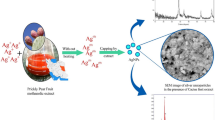
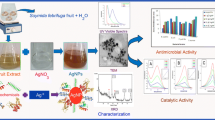
Abstract
A simple, facile and rapid microwave irradiated system was applied to synthesize silver nanoparticles using 'Golden Delicious' apple pulp (Malus domestica) and cumin (Cuminum cyminum) seed extracts. The phytosynthesized AgNPs were characterized by Ultraviolet–Visible Spectroscopy (UV–vis), Fourier transform infrared (FTIR), X-ray Diffraction (XRD) Transmission Electron Microscopy (TEM) and Zeta sizer analysis. In the study, the presence of face-centered cubic crystalline structured metallic silver in AgNPs from apple and cumin extracts and the monodisperse nature of AgNPs with the size distribution range of 5.46–20 nm and 1.84–20.57 nm were confirmed, respectively. This study established an efficient green synthesis approach that created so far, the smallest silver nanoparticles by using these two extracts. According to the results obtained, AgNPs synthesized using both extracts were non-toxic against L929 mouse fibroblast cells, while they were effective against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria with a greater effect on S. aureus. Moreover, AgNPs synthesized through cumin extract exhibited a higher ABTS scavenging ability (96.43 ± 0.78% at 160 μg/mL) in comparison to apple pulp extract mediated AgNPs, while both AgNPs showed lower activity for DPPH (27.84 ± 0.56% and 13.12 ± 0.32% from cumin seed and apple pulp extracts, respectively). In summary, our results suggest the green non-cytotoxic AgNPs synthesized in this study could be a promising template for further biological and clinical applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology consists of many fields such as physics, chemistry, pharmacy, biology, materials science and is a rapidly developing multidisciplinary field of science which has become a general purpose technology that benefits society [1]. In the past few decades, silver nanoparticles have attracted tremendous attention due to their excellent anti-pathogenic mechanism, thanks to their unique and characteristic physical, chemical and biological properties [2]. Antimicrobial prophylaxis of AgNPs widens their application in many aspects of medical science, i.e., sterilization of medical devices, drug delivery system, oral health protection, and wound treatment [3]. Simultaneously, nano-silver are also being utilized in different other fields, including, water treatment, cosmetics, textiles, biomedicine, DNA sequencing, food sanitation and packaging, sensing, biosensing, surface-enhanced Raman scattering (SERS), optoelectronics, and electronics [4,5,6,7,8]. Therefore, a range of techniques have been adopted for synthesizing silver and other metallic nanoparticles, including chemical, physical and biological methods. The use of certain parts or ingredients of many organisms ranging from bacteria to fungi and even plants for the biosynthesis of metallic NPs has been reported [9]. Among all, environmentally benign biological synthesis methods using plant extract possess many advantages over other conventional methods. Plant-mediated synthesis of metallic nanoparticles offers cost-effective and ecofriendly approach by eliminating the use of expensive instruments, high pressure, hazardous chemicals [10, 11]. Various biomolecules available in plants have been reported to show their potentiality in the reduction of metallic ions to nano-scaled particles [12, 13]. Besides, plant based fabrication of NPs is being taken a better option since plants are boasted with some advanced features, including biocompatibility and scalability [14]. A wide range of plant materials, such as whole plants, leaves, barks, stems, roots, flowers, fruits and fruit peels, fruit pulps, seeds as well as different secretory substances and pigments [15, 16] have already been found to be utilized for the fabrication of AgNPs aiming to achieve desired unique magnetic, optical, catalytic, and electrical properties, and wide range applicability [17, 18]. However, the multidisciplinary applications of silver nanoparticles demand their rapid and mass production, and scientists are trying to design faster, well-established and more inexpensive approaches for the fabrication of AgNPs on a large scale. Aiming this, plant mediated synthesis with microwave irradiation could be the fast and facile option for nanoparticle production. Microwave irradiation provides a fast and homogeneous heating system which confirms consistent nucleation and growth of nanoparticles in the reaction medium [19]. Besides, compared to the conventional heating, electromagnetic radiations in the microwave can decrease the reaction time by a factor of ~ 20 without disturbing the reaction condition [20, 21]. During the synthesis, the growth and the capping of a particle are antagonistic against each other, and the binding affinity of the capping agent greatly influence the final sizes, shapes and dispersity of NPs [22]. Previous studies indicated that higher and uniform heating of a microwave system accelerate the reaction kinetics in the synthesis medium, which increase the rate of capping; and thereby, produce nanoparticles with smaller size distribution [23].
Considering all these above mentioned facts and reasons, this study was designed to establish a fast and facile microwave accelerated (with two optimized parameters, i.e., time and temperature) green synthesis of silver nanoparticles using golden delicious apple (Malus domestica 'Golden Delicious') pulp and cumin (Cuminum cyminum) seed extracts without involving any supplementary chemicals. The reasons for choosing these two plant-based materials were because of their availability and being potential sources of different phytochemicals, which might be very effective reducer and stabilizer during the synthesis process. Apple fruits are rich in water soluble hydrocarbons, proteins, tartaric acid, polyphenolics, flavonoids, phytonutrients and antioxidant [24]. On the other hand, cumin seeds are popular as spice and herbal medicine. The presence of different essential volatile oil (5%) in cumin seed are the reason for their distinctive flavor, warm, and strong aroma. Some important essential oil components available in cumin seed are cymene, cuminaldehyde, and different terpenoids [25].
