Abstract
In the Anthropocene era, an immersion of toxic substances, i.e., trace metals, has been enhanced in the marine environment not only due to urban sprawl and industrial development but predominantly owing to incongruous management and lack of sustainable approaches. The coastal region of Pakistan shares a similar obstacle as most of the developing countries confronted. Therefore, this study was designed to investigate concentrations of eight metals (Cu, Fe, Zn, Ni, Co, Pb, Cr, and Cd) in sediment and Dotillid crab, Ilyoplax frater, at three tidal creeks in Karachi, Pakistan. All metals in sediments and crabs were analyzed by atomic absorption spectrometer. The sediment pollution was evaluated by contamination degree (CD) and potential ecological risk index (RI). After depicting the metal pollution in sediments, metal accumulation, and contamination in benthic crab were investigated through total metal concentrations in crabs, accumulation factor (AF), correlation analysis, and regression analysis. The results exhibited substantial differences in the concentrations of Zn, Ni, Pb, Cr, and Cd among the tidal creek sediments. Contamination factors indicated that the Cd and Pb had the highest sharing in sediment pollution, and the tidal creeks classified as moderately contaminated. All metal accumulations in Dotillid crabs showed notable spatial variations, and accumulation factors (AFs) for most of the metals were > 1.0, signifying the strong bioaccumulation of metals in crabs. Particularly, Cu, Co, and Cd levels were considerably greater (two to three times) in Dotillid crabs compared to creek’s sediments, even though they don’t share any relationship between two matrixes. Hydrographical and sedimentological traits also revealed significant interactions with metal levels in sediments and crabs. A substantial association was also noticed in Fe, Ni, and Pb between sediments and crabs. Interestingly, most of the metal AFs showed a notable inverse correlation with the environmental matrix. Exceptionally, a strong positive correlation found between the Pb concentration in crabs and sediments suggested that I. frater probably acts as an indicator of Pb pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Trace metals are among the most hazardous contaminants in the environment because of their perseverance, biogeochemical cycling, and the ecological risks they pose [12, 64, 81]. Marine environments receive trace metals from both natural leaching continental progressions as well as mining, industrial, and urban activities. Coastal sediments, particularly organic-rich sediments that have high potential to accumulate contaminants, act as a primary sink and substantial source of many pollutants [15, 18, 74]. In fact, sediments are a significant carrier for trace metals in marine environments, and heavy metals may be transported into these environments associated with deposits. Consequently, it can influence the water quality and may cause potential hazards to the well-being of humans and the environment [5, 50]. For example, after resuspension of sediments, dissolved and particulate fractions of metals are released into the water. Marine organisms can integrate metals easily in their dissolved form and initiate the process of bioaccumulation. Additionally, particulate metals gradually settle down into the sediment after resuspension and can then also be absorbed by aquatic organisms [32, 81]. Given the potential adverse effects of trace metals in a range of aquatic organisms, there is a critical need for biomonitoring of these contaminants, as a prerequisite to initiating management actions.
Trace metal bioavailability and accumulation potential are greatly affected by spatial and temporal variations in sediment characteristics in a marine environment. Nevertheless, sediments offer a degree of time integration to dampen the effects of temporal variability [38]. Additionally, sediments generally have much higher metal concentrations than the overlying water column, allowing more easy measurement and a lower susceptibility toward accidental contamination [15, 18]. These characteristics mean that the measurement of trace metals in sediments provides a good estimate of the potential contamination that benthic organisms, such as crabs, are exposed to over an intermediate timeframe of a few weeks to months. Some metals (such as Cu, Fe, Zn, etc.) are essential nutrients for aquatic biota, but can show toxicity if they are above a threshold level. By contrast, other metals such as Ag, Hg, Cd, and Pb are already toxic in low concentrations to marine life [11, 64, 70].
