Abstract
This study highlights and combines the advantages of quorum sensing inhibition using scientifically untapped medicinal plants, the ethnobotanicals, through an effective drug delivery system, nanotechnology, to develop antivirulence drugs to control aquaculture pathogens. The ethnobotanical extracts and biologically synthesized gold nanoparticles of the Ilongot-Eǵongot community show antibacterial activity against gram-negative Aeromonas hydrophila. The ethnobotanical crude extracts (CEs) and crude extracts + gold nanoparticles (CEs + AuNPs) exhibit Quorum Sensing Inhibition activity through inhibition of the biofilm formation in A. hydrophila. Moreover, ethnobotanical CEs + AuNPs show much greater activity than its counterpart CEs in antibacterial and biofilm formation assay in A. hydrophila. Thus, this study supports the use of biological synthesis of gold nanoparticles to improved drug delivery which indicate the potential of these ethnobotanicals for therapeutic approach in inhibiting bacterial virulence without developing resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The intense growth in aquaculture in the past years has led to fish rearing conditions in high densities. Subsequently, high rearing conditions in caged culture, cause susceptibility of fishes to disease outbreaks caused by bacterial pathogens that impact mortality rates and productivity efficiency and generate high economic losses to aquaculture production [7].
Among the bacterial diseases, Aeromonas is one of the most significant fish pathogens influencing aquaculture production globally and outbreaks have intensified especially in farmed fresh water fishes. This pathogen mainly infects freshwater fishes with reports on the potential of being zoonotic agents that pose potential risks to consumers and possess a range of virulence determinants. The expression of their exoproducts is associated with high cell densities in the late exponential phase [3, 22].
Deficits from infections caused by emergent bacterial diseases on aquaculture are paving the way for discovering antipathogenic agents. The main treatment for the control of bacterial diseases in fish includes the use of antibiotics [7, 32]. However, the extensive and indiscriminate use of antibiotics in aquaculture has been implicated to the emergence of antibacterial resistance [7] which is mainly caused by the treatment of fish diseases in steady intensive fish production.
The increasing demand for novel strategies of controlling infectious diseases in tilapia addresses the major issue of continual development of resistance to antibiotics with the extensive presence of antibiotic-resistant bacteria [15]. New approaches that take on the concern of antibiotic resistance is through inhibition of quorum sensing (QS), a cell–cell communication process that allows an expanding bacterial population communities to control gene expression [13]. This communication system in bacteria has been the target to control their pathogenicity and indicates an advanced and promising target for antimicrobial drugs [36]. As QS contributes to behaviors of bacteria such as biofilm development that facilitate bacterial defense against antimicrobial compounds, the vital strategic approach is to selectively block the control apparatus of virulence [35]. Disabling QS circuits with small molecules has become the new focus as a potential strategy to prevent bacterial pathogenicity [26] without developing antimicrobial resistance.
The use of ethnobotanical resources of the Ilongot community of Maria Aurora, Aurora, Philippines holds a promising defense against bacterial antibiotic resistance and epitomizes a huge potential to the discovery of drugs that can append newer application and strategies as alternative medicine in aquaculture.
To effectively deliver these anti-pathogenic drugs, nanotechnology has gained substantial interest and applications in drug delivery. Nanotechnology is utilized to enhance treatment of diseases due to their reduced dimensions, extremely small size and large relative surface area [17] while allowing production of non-toxic and eco-friendly particles. In applications to antimicrobial agents, these properties lead to their increased contact with bacteria significantly improving bactericidal activity.