After the completion of synthesis, identification and characterization were completed using different analytical methods i.e., Ultraviolet–Visible Spectroscopy (UV–vis), Fourier transform infrared (FTIR), X-ray Diffraction (XRD) Transmission Electron Microscopy (TEM) and Zeta sizer analysis. Moreover, antimicrobial potentiality, cytotoxicity and antioxidant activity of fabricated AgNPs were examined to determine their suitability for wider range of applications.
2 Materials and methods
2.1 Materials
All chemicals used in the study were of analytical grade and were used to conduct all experiments without further modification or purification. Silver nitrate (AgNO3) and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).Golden delicious apples and dried cumin seeds were purchased from local grocery store. Ultra-purified water from the water purification system (Purelab flex, Veolia Water Solutions and Technologies, Tienen, Belgium) was used for all solutions of reacting materials, and other purposes. All the glass containers were washed using ultra-purified water and dried appropriately before use. Properly autoclaved instruments were used for antibacterial, antioxidant and cytotoxicity studies.
2.2 Preparation of apple (Malus domestica 'Golden Delicious') pulp extract
Fresh apple fruits were washed separately with running tap water to eliminate the unwanted dust particles and then, thoroughly washed several times with ultra-purified water. Using a sterilized kitchen paring knife, the fruits were peeled off and 100 g of its seedless pulp was sliced into small pieces. Then, these pieces were put into a food grade kitchen blender, ground well to make pulp paste. After adding equal volume of ultra-purified water, the paste was transferred into a conical flask, mixed well, and seated into a microwave (laboratory-grade) for 3 min. with a maximum power level of 700 W for irradiation to extract the biomolecules present in apple pulp. After cooling down at room temperature, the pulp suspension was centrifuged at 5000 rpm for 15 min. Finally, the collected pale-yellow colored supernatant was filtered using Whatman No. 1 filter paper to eliminate the impurities and stored in the freezer at 4 °C for further experiments.
2.3 Preparation of cumin (Cuminum cyminum) seed extract
Dried cumin seeds were crushed into fine powder. About 10 g of this powder was added into 100 mL of ultra-purified water and placed into an ultrasonic bath at 70 °C for 20 min. Then, the solution was put at room temperature for cooling down, and centrifuged at 5000 rpm for 15 min. After the centrifuge, a visible yellowish-brown colored supernatant was collected and filtered well using Whatman No. 1 filter paper to remove the stringy discarded particles. Lastly, the final cumin seed extract was stored at 4 °C for further usages.
2.4 Synthesis of silver nanoparticles
The fabrications of silver nanoparticles were conducted separately for each plant extract. For optimizing the synthesis protocol, different synthesis cycles were designed according to the variation of the ratio of plant extract and salt solution as well as temperature at different wavelengths and time durations. Successful synthesis were accomplished when silver nitrate salt (0.017 g AgNO3; 1 mM) was integrated with 90 mL of ultra-purified deionized water and 10 mL of each plant extract. With magnetic stirring bars, the solutions were transferred into the microwave (laboratory-grade) at 90 °C for 15 min with a highest heating level of 300 W. After finishing the microwave irradiations, the color changes in the synthesis media primarily indicated the completion of the fabrication cycle, and the production of AgNPs.
2.5 Purification of fabricated nanoparticles
After the synthesis was successfully completed, the silver nanoparticles produced from both plant extracts were filtered using 2.5 µm pore sized Whatman No.5 filter paper to remove large discarded particles, and the remaining solutions were centrifuged at 5000 rpm at 4 °C for 15 min. The precipitated solid forms in this process were washed several times with ultra-purified H2O to eliminate any undesired plant extract remaining. Finally, under vacuum conditions, AgNPs without plant debris were placed in a laboratory-class dryer to collect dust-free NPs. At the end of all procedures, the nanoparticles were transferred to dark colored bottles and stored in the refrigerator (4 °C) for further studies.
2.6 Characterization of silver nanoparticles
The optical properties of synthesized particles were screened using UV–vis spectrophotometer (Shimadzu UV-1700) to monitor the bioreduction of Ag+ ions and confirm the formation of nano-silver from silver ions over a range of 200–800 nm. IR spectroscopic measurements were conducted using Shimadzu IR Prestige-21 FTIR-ATR instrument. For evaluating the crystallinity of the NPs, the phytosynthesized silver nanoparticles were examined through an X-ray diffraction scheme (PANalytical Empyrean model, UK) where the XRD patterns were calculated over the range of 2 h from 10° to 90° with a step size of 0.02. The Origin 8.5 software (Origin Lab Corporation, Northampton, MA, USA) was used to regenerate the XRD graphs. The morphology of silver nanoparticles was revealed by using Transmission Electron Microscopy (TEM 1400, JEOL, Tokyo, Japan) set at increased speed voltage of 120 kV. In this case, the samples were prepared by taking small amount of nanoparticle suspensions which were placed drop by drop on the copper grids, and then kept for drying at room temperature, and used for TEM imaging. Besides, measurements of zeta potential and size distribution for AgNPs were performed via particle/zeta analyzer (Zetasizer nano ZS, Malvern Instruments Ltd., UK).