Benthic crustaceans are often a target of contaminants due to their limited mobility or close association with the substratum. As a resident associate, they are particularly important in the accumulation of trace metals from a local environment and as such can act as an exposure indicator that reflects the pollution status of an environment [20, 41, 55, 66]. High levels of various metals in sediments can affect marine fauna, with knock-on effects on the food chain and public health due to seafood contamination [12, 37, 64, 68]. Accumulation of trace metals in organisms is a complicated process, and it usually acts as a good indicator of metal exposures in the environment because most of the metals cannot be metabolized. Accumulation in organisms is stimulated through multiple routes of exposure (such as diet or solution) as well as geochemistry of the region [39]. In fact, a dominant dispute in the prediction of toxicity is the bioavailability of trace metals in organisms [14]. High concentrations of a certain metal in an organism indicate the bioavailability of this metal within a location. Moreover, it can be indicative of bioaccumulation or bioconcentration, the process in which trace metals are accumulated into biota from its surrounding phases [46], if the values in biota surpass those in sediments. Different aquatic invertebrates accumulate trace metals with different concentrations in their tissues predominantly via their diets, even if these metals are not necessary for metabolism [22].
The Dotillid crab, Ilyoplax frater, Stimpson 1858 (Brachyura: Ocypodoidea: Dotillidae), is a small-sized crab that inhabits intertidal mudflats of mangrove forests in temperate to tropical Indo-Western Pacific regions [33, 60]. As deposit feeders, they feed upon sediments by sorting organic matter and detritus [60]. These crabs also construct and reconstruct their burrows during low tide like other Ocypodid and Dotillid crabs. They emerge from their burrows to engage in feeding and social activities during low tide, and they return to their burrows when the tide covers their habitat again. Furthermore, during low tide, they renovate their burrows by creating mud balls. The biotic activities of crabs (such as feeding and burrowing) play an important role in nutrient cycling and energy flow in the ecosystem and contribute to the ecological engineer of mangroves and estuaries [36, 61, 63]. Moreover, spatial and temporal differences in the distribution of crab communities as a result of natural factors and human impacts (such as trace metal pollution) can affect the structure and function of local habitat units within the coastal habitat mozaic [67, 76,77,78].
The aims of the current study were (1) to scrutinize contamination by eight metals (Cu, Cd, Cr, Co, Ni, Zn, Pb, and Fe) in sediments from three contaminated tidal creeks, Karachi, Pakistan, (2) to analyze the bioaccumulation of these metals in the resident deposit feeder crab species I. frater related to the sediment concentrations, (3) to evaluate the impacts of environmental heterogeneities on metal the distribution in sediments as well as crab, and (4) to identify the potential of this crab species as a biomonitor for heavy metal contaminated environments, by evaluating the relationship between metal concentrations in crabs and exposure (surroundings) concentrations in the sediment.
2 Materials and methods
2.1 Study sites
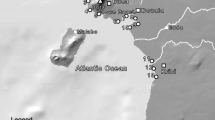
Karachi, a megacity of Pakistan and a hub of industrial and commercial activities, is situated on the northern boundary of the Arabian Sea with a coastal zone of about 167 km. Discharge of untreated sewage and industrial effluent via Lyari and Malir rivers have dramatically increased with intensified industrial development and expansion of the city. These rivers directly drain into estuaries, mangroves, and beaches adjacent to the Arabian Sea, resulting in numerous sorts of pollution (organic and inorganic), with demonstrated environmental problems for resident biota [43, 57, 64, 66, 68]. In this study, three tidal creek areas were selected from the coast of Karachi, Pakistan (Fig. 1). The first site (S1) was situated at 24° 46′ N, 67° 18′ E near Port Bin Qasim, in an intertidal area with mangrove vegetation. Port Bin Qasim is exposed to numerous anthropogenic effluents originating from the largest steel mills, industrial states, auto manufacturing plants, chemical and fertilizer plants, and the largest container terminal and seaport in Pakistan [68].
The second site (S2) was located near Kadiro Creek at 24° 46′ N, 67° 14′ E. It is an intertidal mudflat area with no mangrove vegetation. The third site (S3) was situated at 24° 47′ N, 67° 10′ E, near the Korangi Creek area. It is the northernmost creek of the Indus Delta located in the East near the fishing village of Ibrahim Hyedri. It is associated at its northeastern end with Phitti and Kadiro Creek and its southwestern end with Gizri Creek and is bounded by the open sea and extensive Avicennia marina mangrove vegetation. The coastal mangrove system is a nutrient-rich environment and functions as a spawning and nursery ground for several species of economically important marine fauna, including shrimp and crab species [60]. This area is greatly influenced by several contaminations through point and non-point sources. It receives untreated industrial effluents from Korangi and Landhi industrial states and domestic wastewater from the metropolis of Karachi. When these pollutants spread along the mangrove swamp, they may produce toxicity at different levels in the resident biota which ultimately threatens the fisheries sector [17].