2 Materials and methods
2.1 Collection of plant samples and extraction procedure
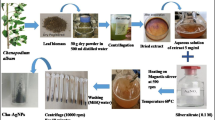
Fifteen (15) plant parts from eleven (11) ethnobotanical plants at the Ilongot- Eǵongot community of Maria Aurora, Aurora, Philippines were collected through the permission of the provincial and tribal chieftains [4]. Plant parts were cleaned and disinfected. Washed plant materials were air dried and pulverized. Fifty (50) grams of each pulverized plant material were soaked in 500 ml of 80% ethanol for 72 h. The mixture was then filtered and subjected to rotary evaporator. The ethnobotanical crude extracts (CEs) were sterilized by centrifugation at 10,000×g for 30 min, membrane filtration and were stored at 2–8 °C prior to use.
2.2 Biological synthesis of AuNPs using Ilongot-Egongot ethnobotanicals
The biological synthesis of AuNPs was done following the protocol of Fernando et al. [12] with modifications. Ethnobotanical extract was mixed with 10–3 M gold chloride individually for the synthesis of AuNPs. The mixture was incubated under dark conditions at room temperature with constant stirring for 60 min until it turned into pink red color indicating the formation of CE + AuNPs and to ensure the stability of the nanoparticles. The CE + AuNPs obtained from the solution were purified by centrifugation to remove the water soluble biomolecules.
2.3 Characterization of CE + AuNPs and UV–visible spectroscopy analysis
The synthesized CE + AuNPs were kept stationary in dark for all concentrations and evaluated visually for probable colloid, aggregate or precipitate formation to ensure that the synthesized CE + AuNPs were stable. The synthesized CE + AuNPs was analyzed by UV–Vis Spectroscopy analysis using Nano drop spectrophotometer by evaluating the excitation due to the applied electromagnetic field and absorption values were recorded.
2.4 Disk-diffusion assay for antibacterial activity of ethnobotanical CEs and CE + AuNPs against A. hydrophila
Sterile paper discs (6 mm) with 20 µl of CEs and CE + AuNPs were dispensed individually and air dried. Prepared media on petri plates of Mueller–Hinton agar swabbed with A. hydrophila were used. Air-dried disc was individually seeded on plates in a three-plant per plate manner with five (5) replications. Sterile distilled water served as negative control; Tetracycline was used as the positive control. Plates were incubated at 37 °C for 24 h. Plant extracts without antibacterial activities were used in the detection of quorum sensing inhibition through modified virulence assays.
2.5 Microtiter plate biofilm formation assay
The effect of plant extracts on the attachment phase of biofilm formation was measured using microtiter plate assay. Briefly, 180 μl of overnight cultures of A. hydrophila added with 20 μl of CEs or CE + AuNPs individually in triplicates were dispensed in each well and incubated at 30 °C for 40 h [9]. MH Broth with A. hydrophila treated with distilled water served as the positive control.
After incubation, the microtiter plates were rinsed with sterile distilled water, air dried for 45 min and stained with 150 μl of 1% crystal violet solution. Plates were rinsed to remove excess stain and biofilm production was quantified by adding 200 μl of 95% ethanol to destain the wells and OD values was measured. Growth inhibition were then quantified using MultiSkan FC, UV–visible Spectrophotometer of Thermo Scientific at 595 nm to evaluate the biofilm formation.
2.6 Gene expression analysis
Plant extracts from the QSI virulence assay in A. hydrophila that showed significantly lower OD values in biofilm formation was chosen for the quantification of gene expression and RNA extraction was done using the Promega protocol with some modifications. Plant extracts with QSI activity against A. hydrophila was subjected to relative gene expression analysis. The expression of AhyR in A. hydrophila were determined to evaluate QSI activity through qRT-PCR analysis.
Amplifications of specific primers for AhyR and internal standard 16S rRNA (Table 1) with qRT-PCR program as follows: 1 cycle at 42 °C for 5 min and 95 °C for 2 min for initial denaturation, followed by 45 cycles at 95 °C for 20 s for denaturation, 55 °C for 20 s for annealing and 72 °C for 20 s for the extension were used.