2.7 Antibacterial activities of biosynthesized silver nanoparticles
The antibacterial potentials of phytosynthesized AgNPs were studied by agar well diffusion assay for both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacterial strains. The freeze-dried cultures of E. coli (ATCC 25,922) and S. aureus (ATCC 25,923) were collected from Microbiologics Inc. (Saint Cloud, MN, USA). The bacterial suspensions were adjusted using 0.5 McFarland turbidity (1.5 × 108 CFU/mL). Using gel puncture, several wells (around 7 mm in diameter) were created on Muller–Hinton Agar (MHA, Merck), and then, 100 µL bacterial inocula were spread onto these agar plates. Afterwards, these plates were kept for air-drying at room temperature. Exactly 5 µg of each biosynthesized powdered NPs was added into 5 mL of ultra-purified H2O which were applied as the working suspensions. Then, 50 µL of aliquot parts from the suspensions were poured into every single well of the medium and then incubated at 35 °C for 24 h. Subsequently, after the incubation, clear and visible regions around the wells indicated the zones of inhibition by nanoparticles were calculated in diameters (mm).The antibacterial potential of silver nanoparticles against these bacterial strains were compared using the standard antibiotic discs of gentamicin (Oxoid, 10 µg/sensidisc). The experiments were repeated in triplicate.
2.8 Cytotoxicity of phytosynthesized silver nanoparticles
In vitro cytotoxicity of phytosynthesized silver nanoparticles was tested by evaluating cell viability on the L929 mouse fibroblast cell line using the XTT assay. DMEM-F12 medium was used to maintain L929 cell line culture supplemented with penicillium-streptomycin and 10% fetal bovine serum. The cells containing media were incubated at 37 °C with 5% CO2. The cells enriched using trypsin were separated from the vessels, followed by counting viable cells stained with Trypan blue. The density of obtained viable cells was adjusted to 106 live cells in 1 mL medium and followed by seeding of plating 100 µL of cell suspension in every well of sterile 96-well flat bottom microplate (BD, Biosciences).
Silver nanoparticles of varying concentrations (0, 0.1, 0.25, 0.5, 1, 2.5 and 5 µg/mL) were added to the cultured cells and incubated at 37 °C for 24 h. Subsequently, the old medium was replaced with fresh medium (100 mL) containing 100 µL XTT (2, 3–Bis–(2–Methoxy–4–Nitro–5–Sulfophenyl)–2H–Tetrazolium–5–Carboxanilide) solution in DMEM (0.5 mg/mL concentration with 7.5 μg/mL Phenazine methosulfate). Then plates containing medium suspensions were incubated at 37 °C for 4 h. Finally, optical densities of live cell suspensions at 450 nm were measured using a multi-plate reader (Lab-Line Instruments, Melrose Park, IL, USA).
2.9 Antioxidant activity of biosynthesized silver nanoparticles
The free radical quenching property of nanoparticles was measured by using stable free radical chemical 2, 2–diphenyl–1–picrylhydrazyl (DPPH) in accordance with Phull [26] and using stable chemical 2, 2′–azino–bis(3–ethylbenzothiazoline–6–sulphonic acid) (ABTS) in accordance with Arnao [27]. Briefly, different concentration of nanoparticles was mixed with 2 mL of methanolic DPPH solution (40 mg/mL) and 1 mL of 50 mM tris HCl. The reaction mixture was incubated at room temperature in dark for 30 min and absorbance was recorded at 517 nm. Also, ABTS stock solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium per sulphite in methanol and incubated in a dark at room temperature. Different concentration of nanoparticles was mixed with ABTS working solution. Absorbance was recorded after 30 min of incubation in a dark at 734 nm. All the experiments were carried out in triplicates. The percentage of free radical quenching property was calculated as follows:
3 Results and discussion
Silver nanoparticles were fabricated from silver nitrate (AgNO3) salt using Malus domestica pulp and Cuminum cyminum seed extracts. Formation and fabrication of AgNPs were followed by an immediate color change of the reaction medium after a certain time of period (Fig. 1).
3.1 UV-Vis spectrographic analysis
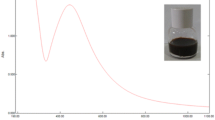
The absorption maxima of biosynthesized silver nanoparticles from apple pulp extract was observed at 440 nm whereas form cumin seed extract was found at 439 nm (Fig. 2). Metallic nanoparticles show UV–Vis spectrograph peaks in specific range of electromagnetic wave due to their surface plasmon resonance (SPR). It has observed that silver nanoparticles provide the characteristic sharp peak in the range of 400–475 nm [28]. SPR is the expression of a resonance effect that causes as a result of the interaction of free and highly mobile electrons of metallic nanoparticles with incident photons of the visible light during the UV–vis spectroscopy [29]. The interaction depends on the size and shape of the NPs, which shifts to a longer wavelength as the particle size increases [30]. Additionally, morphological features of NPs and their dispersity in the suspension could also be monitored by this spectroscopic analysis [31]. Characteristic single sharp peak for both the samples indicated the presence of monodispersed, smaller sized silver nanoparticles [32], which was confirmed by TEM imaging and Zeta analysis.