2.2 Sampling procedure
Sampling of crabs and sediments was carried out during low tide from three creeks of Karachi. To determine the trace metal contamination in sediments, three sediment samples were collected using a PVC core (20 cm deep) at each site. The samples were kept in separate tagged polyethylene bags until analysis. To evaluate the bioaccumulation of trace metals in resident biota, crabs were collected by transect and quadrat methods. At each monitoring site, two transects were placed from the low to the high tide mark, while the area was exposed during low tide. On each transect, 0.25 m2 quadrats (4–6 at each site) were placed and excavated (about 30 cm deep), after which all crabs were hand-picked. The crabs present in each quadrat were bagged in separate pre-washed and labeled polyethylene bags. The hydrographic properties such as temperature, salinity, and pH were determined through a portable thermometer, refractometer, and pH meter from pore water after excavating the each quadrate. Crab and sediment samples were stored in the icebox for transport to the laboratory.
2.3 Laboratory analysis
Physical (organic matter and grain size) and chemical (metal concentrations) properties of the sediment were analyzed for each site. The percent organic matter (OM%) was determined by the difference in weight loss before and after the combustion of a 3–5 g dry sediment sample at 450 °C for 3 h in a furnace [60]. Grain size analysis was performed using a standard dry sieving method [26]. Specifically, dried sediment samples (100 g) were homogenized thoroughly, then mechanically sorted through a set of US mesh sieves using a mechanical shaker. Sediments passing through a 63-µm sieve were used for acid digestion. Approximately 1.0 g of sediment samples were mixed with 10 ml of aqua regia and digested on a hot plate at 80 °C for an hour. Following cooling at room temperature, samples were filtered and diluted for metal analysis [64].
From the crab samples, individuals of I. frater were identified, sorted, washed with plenty of distilled water to remove sediment and debris, and stored in freezer for further processing. All individuals from one creek site were assembled to make a composite sample from each location for trace metal analysis due to the small size and low weight of crabs. All crab samples were oven-dried to constant weight, grounded, and homogenized. Approximately, 0.5 g of dry sample was added to 10 ml distilled water and mixed with 5 ml of concentrated hydrochloric acid (HCl) and 2 ml of nitric acid (HNO3) in sequence. The mixture was heated to near dryness, resulting in a concentrated yellow fluid. Subsequently, 10 ml hydrogen peroxide (H2O2) was added. Samples were diluted to 50 ml using distilled water, after which the resulting solution was filtered and analyzed for metals [35, 49].
Samples (crab and sediment) were analyzed for metals using an atomic absorption spectrometer (Perkin Elmer (USA) A Analyst 700). The quality of the analytical methodology was assured through the use of calibration curves with standards, blanks, and replicates. Firstly, metal standard solutions of different concentrations such as 2, 4, and 6 ppm were prepared from a certified stock solution of 1000 mg/L for each metal via dissolving their suitable salts in deionized water. Secondly, the blank sample was prepared by adding the acid solution without a real sample. Thirdly, replicate samples were also used to assure the analytical quality. All analytical grade chemicals were utilized in the study. All metals detected in sediment and crabs by AAS achieved good precision (1–5% RSD), except Co for which precision (moderate) ranged from 5 to 16% RSD. The achieved % RSD were: Fe (0.96–1.26%), Zn (0.33–1.65%), Cu (0.81–3.72%), Ni (0.99–4.20%), Co (5.33–15.55%), Cr (1.04–3.79%), Pb (0.69–3.7%), and Cd (0.35–4.24%).
2.4 Sediment contamination and risk analyses
Contamination factors (Cf) and contamination degrees (Cd) were evaluated following Hakanson [28] to identify the contamination levels by eight metals in the coastal sediment of Karachi, Pakistan.
The Cd is the sum of all contamination factors, and Cs and Cb are the concentration of a specific metal in the sample and background, respectively. The pre-industrial or baseline values of studying metals are not available for the selected site; therefore, the reference values of studying metals were taken from the Earth’s average values of clays from sedimentary rocks (AVCSR) stated by Turekian and Wedepohl [75]. The contamination factor is categorized into four classes according to contamination severity: Class I = low contamination (Cf = < 1; Cd = < 8), Class II = moderate contamination (Cf = 1–3; Cd = 8–16), Class III = considerable contamination (Cf = 3–6; Cd = 16–32) and Class IV = very high contamination (Cf = > 8; Cd = > 32).