3 Results and discussions
3.1 Biological synthesis of AuNPs using Ilongot-Egongot ethnobotanical CEs
Fifteen (15) CE + AuNPs were successfully synthesized following the protocol of Fernando et al. [12]. The change in color from yellowish to pink red indicates the formation of CE + AuNPs in the solution due to excitation of surface Plasmon vibration in the metal nano particles (NPs) confirmed by the UV-spectroscopy analysis.
3.2 Characterization of CE + AuNPs and UV–visible spectroscopy analysis
The synthesized CE + AuNPs were kept stationary in dark condition and evaluated visually. Colloid, aggregate and precipitate formation were not observed. It was then monitored by UV–visible spectroscopy by analyzing the excitation due to the applied electromagnetic field and absorption values. Surface Plasmon Resonance (SPR) peaks attained in UV–Vis spectroscopy is one of the versatile techniques to confirm the formation of metal NPs and it was generated due to the coherence of electrons on the surface of AuNPs.
The study of Emmanuel et al. [11] demonstrated that the decrease in the conjugation length and intensities of AuNPs indicates the decrease in particle size. Hence, the CE + AuNPs conjugation length and intensities decreased from 595 to 544 nm which signifies the decrease in size. As depicted form spectra, the color change in reaction from yellowish to pink red and decreased conjugation length confirms the formation of CE + AuNPs [25, 27].
3.3 Antibacterial activity of plant extracts against Aeromonas hydrophila
Disk diffusion assay was performed to determine the antibacterial activity of 15 CEs and 15 CE + AuNPs to select the qualified extracts for the quorum sensing inhibition (QSI) assay.
Against A. hydrophila, only one CE, Cassia alata leaves, showed antibacterial activity as well as ten CE + AuNPs namely: Mikania micrantha; Premna odorata leaves; P. odorata bark; C. alata; Cymbopogan winterianus; Phyllanthus urinaria; Dillenia philippinensis bark; Hydrocotyle vulgaris; Ceiba pentandra; Eleusine indica roots (Table 2). Each plant extract in the study should not exhibit clearing to rule out antibacterial-mediated decrease in virulence factor production, which was required for accuracy of the subsequent assays. Since no antibacterial activity or no zone of bacterial inhibition was noted in fourteen (14) ethnobotanical CEs and five (5) CE + AuNPs against A. hydrophila these extracts qualified for the biofilm virulence assay.
Each plant extract in the study should not exhibit clearing to rule out antibacterial-mediated decrease in virulence factor production, which was required for accuracy of the subsequent assays. Only plant extracts without antibacterial activities were used in the detection of quorum sensing inhibition through modified virulence assays. The antibacterial activity of C. alata against A, hydrophila confirms previous reports. Khan et al. [16] observed the antibacterial effect of C. alata leaves, stem, flowers and root bark against 28 bacterial and fungal species including Gram-negative and Gram-positive bacteria. Other reported activities and traditional uses of C. alata leaves include treatment for ringworm, liver diseases [31], wound healing [28], analgesic [29] and treatments for gastrointestinal problems as well as haemorrhoids and constipation [31].
This antimicrobial activity of C. alata may be accounted to its active constituents. C. alata contains alquinone, 3,5,7,4-tetrahydroxy flavone and flavonoid glycosides [34]. The observed antibacterial effect of CE + AuNPs may be accounted to their reduced particle size enabling efficient delivery [11].
The high efficiency of plant extracts conjugated with AuNPs is explained by the mechanism on the hydrophobic nature of bacterial cell membrane made of phospholipids and glycoproteins that may facilitate the transfer of drug loaded with AuNPs, which are hydrophilic, across the membrane efficiently [33, 37–39]. The interference of nanoparticles with growth-signaling pathway inside the cell via tyrosine phosphorylation modulation of growth essential peptides substrate inhibits the bacterial growth [18].
