüskes R, Levsen K (1997) Pesticides in rain. Chemosphere 35:3013–3024
Majewski MS, Foreman WT, Goolsby DA (2000) Pesticides in the atmosphere of the Mississippi River Valley, part I—rain. Sci Total Environ 248:201–212
Vogel JR, Majewski MS, Capel PD (2008) Pesticides in rain in four agricultural watersheds in the United States. J Environ Qual 37:1101–1115
Battaglin WA, Smalling KL, Anderson C, Calhoun D, Chestnut T, Muths E (2016) Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Sci Total Environ 566–567:320–332
Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G (2009) EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157:561–568
De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa JL (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290
Loos R, Locoro G, Comero S, Contini S, Schwesig D, Werres F, Balsaa P, Gans O, Weiss S, Blaha L, Bolchi M, Gawlik BM (2010) Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res 44:4115–4126
Cabrera AL (1971) Phytogeography of the Argentinean Republic. Bull Argent Bot Soc 14:1–42 (in Spanish)
Boletta PE, Ravelo AC, Planchuelo AM, Grilli M (2006) Assessing deforestation in the Argentine Chaco. For Ecol Manag 228(1–3):108–114
Boletta P, Acuña L, Juárez De Moya M (1989) Analysis of the climatic characteristics of the Province of Santiago del Estero. INTA–UNSE Agreement. Santiago del Estero
INTA (2016) Monthly precipitation data of the towns of Sachayoj and Bandera, from 1949 to 2015. http://santiago.inta.gov.ar/meteo. Accessed 20 Oct 2016 (in Spanish)
Aparicio VC, De Gerónimo E, Marino D, Primost J, Carriquiriborde P, Costa JL (2013) Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 93(9):1866–1873
Shi W, Zhang F, Zhang X, Su G, Wei S, Liu H, Cheng S, Yu H (2011) Identification of trace organic pollutants in freshwater sources in Eastern China and estimation of their associated human health risks. Ecotoxicology 20(5):1099–1106
Hu Y, Qi S, Zhang J, Tan L, Zhang J, Wang Y, Yuan D (2011) Assessment of organochlorine pesticides contamination in underground rivers in Chongqing, Southwest China. J Geochem Explor 111:47–55
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E (2015) A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9
Institute SAS (2002) User’s guide: statistics. SAS Institute, Cary, NC
Majewski MS, Coupe RH, Foreman WT, Capel PD (2014) Pesticides in Mississippi air and rain: a comparison between 1995 and 2007. Environ Toxicol Chem 33(6):1283–1293
Chang F-C, Simcik MF, Capel PD (2011) Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem 30(3):548–555
Alonso LL, Demetrio PM, Etchegoyen MA, Marino DJ (2018) Glyphosate and atrazine in rainfall and soils in agroproductive areas of the pampas region in Argentina. Sci Total Environ 645:89–96
Quaghebeur D, De Smet B, De Wulf E, Steurbaut W (2004) Pesticides in rainwater in Flanders, Belgium: results from the monitoring program 1997–2001. J Environ Monitor 6(3):182–190
Mörtl M, Németh G, Juracsek J, Darvas B, Kamp L, Rubio F, Székács A (2013) Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem J 107:143–151
Aparicio VC, Aimar S, De Gerónimo E, Méndez MJ, Costa JL (2018) Glyphosate and AMPA concentrations in wind-blown material under field conditions. Land Degrad Dev 29:1317–1326
University of Hertfordshire (2019) Pesticide properties database (PPDB) developed by the Agriculture & Environment Research Unit. http://sitem.herts.ac.uk/aeru/footprint/es/index.htm. Accessed 20 May 2019
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Sidoli P, Baran N, Angulo-Jaramillo R (2015) Glyphosate and AMPA adsorption in soils: laboratory experiments and pedotransfer rules. Environ Sci Pollut Res 23:5733–5742
De Jonge H, De Jonge LW, Jacobsen OH, Yamaguchi T, Moldrup P (2001) Glyphosate sorption in soils of different pH and phosphorus content. Soil Sci 166:230–238