3.2 Fourier transforms infrared (FTIR) analysis
The Fourier transforms infrared (FTIR) spectrum of phytosynthesized silver nanoparticles from apple pulp extract (Fig. 3a) provided the band at 3381.21 cm−1 corresponds to aliphatic primary amine stretching (N–H). The band at 1641.42 cm−1 is responsible for strong alkene monosubstituted (C = C) bond. A strong C–O stretching primary alcohol bond was found at the peak of 1055.06 cm−1. The IR band at 972.12 cm−1 represents a strong alkene disubstituted (trans-) bond whereas the stretch of medium alkene (C = C) trisubstituted was found at 794.67 cm−1.
On the other hand, Fig. 3b represents IR-spectrum of biosynthesized silver nanoparticles from cumin seed extract. The broad peak was observed at 3373.50 cm−1 represents the meadium aliphatic primary amine (N–H) stretch. The band at 1639.49 cm−1 indicates a strong alkene monosubstituted (C = C) stretching. The absorption peak at 1415.75 cm−1 could be identified as the –OH stretching of H2O or ethanol present in the sample. The peak at 1058.92 cm−1 is due to the strong C–O stretching of primary alcohol vibration. The spectrum at 972.12 cm−1 represents a strong alkene disubstituted (trans) bond whereas the peak at 794.67 cm−1 is owing to the stretch of medium trisubstituted alkene (C = C) stretching and finally, 655 cm−1 is for strong C–Br stretching (halo compound). FTIR spectra therefore suggested that some amino acid residues, proteins, reducing sugars, polyphenols, flavanones, and terpenoids available in plant extracts played the vital rules in the reduction of silver ions into AgNPs and interacted with phytosynthesized silver nanoparticles to stabilize these particles [33, 34].
3.3 X-ray diffraction
X-ray diffraction (XRD) studies were utilized to exhibit the crystalline structure of green synthesized silver nanoparticles. The XRD spectrum of biosynthesized AgNPs by fresh Malus domestica pulp extract is illustrated in Fig. 4a. Strong peaks were observed at 38.13°, 44.29°, 64.48° and 77.49° with the interplanar spacing (dcalculated) values are 2.360, 2.046, 1.446 and 1.230 Å. Moreover, Fig. 4b reveals the XRD pattern of biosynthesized AgNPs using Cuminum cyminum seeds extract. Strong peaks were detected at 38.10°, 44.37°, 64.50° and 77.44° together with the interplanar spacing (dcalculated) values are 2.362, 2.043, 1.444 and 1.233 Å. For both samples, the presence of these four strong reflections at 2θ values attributed to the characteristic Bragg's diffraction plans regarded as (111), (200), (220) and (311), respectively. The outcomes from XRD studies signify that both AgNPs specimens are consist of face-centered cubic crystalline structured metallic silver, which correspond coordinate the catalog of the JCPDS (Joint Committee on Powder Diffraction Standards) file no: 04–0783 [35].
3.4 Transmission electron microscopy (TEM)
Morphological structure and size distribution of reduced phytosynthesized silver nanoparticles were analyzed by TEM. TEM profile of biosynthesized AgNPs by fresh Malus domestica pulp extract showed that the nanoparticles at 100 nm scales are morphologically spherical or globular in shape with the distribution range of 5.46–20 nm in diameter (Fig. 5a). The TEM micrograph of the biosynthesized AgNPs using Cuminum cyminum seeds extract at 50 nm scales was revealed in Fig. 5b.The result confirmed that the nanoparticles are almost globular in shape with maximum particles in the size ranged from 1.84 to 20. 57 nm. Moreover, Both the synthesized nanoparticles are distributed uniformly i.e. monodisperse nanoparticles.
3.5 Particle size distribution and zeta potential measurement
Characterization of nanoparticles using particle size distribution and zeta potential measurement reveals information regarding the size distribution, surface charge, colloidal behavior and stability of NPs [36]. The zetasizer analysis of biosynthesized silver nanoparticles from apple pulp extract revealed that the average value of nanoparticle size distribution was 20.70 nm whereas the average zeta potential value was found as − 25.80 mV as shown in Fig. 6. Besides, the result of AgNPs obtained from cumin seed extract indicated the average particle size as 14.30 nm with the zeta potential value of − 27.8 mV (Fig. 7). However, the average particle size distribution of both nanoparticles also supports the results of TEM analysis by providing the average size values closer to the size distribution ranges of TEM profiles. Overall, the size distribution by zeta sizer indicated the absence of aggregation. Moreover, the negative zeta potential values suggested the presence of the possible capping and stabilization of NPs by the biomaterials available in the plant extracts as well as present of strong agglomeration by retaining the particles separate from each other, which enhanced the negativenegative repulsion among the particles and consequently, confirmed higher stability [36].