3.4 Inhibitory effect of plant extracts on biofilm formation
The extracts for the QSI screening should not exhibit inhibition of bacterial growth to rule out antimicrobial-mediated decrease in virulence factor production in the later tests, which is required for accuracy of the subsequent assays. Hence, CEs and CE + AuNPs that did not exhibit antibacterial activity were qualified for the screening of quorum sensing inhibition (QSI).
3.5 CEs against A. hydrophila biofilm formation
Significant inhibition of biofilm formation in A. hydrophila was observed in the CEs of 13 plants namely H. vulgaris (0.066); M. micrantha (0.067); D. philippinensis bark (0.064); C. pentandra (0.066); C. winterianus (0.063); U. lobata (0.065); D. philippinensis leaves (0.066); P. odorata bark (0.065); S. jamaicensis (0.070); E. indica roots (0.066); D. esculentum (0.093); P. odorata leaves (0.071); E. indica leaves (0.069); P. urinaria (0.062) in which it showed lower optical density (OD) values compared to the control (no extract) with a value of 0.218 mg/ml.
3.6 CEs + AuNPs against A. hydrophila biofilm formation
Five (5) CE + AuNPs showed significantly lower OD values than the control as well as compared to the OD values of ethnobotanical CEs: U. lobata (0.058); D. philippinensis leaves (0.060); S. jamaicensis (0.059); D. esculentum (0.063) and E. indica leaves (0.060). A number of CE + AuNPs showed antibacterial activity against A. hydrophila, hence, the lower number in treatments showing QSI in CE + AuNPs is prevalent as compared to CEs (Fig. 1).
3.7 CEs versus CEs + AuNPs in A. hydrophila biofilm formation
The observed lower biofilm formation of CEs + AuNPs as compared to ethnobotanical CEs against A. hydrophila (Table 3), indicating the improved delivery system of the compounds through the reduction of particle size and increased surface area of the nanoparticles which enables the efficient penetration of the nanoparticle in the cell membrane of the bacteria [37, 38].
Aeromonas, a model bacterium for biofilm research, live attached to biofilms on biotic surfaces which contributes to its virulence. Natural biofilms are developed and differentiate themselves to build a packed community and embedded in a polymeric extracellular matrix of their own production that contains channels involved in the circulation of nutrients and water [10].
A.hydrophila is one of the opportunistic bacterial pathogens on tilapia aquaculture and an extensive range of diseases are attributed to their bacterial biofilms that harbor some inherent degree of structural heterogeneity in response to chemical gradients and adaptation to local microenvironments, including Motile Aeromonas Septicemia (MAS), Hemorrhagic Septicemia and Red-Sore Disease [40]. Thus, its mode of action is by repressing several surface that mediate contact with the host matrix [42]. The A. hydrophila surface α-glucan independent of the lipopolysaccharides (LPS) also improves biofilm formation [24]. The divalent cation and its transporter mgtE are directly involved in adherence to epithelial cells in swarming and in biofilm formation of A. hydrophila [23].
The suppression of virulence factors such as biofilm formation may indicate constraint in the QS mechanism of the bacteria upon exposure to the ethnobotanical CE. The virulence of the fish bacterial pathogen A. hydrophila is through multifactorial expression [2] and can be interfered to attenuate the bacterial pathogenicity. Virulence factors in Aeromonas include extracellular enterotoxins, endotoxins, haemolysins, cytotoxins, proteases, S-layer, fimbriae, adhesins and biofilm formation [14, 41]. Hence, the mechanism of inhibition, however, cannot be elucidated but may be assumed in manners such as (1) inhibition of AHL autoinducer synthesis, (2) enzymatic destruction of AHLs molecules and (3) interference with signal receptors or blockage of formation of receptor complex. These strategies, similarly can be applied to the inhibition of AIPs-mediated quorum sensing in gram-positive bacteria [19].