Mamy L, Barriuso E (2005) Glyphosate adsorption in soils compared to herbicides replaced with the introduction of glyphosate-resistant crops. Chemosphere 61:844–855
Bento CPM, Goossens D, Rezaei M, Riksen M, Mol HGJ, Ritsema CJ, Geissen V (2017) Glyphosate and AMPA distribution in wind-eroded sediment derived from loess soil. Environ Pollut 220:1079–1089
Yang X, Wang F, Bento CPM, Xue S, Gai L, van Dam R, Mol H, Ritsema CJ, Geissen V (2015) Short-term transport of glyphosate with erosion in Chinese loess soil—a flume experiment. Sci Total Environ 512–513:406–414
Araújo ASF, Monteiro RTR, Abarkeli RB (2003) Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 52(5):799–804
Forlani G, Mangiacalli A, Nielsen E, Suardi CM (1999) Degradation of the phosphonate herbicide glyphosate in soil: evidence for a possible involvement of unculturable microorganisms. Soil Biol Biochem 31:991–997
Lamprea K, Ruban V (2011) Characterization of atmospheric deposition and runoff water in a small suburban catchment. Environ Technol 32(10):1141–1149
Mercurio P, Flores F, Mueller JF, Carter S, Negri AP (2014) Glyphosate persistence in seawater. Marin Pollut Bull 85(2):385–390
Mallat E, Barceló D (1998) Analysis and degradation study of glyphosate and of aminomethylphosphonic acid in natural waters by means of polymeric and ion-exchange solid-phase extraction columns followed by ion chromatography–post-column derivatization with fluorescence detection. J Chrom A 823:129–136
Struger J, Thompson D, Staznik B, Martin P, McDaniel T, Marvin C (2008) Occurrence of glyphosate in surface waters of southern Ontario. Bull Environ Contam Toxicol 80:378–384
Primost JE, Marino DJ, Aparicio VC, Costa JL, Carriquiriborde P (2017) Glyphosate and AMPA, “pseudo-persistent” pollutants under real world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ Pollut 229:771–779
Okada E, Pérez DJ, De Gerónimo E, Aparicio VC, Massone H, Costa JL (2018) Non-point source pollution of glyphosate and AMPA in a rural basin from the southeast Pampas, Argentina. Environ Sci Pollut Res 25(15):15120–15132
Mac Loughlin TM, Peluso ML, Aparicio VC, Marino DJG (2020) Contribution of soluble and particulate-matter fractions to the total glyphosate and AMPA load in water bodies associated with horticulture. Sci Total Environ 703:134717
Masiá A, Campo J, Vázquez-Roig P, Blasco C, Picó Y (2013) Screening of currently used pesticides in water, sediments and biota of the Guadalquivir river basin (Spain). J Hazard Mater 263(1):95–104
Battaglin WA, Rice CK, Foazio MJ, Salmons S, Barry RX (2009) The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa and Wyoming, 2005–2006. Environ Monit Assess 155:281–307
Caprile AC, Aparicio VC, Portela SI, Sasal MC, Andriulo AE (2017) Drainage and vertical transport of herbicides in two mollisols of Argentine Rolling Pampas. Ciencia del Suelo 35(1):147–159 (In Spanish)
Lutri VF, Matteoda E, Blarasin M, Aparicio V, Giacobone D, Maldonado L, Becher Quinodoz F, Cabrera A, Giuliano Albo J (2020) Hydrogeological features affecting spatial distribution of glyphosate and AMPA in groundwater and surface water in an agroecosystem, Córdoba, Argentina. Sci Total Environ 711:134557
Hang S, Bocco M, Sereno R (2000) Adsorption of atrazine in two soil profiles of Argentina under no till management. Agrochimica 44(3–4):115–122 (In Spanish)
Bottoni P, Grenni P, Lucentini L, Barra Caracciolo A (2013) Terbuthylazine and other triazines in Italian water resources. Microchem J 107:136–142
Barchanska H, Sajdak M, Szczypka K, Swientek A, Tworek M, Kurek M (2017) Atrazine, triketone herbicides, and their degradation products in sediment, soil and surface water samples in Poland. Environ Sci Pollut Res 24(1):644–658