The potential ecological risk index is the summation of all risk factor for each metal and utilized as a sign to highlight the harmful effects of all studied metals [28, 53]:
The ecological risk factor (ERi) is classified into five grades according to risk: Class I = low (ERi = < 40), Class II = moderate (ERi = 40–80), Class III = considerable (ERi = 80–160), Class IV = high (ERi = 160–320), and Class V = very high (ERi = > 320), whereas the potential ecological risk index (RI) tier into following categories: Class I = low (RI = < 150), Class II = moderate (RI = 150–300), Class III = considerable (RI = 300–600), and Class IV = high (RI = > 600).
2.5 Accumulation factor (AF)
Accumulation factors (AFs) were used for investigating bioavailability of each trace metal present in the sediment to crabs [69]. It is a ratio of concentrations of a specific metal (MC) present in crabs and sediments (AF = MCCrab/MCSediment). An AF value less than one (< 1.0) designates no bioaccumulation of heavy metals in crabs, while an AF greater than one (> 1.0) indicates active bioaccumulation of heavy metals in crabs.
2.6 Statistical analysis
Spatial differences in trace metal concentrations in sediments and crabs were compared by one-way ANOVA followed by Tukey’s comparison test. Pearson’s correlation analysis was used to determine the relations of environmental characteristics and metal distribution in sediments and crabs. Regression analysis was conducted to compare metal levels in crabs and their exposure concentration in sediments. Moreover, the relationship between accumulation factor (AF) and metal concentration in sediments was also determined. All statistical analysis was performed using Minitab (version 17.0).
3 Results and discussion
3.1 Hydrographic properties
Hydrographic characteristics (temperature, salinity, and pH) of pit water at three locations of the Karachi coast are presented in Table 1. The temperature exhibited evenness among the sites and ranged from 24.60 to 24.70 °C. The results were consistent with the other previous studies [2, 66]. The high salinity of pit water was observed in this study was in the range 52.50 –67.50‰ at S1, 50.0–55.0‰ at S2, and 40–50‰ at S3, suggesting diminutive influence of freshwater in the environment as mentioned in the earlier studies [48, 66]. The spatial distribution of pH varied in the range 6.85–7.10, 5.79–5.80, and 6.30–6.50 at S1, S2, and S3 respectively, and showed significant differences (F 2, 8 = 120.94, p < 0.001) among monitoring sites. A slightly acidic environment found at S2 as compared to the other two sites may have resulted from the decaying of the domestic and industrial waste litter in the study site contributing to the acidic nature of the groundwater. The reported results also showed alignment to the previous studies [48, 66, 72].
3.2 Physical properties of sediments
The percentage of organic matter (OM) in tidal creek sediments ranged from 1.26% to 2.59%. The lowest percentage of OM was examined at S3, and the highest mean value was observed at S2, but no noteworthy differences were detected among sites (Table 1). According to Marin et al. [42], the high-quality sediments have less than 5% of organic matter, conversely poor-quality sediments have > 10% of organics. In comparison with this ecological quality status, all three sites revealed good quality of sediments. The grain size analysis of sediments among the three coastal sites revealed different comparative proportions of granules, sand, and mud (Table 1). Granules varied from 1.86 to 4.36% and decreased from S1 to S3. Conversely, the percentage of sand ranged from 54.72 to 85.70% and increased from S1 to S3. Like granule content, the percentage of mud also showed a decreasing trend from S1 to S3 (range across the three sites: 12.75–41.32%). Significant variations (p < 0.05) were observed in grain size composition among the creeks (Table 1). Granulometric analysis contributes to the elucidation of sediment origination, their transport history, and depositional conditions in a certain area [26]. In general, trace metal concentrations and grain size are inversely related, as metal concentrations increase with reducing the size of sediment particles, mainly due to their high surface area and organic matter content [17, 30].