It is worthy to note that a number of CEs and all of the CE + AuNPs showed QSI. Fourteen (14) ethnobotanical CEs and five (5) CE + AuNPs showed significant lower biofilm formation than the control in gram-negative bacteria. All of the CE + AuNPs significantly lowered biofilm formation than its counterpart CEs against A. hydrophila, indicating the improved delivery system of the compounds through the reduction of particle size and increased surface area of the nanoparticles. This facilitates efficient entry of the compounds across the phospholipids and glycoproteins that make up the cell membrane [33, 37–39].
3.8 AhyR in Aeromonas hydrophila as affected by CEs and CEs + AuNPs
AhyR showed downregulated expression in CEs and CE + AuNPs against A. hydrophila confirming the results in biofilm quantification. All plant extracts that showed biofilm formation inhibition in the virulence assay have significantly lower AhyR expression (Fig. 2).
The CEs and CE + AuNPs may prevent the expression of the QS-regulated gene but minimal expression of genes linked in biofilm formation were shown in the CEs + AuNPs of U. lobata, D. philippinensis leaves, S. jamaicensis leaves and E. indica leaves (Table 4) which connotes maximal inhibition of QS in the gram-negative bacteria, A. hydrophila. The downregulated expressions of the QS-related gene, AhyR in A. hydrophila involved in the production of homoserine lactone, may have acted by interrupting the QS system or by deregulating the synthesis of homoserine lactone thereby inhibiting the formation of bacterial biofilms.
The AhyI and AhyR genes comprise a divergon with a 62 bp intergenic region that contains a 12 bp symmetrical sequence making it a potential binding site for AhyR that can interact and function as both a positive and negative regulator of AhyI [21]. Moreover, AhyR is not required for the transcription of AhyI but performs to control both timing of AhyI expression that greatly contributes into the formation of biofilm and turnover of AhyI in stationary phase inhibits the expression of the virulence factor, biofilm formation. This may mean that the components in CEs and CE + AuNPs may have blocked the pathway for AhyR marked by its significant downregulation and confirming the phenotypic characters exhibited in the biofilm formation assay.
A clear approach to screen the action of compounds is to prevent the signal molecules from being synthesized by the AHL synthase and it is possible that AhyR controls the production of a protease responsible for controlling AhyI levels in stationary phase on the biofilm formation, therefore blocking the QS-system on the bacterial pathogen. In this case, the failure of producing AHL resulted the bacterial community to disabled the communication even in a quorum concentration, hence, QS-controlled genes will not be activated and allowing the community to exist, but the mechanism is in silence [35].
AhyR coordinates pathogenicity in gram-negative bacteria, such as in A. hydrophila, [1] and activates virulence genes AhyI, AhyRI, gyrA and gyrC [30]. Hence, if QSI compounds can prevent AhyR expression, then other genes in the QS cascade would be controlled as well, including those involved in biofilm formation [8].
It has been found that these virulence system receptors can be inhibited from producing chemical signals by mimicking their structure therefore confusing the quorum sensing of the bacteria, as may be exhibited when these plant extracts were applied [20].
The gene expression values of AhyR as affected by the ethnobotanicals CE and CE + AuNPs was significantly downregulated thereby exhibiting antagonistic effect on AhyR signifying that it can interact and function as a regulator of ahyI [21]. When the pathway for AhyR is blocked, many other virulence productions formed by other signal receptors may also be affected due to the overlapping mechanisms of such receptors. As AhyR behaves as a repressor of ahyI, it is projected to bind DNA in the absence of AHLs and to disassociate in the presence of C4-HSL [6].
4 Conclusion
The study supports the use of biological synthesis of gold nanoparticles to improved drug delivery which indicate the potential of these ethnobotanicals for therapeutic approach in inhibiting bacterial virulence of aquatic pathogen without developing resistance.