Solomon KR, Carr JA, Du Preez LH, Giesy JP, Kendall RJ, Smith EE, Van Der Kraak GJ (2008) Effects of atrazine on fish, amphibians, and aquatic reptiles: a critical review. Crit Rev Toxicol 38:721–772
Vonberg D, Vanderborght J, Cremer N, Pütz T, Herbst M, Vereecken H (2014) 20 years of long-term atrazine monitoring in a shallow aquifer in western Germany. Water Res 50:294–306
Commission of the European Communities (2004) Commission Decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance (2004/248/EC). Off J Eur Union L78:53–55
Sassine L, Le Gal La Salle C, Khaska M, Verdoux P, Meffre P, Benfodda Z, Roig B (2017) Spatial distribution of triazine residues in a shallow alluvial aquifer linked to groundwater residence time. Environ Sci Pollut Res 24(8):6878–6888
Schwab AP, Splichal PA, Banks MK (2006) Persistence of atrazine and alachlor in ground water aquifers and soil. Water Air Soil Pollut 171(1–4):203–235
Thurman E, Cromwell A (2000) Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ Sci Technol 34:3079–3085
Goolsby DA, Thurman EM, Pomes ML, Meyer MT, Battaglin WA (1997) Herbicides and their metabolites in rainfall: origin, transport, and deposition patterns across the Midwestern and Northeastern United States, 1990–1991. Environ Sci Technol 31:1325–1333
Foreman WT, Majewski MS, Goolsby DA, Wiebe FW, Coupe RH (2000) Pesticides in the atmosphere of the Mississippi River Valley, Part II—air. Sci Total Environ 248:213–226
Mickelson SK, Boyd P, Baker JL, Ahmed SI (2001) Tillage and herbicide incorporation effects on residue cover, runoff, erosion, and herbicide loss. Soil Tillage Res 60:55–66
Hang S, Andriulo A, Sasal C, Nassetta M, Portela S, Cañas I (2010) Integral study of atrazine behaviour in field lysimeters in Argentinean Humid Pampas soils. Chil J Agric Res 70(1):104–112
Vryzas Z, Papadakis NΕ, Papadopoulou-Mourkidou E (2012) Leaching of Br-, metolachlor, alachlor, atrazine, deethylatrazine and deisopropylatrazine in clayey vadose zone: a field scale experiment in north-east Greece. Water Res 46:1979–1989
Zablotowicz RM, Locke MA, Krutz LJ, Lerch RN, Lizotte RE, Knight SS, Gordon RE, Steinriede RW (2006) Influence of watershed system management on herbicide concentrations in Mississippi Delta oxbow lakes. Sci Total Environ 370:552–560
Potter TL, Coffin AW (2017) Assessing pesticide wet deposition risk within a small agricultural watershed in the Southeastern Coastal Plain (USA). Sci Total Environ 580:158–167
Fairbairn DJ, Karpuzcu ME, Arnold WA, Barber BL, Kaufenberg EF, Koskinen WC, Novak PJ, Rice PJ, Swackhamer DL (2016) Sources and transport of contaminants of emerging concern: a two-year study of occurrence and spatiotemporal variation in a mixed land use watershed. Sci Total Environ 551–552:605–613
Vryzas Z, Papadakis E, Vassiliou G, Papadopoulou-Mourkidou E (2012) Occurrence of pesticides in transboundary aquifers of northeastern Greece. Sci Total Environ 441:41–48
Baran N, Gourcy L (2013) Sorption and mineralization of S-metolachlor and its ionic metabolites in soils and vadose zone solids: consequences on groundwater quality in an alluvial aquifer (Ain Plain, France). J Contam Hydrol 154:20–28
Novak SM, Banton O, Schiavon M (2003) Modelling metolachlor exports in subsurface drainage water from two structured soils under maize (eastern France). J Hydrol 270:295–308
Lerch RN, Blanchard PE (2003) Watershed vulnerability to herbicide transport in northern Missouri and southern Iowa streams. Environ Sci Technol 37:5518–5527
Ferenczi J, Ambrus Á, Don Wauchope R, Sumner HR (2002) Persistence and runoff losses of 3 herbicides and chlorpyrifos from a corn field in the Lake Balaton watershed of Hungary. J Environ Sci Health B 37(3):211–224
Herrero-Hernández E, Andrades MS, Álvarez-Martín A, Pose-Juan E, Rodríguez-Cruz MS, Sánchez-Martín MJ (2013) Occurrence of pesticides and some of their degradation products in waters in a Spanish wine region. J Hydrol 486:234–245