Without any microwave irradiation, several previous studies have established different protocols for synthesizing AgNPs by food extracts as reducing and stabilizing agents. For instance, comparatively higher concentrated salt solution (0.1 M/100 mM AgNO3) and red apple fruits were used for Ag nanoparticle synthesis; and in such case, 20 mL of the red apple fruit extract was added into 180 mL aqueous silver nitrate solution, and heated at 60 °C for an hour for synthesizing silver nanoparticles. Laser Dynamic Light Scattering (DLS) analysis estimated the average size of the spherical shaped nanoparticles which was found to be 30.25 ± 5.26 nm. However, particle size distribution indicated the existence of aggregation [37]. Similar concentration of salt (0.1 M/100 mM) was also used in other study; however, AgNPs were synthesized at room temperature by mixing 5 mL of red apple fruit extract with 50 mL of aqueous AgNO3 solution, which was examined after 168 h reaction time [38]. The DLS assessment of the synthesized silver nanoparticles showed polydispersity with the particle size range of 50–300 nm. Furthermore, use of red apple as reducing agent for Ag nanoparticle synthesis was also evidenced by another literature. Following drop-wise addition method, 10 mL of red apple fruit extract was combined with 100 mL silver nitrate salt (20 mM) solution, and the reduction of silver ions to nano particles was confirmed in between 18 and 24 h of reaction time. TEM imaging revealed the presence of spherical shaped nanoparticles with dia of 20 nm [39]. In the study of cumin, AgNPs were synthesized from aqueous AgNO3 solution using C. cyminum leaf extract and they found the maximum rate of synthesis at 240 min after reaction [40].
While comparing with previously used protocols for synthesizing AgNPs by apple fruit and cumin seed extracts, it is the fact that utilization of microwave-assisted green synthesis applied in this study is more rapid, advantageous and easier approach. Additionally, being the fastest process, this protocol also produced so far, the smallest silver nanoparticles from these plant extract, which are distributed uniformly i.e. monodisperse in nature, without any aggregation. Here, the obtained results in this study occurred due to the fact that rapid and uniform heating process during microwave irradiation synthesis facilitated homogenous nucleation and faster capping rate, which significantly influenced the sizes, shapes and dispersity of NPs [22].
3.6 Analysis of antibacterial activities of biosynthesized silver nanoparticles
Antibacterial potentials of biosynthesized AgNPs using Cuminum cyminum seed extracts with the inhibition zones of 12.53 ± 0.45 and 10.30 ± 0.36 mm, and AgNPs by Malus domestica pulp extract with the inhibition zones of 10.20 ± 0.30 and 9.90 ± 0.50 mm were found against S. aureus and E. coli, respectively (Fig. 8, Table 1).
It is significant that stronger antibacterial activities were demonstrated by the silver nanoparticles with smaller sized particles and higher potential value, which were biosynthesized using cumin seed extract. Morphological and physiochemical properties of nanoparticles are the vital factors for exhibiting their antibacterial potential [41]. Nanoparticles with smaller size distribution have high reactive surface to volume ratio compared to their bulk macromolecules [42, 43]. This distinctive feature of NPs might facilitate them to contact and interrelate easily with other particles. Hence, they are capable of interacting with the bacterial cell and trend to show stronger antimicrobial effect [44, 45]. Furthermore, the potential values of NPs also influence their bactericidal properties. Nano-metallic particles with high potential charge could rapidly bind with surfaces of bacterial cells which might increase of the bactericidal effect as well [46, 47].
From a study, it was observed that bactericidal activities of powdered silver nanoparticles under varying concentrations against E. coli and S. aureus were almost identical [48]. AgNPs significantly increased the cell membrane permeability that caused protein leakage. It also induced the formation of bactericidal reactive oxygen species (ROS) which permanently deactivated bacterial respiratory chain lactate dehydrogenase (LDH) [48]. At the same time, the inhibition effect of nanoparticles on S. aureus was more than E. coli in general, and these results were in line with the studies in the literature [49, 50]. This study suggested that nano-silver can be a competent antibacterial agent against various pathogenic microbes. At the same time, the use of biologically synthesized silver nanoparticles in many film applications with higher antifungal activities compared to chemically synthesized forms has also been reported. This shows that nanoparticles obtained by green synthesis in many areas, especially in the food industry, will be a promising tool in the future [51].
3.7 Cytotoxicity study
The in-vitro cytotoxic effects of both silver nanoparticle samples were monitored against healthy mouse fibroblasts cell line (L929) through XTT cell viability assay. Different concentration of nanoparticles (0, 0.1, 0.25, 0.5, 1, 2.5 and 5 μg/mL) were applied to test the cell viability by observing the activity of mitochondrial enzymes in response to XTT reagent. Mitochondrial enzymes of viable cells can convert XTT reagent into visible orange color, which can be measured through absorbance detection. The absorbance peak of optical intensity is directly proportional to the cell viability, and therefore, the optical density can indicate the percentage of cell viability [19, 52]. Figure 9 showed the in vitro cytotoxic effects of fabricated AgNPs by using Malus domestica pulp and Cuminum cyminum seed extracts. The result indicated that the optical density did not decline drastically with the increased concentration of NPs. Hence, in the present study, there are no cytotoxic effects of phytosynthesized silver nanoparticles on regular mouse fibroblasts cell line (L929) at the given concentrations. Nevertheless, it is remarkable that both AgNP samples exhibited antibacterial activities against two important pathogenic bacterial strains (Staphylococcus aureus and Escherichia coli) at very low concentration (1 μg/mL).