References
Adonizio A, Kong KF, Mathee K (2008) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother 52(1):198–203
Allan BJ, Stevenson RMW (1981) Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can J Microbiol 27:1114–1122
Anguita J, Rodriguez-Aparicio LB, Naharro G (1993) Purification, gene cloning, amino acid sequence analysis, and expression of an extracellular lipase from an Aeromonas hydrophila human isolate. Appl Environ Microbiol 59:2411–2417
Balberona AN, Noveno JJ, Angeles MGB, Santos RI, Cachin E (2018) Ethnomedicinal plants utilized by the Ilongot-Eǵongot Community of Bayanihan, Maria Aurora, Aurora, Philippines. Int J Agric Technol 14(2):145–159
Bi ZX, Liu YJ, Lu CD (2007) Contribution of AhyR to virulence of Aeromonas hydrophila J-1. Res Vet Sci 83(2007):150–156
Beck von Bodman S, Majerczak DR, Coplin DL (1998) A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. Stewartii. Proc Natl Acad Sci USA 95:7687–7692
Castro SBR, Leal CAG, Freire FR, Carvalho DA, Oliveira DF, Figueiredo HCP (2008) Antibacterial activity of plant extracts from Brazil against fish pathogenic bacteria. Braz J Microbiol 39:756–760
De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH (2001) Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl Environ Microbiol 67(4):1865–1873
Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter plate assay for assessment of listeria monocytogenes biofilm formation. Appl Environ Microbiol. https://doi.org/10.1128/aem.68.6.2950-2958.2002
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. https://doi.org/10.1128/CMR.15.2.167-193.2002
Emmanuel R, Saravanan M, Ovais M, Padmavathy S, Shinwari ZK, Prakash P (2017) Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: a nanoantibiotic approach. Microbial Pathog. https://doi.org/10.1016/j.micpath.2017.10.055
Fernando SID, Judan-Cruz KG, De Guia ACM (2017) Biologically synthesized gold nanoparticles (Aunp) using pine (Pinus kesiya) pollen extract show antifungal activity against Candida albicans. Int J Agric Technol 13(7.3):2615–2622
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176(2):269–275
González-Serrano CJ, Santos JA, García-López ML, Otero A (2002) Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J Appl Microbiol 93:414–419
Kalia VC, Rani A, Lal S, Cheema S, Raut CP (2007) Combing databases reveals potential antibiotic producers. Expert Opin Drug Discov 2(2):211–224
Khan R, Takahashi E, Nakura H, Ansaruzzaman M, Banik S, Ramamurthy T, Okamoto K (2008) Toxin production by Aeromonas sobria in natural environments: river water vs. seawater. Acta Med Okayama 62:363–371
Khatami M, Heli H, Jahani PM, Azizi H, Lima NM (2017) Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol 2017:11. https://doi.org/10.1049/iet-nbt.2016.0189
Kumar PSM, MubarakAli D, Saratale RG, Saratale GD, Pugazhendhi A, Gopalakrishnan K, Thajuddin N (2017) Synthesis of nano-cuboidal gold particles for effective antimicrobial property against clinical human pathogens. Microb Pathog. https://doi.org/10.1016/j.micpath.2017.10.032
Lade H, Paul D, Kweon JH (2014) Quorum quenching mediated approaches for control of membrane biofouling. Int J Biol Sci 10(5):550–565. https://doi.org/10.7150/ijbs.9028
Livorsi DJ, Stenehjem E, Stephens DS (2011) Virulence factors of gram-negative bacteria in sepsis with a focus on Neisseria meningitidis. Contrib Microbiol 17:31–47. https://doi.org/10.1159/000324008
Lynch MJ, Swift S, Kirke DF, Keevil CW, Dodd CER, Williams P (2002) The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ Microbiol 4:18–28
MacIntyre S, Buckley JT (1978) Presence of glycerophospholipid cholesterol acyltransferase and phospholipase in culture supernatants of Aeromonas hydrophila. J Bacteriol 135:402–407
Merino S, Gavín R, Altarriba M, Izquierdo L, Maguire ME, Tomás JM (2001) The MgtE Mg2+ transport protein is involved in Aeromonas hydrophila adherence. FEMS Microbiol Lett 198:189–195