Herrero-Hernández E, Rodríguez-Cruz MS, Pose-Juan E, Sánchez-González S, Andrades MS, Sánchez-Martín MJ (2017) Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Sci Total Environ 609:161–171
Potter TL, Bosch DD, Stricklan TC (2014) Comparative assessment of herbicide and fungicide runoff risk: a case study for peanut production in the southern Atlantic coastal plain (USA). Sci Total Environ 490:1–10
Glinski DA, Purucker ST, Van Meter RJ, Black MC, Henderson WM (2018) Analysis of pesticides in surface water, stemflow, and throughfall in an agricultural area in South Georgia, USA. Chemosphere 209:496–507
Hladik ML, Corsi SR, Kolpin DW, Baldwin AK, Blackwell BR, Cavallin JE (2018) Year-round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA. Environ Pollut 235:1022–1029
Hernández AF, Gil F, Lacasaña M (2017) Toxicological interactions of pesticide mixtures: an update. Arch Toxicol 91:3211–3223
Bozkaya-Schrotter B, Daines C, Lescourret A-S, Bignon A, Breant P, Schrotter JC (2008) Treatment of trace organics in membrane concentrates I: pesticide elimination. Water Sci Technol Water Supply 8(2):223–230
Jönsson J, Camm R, Hall T (2013) Removal and degradation of glyphosate in water treatment: a review. J Water Supply Res Technol 62:395–408
Assalin MR, De Moraes SG, Queiroz SC, Ferracini VL, Duran N (2010) Studies on degradation of glyphosate by several oxidative chemical processes: ozonation, photolysis and heterogeneous photocatalysis. J Environ Sci Health B 45:89–94
Brosillon S, Wolbert D, Lemasle M, Roche P, Mehrsheikh A (2006) Chlorination kinetics of glyphosate and its by-products: modeling approach. Water Res 40:2113–2124
Sanches S, Barreto Crespo MT, Pereira VJ (2010) Drinking water treatment of priority pesticides using low pressure UV photolysis and advanced oxidation processes. Water Res 44:1809–1818
Sanches S, Penetra A, Granado C, Cardoso VV, Ferreira E, Benoliel MJ, Barreto Crespo MT, Pereira VJ, Crespo JG (2011) Removal of pesticides and polycyclic aromatic hydrocarbons from different drinking water sources by nanofiltration. Desalin Water Treat 27(1–3):141–149
Didoné EJ, Gomes Minella JP, Schneider FJA, Londero AL, Lefèvre I, Evrar O (2019) Quantifying the impact of no-tillage on soil redistribution in a cultivated catchment of Southern Brazil (1964–2016) with 137Cs inventory measurements. Agric Ecosyst Environ 284:106588
Liu XB, Zhang XY, Wang YX, Sui YY, Zhang SL, Herbert SJ, Ding GW (2010) Soil degradation: a problem threatening the sustainable development of agriculture in Northeast China. Plant Soil Environ 56(2):87–97
Dabney SM, Delgado JA, Reeves DW (2001) Using winter cover crops to improve soil and water quality. Commun Soil Sci Plant Anal 32:1221–1250
Morgan RPC (2005) Soil erosion and conservation, 3rd edn. Blackwell Publishing Ltd, New York
Bird PR (1992) The role of shelter in Australia for protecting soils, plants and livestock. Agrofor Syst 20:59–86
Giaccio GCM, Laterra P, Aparicio VC, Costa JL (2016) Glyphosate retention in grassland riparian areas is reduced by the invasion of exotic trees. J Exp Bot 85:108–116
Acknowledgements
The authors thank the populations of Bandera and Sachayoj for allowing the sampling and the professionals of the AERs of Sachayoj and Bandera for field assistance. This work was part of the 2016 Postgraduate Training Program and was funded by INTA-PNSUELO 1134044 and the aforementioned program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mas, L.I., Aparicio, V.C., De Gerónimo, E. et al. Pesticides in water sources used for human consumption in the semiarid region of Argentina. SN Appl. Sci. 2, 691 (2020). https://doi.org/10.1007/s42452-020-2513-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2513-x