In the past few decades, silver nanoparticles have obtained a special interest due to their excellent anti-pathogenic mechanism [53]. Despite having inadequate information about biological behavior and cytotoxicity of nano-silver, they have been used in the field of cosmetics, clinical diagnosis, biomedical evaluation and revaluation, biotechnology, food processing and some environmental aspects [54, 55]. However, by using animal models, several toxicology studies showed the in vitro and in vivo cytotoxicity of conventionally manufactured nanoparticles [56,57,58]. In a previous study, silver nanoparticles (AgNPs) synthesized using biological material were found to be non-toxic to fibroblasts in a wide concentration range (100–1000 μg/ml) and did not compromise cell viability or growth [59]. Also, it was stated in another study that the genotoxicity of biologically synthesized NPs depends on the synthesis parameters, biological source and the test applied [60]. Therefore, productions of nanoparticles without using any hazardous chemicals as well as measuring the cytotoxicity of produced NPs have become the primary and necessary steps before any kind of nano-based applications.
3.8 Antioxidant activity of biosynthesized silver nanoparticles
The antioxidant activity of synthesized AgNPs at the increased concentration (10, 20, 40, 80, 160 μg/mL) was evaluated by DPPH and ABTS radical scavenging assay. Trolox was used as a positive control at the same concentration range. The scavenging ability of the nanoparticles for both radicals increased in a dose dependent manner (Fig. 10). At the highest concentration (160 μg/mL), the recorded DPPH scavenging ability of the biosynthesized AgNPs by cumin seed extract was 27.84 ± 0.56% whereas for the AgNPs by apple pulp extract was found to be 13.12 ± 0.32%. Besides, the inhibition percentage (%) of Trolox was 95.29 ± 0.58% at the same concentration. In previous reports, DPPH scavenging activities of different plants mediated AgNPs were higher than the present study [61, 62].
On the other hand, in the case of the ABTS scavenging activity, the inhibition percentage at the concentration of 160 μg/mL was 96.43 ± 0.78% for AgNPs synthesized by cumin seed extract while the inhibition percentage was 78 ± 0.11% for AgNPs by apple pulp extract. In addition, the inhibition percentage at the same concentration was 99.68 ± 0.06 for Trolox.
Both assay percentages (%) indicated that silver nanoparticles which was produced using cumin seed extract and possess smaller particle size demonstrated higher antioxidant activity compared to the AgNPs from apple pulp extract. Moreover, the results revealed that the synthesized nanoparticles by both extracts exhibited higher radical scavenging activity in ABTS assay than they showed in the DPPH assay. This might be possible due to the difference in sensitivity of ABTS and DPPH radicals [63]. These experiments revealed that this interaction expresses the reducing ability of the nanoparticles and the antioxidant properties of AgNPs synthesized by the plant extract has been retained possibly due to the capping on the AgNPs [64].
4 Conclusion
This study established that the simple microwave-irradiation scheme only with two optimized parameters (time and temperature) is very effective, convenient and advantageous for the biosynthesis of silver nanoparticles using golden delicious apple (Malus domestica 'Golden Delicious') pulp and cumin (Cuminum cyminum) seed extracts. Moreover, using these two plant extracts, this rapid, facile and efficient green synthesis approach were created the smallest and highly stabilized silver nanoparticles, which were found to be monodisperse in nature and without any aggregation. Most importantly, the results of the current study presented that silver nanoparticles possessed the most promising non-cytotoxic mammalian cell behavior with the greatest antibacterial activity offer a rational approach towards their future investigation in a wide range of biomedical and pharmaceutical applications.