Merino S, Bouamama L, Knirel YA, Senchenkova SN, Regué M, Tomás JM (2012) Aeromonas surface glucan attached through the O-antigen ligase represents a new way to obtain UDP-glucose. PLoS ONE 7:35707
Mukherjee S, Sau S, Madhuri D, Bollu VS, Madhusudana K, Sreedhar B, Banerjee R, Patra CR (2016) Green synthesis and characterization of monodispersed gold nanoparticles: toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J Biomed Nanotechnol 12(2016):165–181
O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL (2013) A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci 110(44):17981–17986
Ovais M, Khalil AT, Raza A, Khan MA, Ahmad I, Islam NU, Saravanan M, Ubaid MF, Ali M, Shinwari ZK (2016) Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 11(2016):3157–3177
Palanichamy S, Bhaskar EA, Bakthavathsalam R, Nagarajan S (1991) Wound healing activity of Cassia alata. Fitoterapia 62(2):153–156
Palanichamy S, Nagarajan S (1990) Antifungal activity of Cassia alata leaf extract. J Ethnopharmacol 1990(29):337
Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179(18):5756–5767
Perry LM (1980) Medicinal plants of East and Southeast Asia: attributed properties and uses. The MIT Press, Cambridge, p 209
Pridgeon JW, Yildirim-Aksoy M, Klesius PH, Kojima K, Mobley JA, Srivastava KK et al (2013) Identification of gyr Band rpoB gene mutations and differentially expressed proteins between a novo biocin-resistant Aeromonas hydrophila catfish vaccine strain and its virulent parent strain. Vet Microb Biol 166:624–630
Pugazhendhi A, Dhanarani S, Shankar C, Prakash P, Ranganathan K, Saratale RG, Thamaraiselvi K (2017) Electrophoretic pattern of glutathione S-transferase (GST) in antibiotic resistance Gram-positive bacteria from poultry litter. Microb Pathog 110:285–290
Rahaman MS, Moynul Hasan AJM, Ali MY, Ali MU (2006) A flavone from the leaves of Cassia alata. Bangladesh J Sci Ind Res 41:93–96
Rasmussen TB, Givskov M (2006) Quorum sensing inhibitors: a bargain of effects. Microbiology 152(4):895–904
Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Te MK, Nielsen J, Eberl L, Givskov M (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol 187(5):1799–1814
Saravanan M, Nanda A (2010) Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf B Biointerfaces 77:214–218
Saravanan M, Vemu AK, Barik SK (2011) Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids Surf B Biointerfaces 88:325–331
Shanmuganathan R, MubarakAli D, Prabakar D, Muthukumar H, Thajuddin N, Kumar SS et al (2017) An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9367-9
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210
Thornley JP, Shaw JG, Gryllos IA, Eley A (1997) Virulence properties of clinically significant Aeromonas species: evidence for pathogenicity. Rev Med Microbiol 8:61–72
Yarwood JM, Schlievert PM (2003) Quorum sensing in Staphylococcus infections. J Clin Investig 112:1620–1625
Acknowledgements
The authors acknowledge the support of the Molecular Biology and Biotechnology Laboratory of the College of Fisheries and the Molecular Laboratory of the College of Veterinary Science and Medicine of the Central Luzon State University, Science City of Munoz, Nueva Ecija, Philippines for the use of their facilities and the DOST-Applied Science and Technology Human Resource Development Program (DOST-ASTHRDP).
Funding
Funding was provided by National Research Council of the Philippines (DOST-SEI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernando, S.I.D., Judan Cruz, K.G. Ethnobotanical biosynthesis of gold nanoparticles and its downregulation of Quorum Sensing-linked AhyR gene in Aeromonas hydrophila. SN Appl. Sci. 2, 570 (2020). https://doi.org/10.1007/s42452-020-2368-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2368-1