References
Kanagamani K, Muthukrishnan P, Shankar K, Kathiresan A, Barabadi H, Saravanan M (2019) Antimicrobial, cytotoxicity and photocatalytic degradation of norfloxacin using Kleinia grandiflora mediated silver nanoparticles. J Cluster Sci 30(6):1415–1424
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176(1):1–12
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Cohen-Karni T, Langer R, Kohane DS (2012) The smartest materials: the future of nanoelectronics in medicine. ACS Nano 6(8):6541–6545
Murphy CJ, Gole AM, Hunyadi SE, Stone JW, Sisco PN, Alkilany A, Kinard BE, Hankins P (2008) Chemical sensing and imaging with metallic nanorods. Chem Commun 5:544–557
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592):607–609
Cao Y, Jin R, Mirkin CA (2001) DNA-modified core−shell Ag/Au nanoparticles. J Am Chem Soc 123(32):7961–7962
Matejka P, Vlckova B, Vohlidal J, Pancoska P, Baumruk V (1992) The role of triton X-100 as an adsorbate and a molecular spacer on the surface of silver colloid: a surface-enhanced Raman scattering study. J Phys Chem 96(3):1361–1366
Barabadi H, Tajani B, Moradi M, Kamali KD, Meena R, Honary S, Mahjoub MA, Saravanan M (2019) Penicillium family as emerging nanofactory for biosynthesis of green nanomaterials: a journey into the world of microorganisms. J Clust Sci 30:1–14
Jha AK, Prasad K, Prasad K, Kulkarni A (2009) Plant system: nature’s nanofactory. Colloids Surf, B 73(2):219–223
Annamalai N, Thavasi R, Vijayalakshmi S, Balasubramanian T (2011) A novel thermostable and halostable carboxymethylcellulase from marine bacterium bacillus licheniformisAU01. World J Microbiol Biotechnol 27(9):2111–2115
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed nanotechnol biolo med 6(2):257–262
Rai M, Yadav A (2013) Plants as potential synthesiser of precious metal nanoparticles: progress and prospects. IET Nanobiotechnol 7(3):117–124
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10(3):507–517
Kasthuri J, Kathiravan K, Rajendiran N (2009) Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 11(5):1075–1085
Adelere IA, Lateef A (2016) A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev 5(6):567–587
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 84(2):151–157
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int nano lett 2(1):32
Jahan I, Erci F, Isildak I (2019) Microwave-assisted green synthesis of non-cytotoxic silver nanoparticles using the aqueous extract of Rosa santana (rose) petals and their antimicrobial activity. Anal Lett 52(12):1860–1873
Patel K, Kapoor S, Dave DP, Mukherjee T (2005) Synthesis of nanosized silver colloids by microwave dielectric heating. J Chem Sci 117(1):53–60
Joseph S, Mathew B (2015) Microwave assisted facile green synthesis of silver and gold nanocatalysts using the leaf extract of Aerva lanata. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1371–1379
Ali K, Ahmed B, Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J (2015) Microwave accelerated green synthesis of stable silver nanoparticles with eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS ONE 10(7):131178
Kudle KR, Donda MR, Merugu R, Kudle MR, Rudra MP (2013) Micro wave assisted green synthesis of silver nanoparticles using Boswellia serrata flower extract and evaluation of their antimicrobial activity. Int Res J Pharm 4(6):197–200. https://doi.org/10.7897/2230-8407.04644
Lee KW, Kim YJ, Kim D-O, Lee HJ, Lee CY (2003) Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem 51(22):6516–6520
Mohamed DA, Hamed IM, Fouda KA (2018) Research article antioxidant and anti-diabetic effects of cumin seeds crude ethanol extract. J Biol Sci 18(5):251–259
Phull A-R, Abbas Q, Ali A, Raza H, Zia M, Haq I-u (2016) Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J Pharm Sci 2(1):31–36
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73(2):239–244
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 1(1):3
Jana J, Ganguly M, Pal T (2016) Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv 6(89):86174–86211
Barabadi H, Honary S, Ebrahimi P, Alizadeh A, Naghibi F, Saravanan M (2019) Optimization of myco-synthesized silver nanoparticles by response surface methodology employing box-behnken design. Inorg Nano-Metal Chem 49(2):33–43
Zhang L, Shen Y, Xie A, Li S, Jin B, Zhang Q (2006) One-step synthesis of monodisperse silver nanoparticles beneath vitamin E Langmuir monolayers. J Phys Chem B 110(13):6615–6620
Nagalingam M, Kalpana V, Panneerselvam A (2018) Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible gold nanoparticles from Alternanthera bettzickiana. Biotechnol Rep 19:e00268
Hind AR, Bhargava SK, McKinnon A (2001) At the solid/liquid interface: FTIR/ATR—the tool of choice. Adv Coll Interface Sci 93(1–3):91–114
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7(1):1–37
Hamedi S, Shojaosadati SA, Mohammadi A (2017) Evaluation of the catalytic, antibacterial and anti-biofilm activities of the convolvulus arvensis extract functionalized silver nanoparticles. J Photochem Photobiol, B 167:36–44
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 79(3):594–598
Ali ZA, Yahya R, Sekaran SD, Puteh R (2016) Green synthesis of silver nanoparticles using apple extract and its antibacterial properties. Adv Mater Sci Eng 2016:1–6
Umoren S, Obot I, Gasem Z (2014) Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci 5(3):907–914
Roy K, Sarkar C, Ghosh C (2014) Green synthesis of silver nanoparticles using fruit extract of malus domestica and study of its antimicrobial activity. Dig J Nanomater Biostruct 9(3):1137–1147
Karamian R, Kamalnejad J (2019) Green synthesis of silver nanoparticles using cuminum cyminum leaf extract and evaluation of their biological activities. J Nanostruct 9(1):74–85. https://doi.org/10.22052/jns.2019.01.008
Mohammadi G, Valizadeh H, Barzegar-Jalali M, Lotfipour F, Adibkia K, Milani M, Azhdarzadeh M, Kiafar F, Nokhodchi A (2010) Development of azithromycin–PLGA nanoparticles: physicochemical characterization and antibacterial effect against Salmonella typhi. Colloids Surf, B 80(1):34–39
Matsumura Y, Yoshikata K, Kunisaki S-i, Tsuchido T (2003) Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol 69(7):4278–4281
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? a study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73(6):1712–1720
Fellahi O, Sarma RK, Das MR, Saikia R, Marcon L, Coffinier Y, Hadjersi T, Maamache M, Boukherroub R (2013) The antimicrobial effect of silicon nanowires decorated with silver and copper nanoparticles. Nanotechnology 24(49):495101
Mohammadi G, Nokhodchi A, Barzegar-Jalali M, Lotfipour F, Adibkia K, Ehyaei N, Valizadeh H (2011) Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf, B 88(1):39–44
Seil JT, Webster TJ (2012) Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomed 7:2767
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4(3):316
Kim S-H, Lee H-S, Ryu D-S, Choi S-J, Lee D-S (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol 39(1):77–85
Dahlous KA, Abd-Elkader OH, Fouda MMG, Al Othman Z, El-Faham A (2019) Eco-friendly method for silver nanoparticles immobilized decorated silica: synthesis & characterization and preliminary antibacterial activity. J Taiwan Inst Chem Eng 95:324–331. https://doi.org/10.1016/j.jtice.2018.07.020
Erci F, Torlak E (2019) Antimicrobial and antibiofilm activity of green synthesized silver nanoparticles by using aqueous leaf extract of Thymus serpyllum. Sak Univ J Sci 23(3):333–339. https://doi.org/10.16984/saufenbilder.445146
Fouda MMG, El-Aassar M, El Fawal GF, Hafez EE, Masry SHD, Abdel-Megeed A (2015) k-Carrageenan/poly vinyl pyrollidone/polyethylene glycol/silver nanoparticles film for biomedical application. Int J Biol Macromol 74:79–184. https://doi.org/10.1016/j.ijbiomac.2014.11.040
Erci F, Cakir-Koc R, Isildak I (2018) Green synthesis of silver nanoparticles using Thymbra spicata L var spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity. Artif cells nanomed biotechnol 46(sup1):150–158
Burdușel A-C, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E (2018) Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials 8(9):681
Tavaf Z, Tabatabaei M, Khalafi-Nezhad A, Panahi F (2017) Evaluation of antibacterial, antibofilm and antioxidant activities of synthesized silver nanoparticles (AgNPs) and casein peptide fragments against streptococcus mutans. Eur J Integr Med 12:163–171
Chowdhury NR, MacGregor-Ramiasa M, Zilm P, Majewski P, Vasilev K (2016) ‘Chocolate’silver nanoparticles: synthesis, antibacterial activity and cytotoxicity. J Colloid Interface Sci 482:151–158
El Mahdy MM, Eldin TAS, Aly HS, Mohammed FF, Shaalan MI (2015) Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp Toxicol Pathol 67(1):21–29
Pinzaru I, Coricovac D, Dehelean C, Moacă E-A, Mioc M, Baderca F, Sizemore I, Brittle S, Marti D, Calina CD (2018) Stable PEG-coated silver nanoparticles–a comprehensive toxicological profile. Food Chem Toxicol 111:546–556
Thounaojam MC, Jadeja RN, Valodkar M, Nagar PS, Devkar RV, Thakore S (2011) Oxidative stress induced apoptosis of human lung carcinoma (A549) cells by a novel copper nanorod formulation. Food Chem Toxicol 49(11):2990–2996
Abdel-Mohsen A, Abdel-Rahman RM, Fouda MM, Vojtova L, Uhrova L, Hassan A, Al-Deyab SS, El-Shamy IE, Jancar J (2014) Preparation, characterization and cytotoxicity of schizophyllan/silver nanoparticle composite. Carbohyd Polym 102:238–245
Barabadi H, Najafi M, Samadian H, Azarnezhad A, Vahidi H, Mahjoub MA, Koohiyan M, Ahmadi A (2019) A systematic review of the genotoxicity and antigenotoxicity of biologically synthesized metallic nanomaterials: are green nanoparticles safe enough for clinical marketing? Medicina 55(8):439
Adebayo EA, Ibikunle JB, Oke AM, Lateef A, Azeez MA, Oluwatoyin AO, AyanfeOluwa AV, Blessing OT, Comfort OO, Adekunle OO (2019) Antimicrobial and antioxidant activity of Silver, gold and silver-gold alloy nanoparticles phytosynthesized using extract of opuntia ficus-indica. Rev Adv Mater Sci 58(1):313–326
Lateef A, Folarin BI, Oladejo SM, Akinola PO, Beukes LS, Gueguim-Kana EB (2018) Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L leaf extract. Prep Biochem Biotechnol 48(7):646–652. https://doi.org/10.1080/10826068.2018.1479864
Dauthal P, Mukhopadhyay M (2013) In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. J Nanopart Res 15(1):1366
Mittal AK, Bhaumik J, Kumar S, Banerjee UC (2014) Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci 415:39–47
Acknowledgements
The present study was a part of the PhD thesis of Dr. Israt Jahan. The authors are very greatfull to the Department of Bioengineering, Yildiz Technical University for their lab facilities and research opportunities.Besides, authors must present their sincere gratitude and gratefulness to Dr. Rabia ÇAKIR KOÇ for her support and contibuton to cytotoxicity study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jahan, I., Erci, F. & Isildak, I. Rapid green synthesis of non-cytotoxic silver nanoparticles using aqueous extracts of 'Golden Delicious' apple pulp and cumin seeds with antibacterial and antioxidant activity. SN Appl. Sci. 3, 94 (2021). https://doi.org/10.1007/s42452-020-04046-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-04046-6