3.3 Sediment contamination and ecological risk analyses
The metal concentrations in sediments from the tidal creeks of Karachi are illustrated in Table 1. The concentrations of Fe, Zn, Cu, Cr, Co, Pb, Ni, and Cd varied in the range of 1006–1019, 58.97–104.82, 28.50–37.21, 85.3–137.6, 3.86–5.61, 31.63–157.91, 17.45–47.79, and 0.39–1.21 µg g−1, respectively. The values of Zn, Cr, and Pb were considerably higher at S3, whereas Ni and Cd were significantly lower at S3 compared to the other two sites. No significant variations (p > 0.05) were observed in Fe, Cu, and Co levels in the creek sediments (Table 1). The metal contamination in tidal creek sediments was also evaluated through contamination factors and the contamination degree (Fig. 2). The contamination factor (Cf) for Cu, Z, Ni, Cr, Co, Pb, and Cd varied among the creeks. Moreover, according to Cf classification, there was low contamination by Cu, Zn, Ni, and Co, moderate contamination by Cr, and considerable contamination by Pb and Cd in the creek sediments (Fig. 2a). Noteworthy variations (p < 0.05) were observed in Cf for Zn, Ni, Cr, Pb, and Cd among the creek areas. The high Cf of most of the studied metals disclosed that they play an important role in coastal contamination through adsorbing and accumulating in sediments and are a potential threat to sedimentary biota as well as ecological well-being [37, 66]. Therefore, analysis of sediment contamination increases understanding of past exposure to contaminants as well as current status of vulnerable coastal areas. According to the contamination degree, the three creek areas were categorized as considerably contaminated (Fig. 2b), which ranged from 8.82 to 13.06 and showed significantly higher (p < 0.001) values in sediments of S3 compared to S1 and S2. The entire Korangi/Phitti, Kadiro, and the Gharo creek area forms an interrelated and complex creek system. This creek system receives industrial and domestic effluent waste from various point and non-point sources which contribute the main sources of pollution in the southeast coast of Karachi [56].
Spatial variation in heavy metal contamination factor (a), contamination degree (b), ecological risk factor (c), and ecological risk index (d) in the coastal sediments of Karachi, Pakistan (Roman letters show the class of the sediment contamination and risk, i.e., I = low, II = moderate, III = considerable and, IV = high)
Most of the metals showed a similar pattern in tidal sediments of S1 and S2. S1 was located in Gharo Creek adjacent to Port Bin Qasim and S2 was situated on Kadiro Creek, which is between the Gharo Creek and Korangi Creek. Port Bin Qasim is the biggest port of Karachi, which is comprised of the largest steel mill (Pakistan Steel Mill), fertilizer plants, vehicle manufacturing plants, thermal power plants, and the largest container terminal and seaport, all of which influence concentrations of organic and inorganic pollutions in the nearby environment [68]. In the current study, S3 was categorized as the most contaminated site. It is situated in the Korangi Creek area, which is mostly affected by the Malir River runoffs. This comprises effluents from adjacent industrialized areas such as Landhi Industrial and Trading Estate (LITE) and Korangi Industrial and Trade Estate (KITE) that contain the waste from hundreds of different industries such as oil refineries, chemical industries, petrochemicals, metal industries, pharmaceuticals, tanneries, and textile industries [64, 68].
The results of ecological risk factor (ERi) presented the highest values of Cd at all studied sites (Fig. 2c). At S1 and S2, Cd posed a considerable ecological risk; however, it generates low risk at S3. The results clearly showed the detrimental effects of Cd on the faunal well-being. The potential ecological risk index (RI) indicated that the decreasing trend of risk toward S1 to S3 (Fig. 2d), and all sites exhibited low ecological risk. If we compared the results of contamination degree and ecological risk index, they showed contrasting mainly due to the high-risk factor of Cd, which shifted the burden on the S3 as compared to other sites. The overall trend of both indices revealed the trace metal pollution in sediments and ultimately their transferences and toxicity in the resident biota.
3.4 Metal concentrations in resident crab (Ilyoplax frater)
I. frater is a deposit and bottom feeder crab and directly interacts with the sediments in its habitat over time. All metal concentrations in Dotillid crabs exhibited variable accumulation patterns and were considerably different (p < 0.05) among the three studied populations. Most of the metals (Cu, Fe, Zn, Cr, and Cd) were observed in significantly greater levels in crabs at S2, whereas Ni and Pb showed substantially higher accumulation in crabs at S3 (Table 2). Generally, benthic organisms may take up trace metals from contaminated sediment in relatively large amounts, hence playing an important role in the potential transfer of these contaminants into the food chain [12, 16, 37, 66]. Indeed, as bottom feeders, these crabs are generally expected to concentrate more metals than surface feeders. But in this study, crabs showed different accumulation behavior with respect to sediment contamination. This variation in bioaccumulation of metals is, in part, an indication of the degree to which certain species picks up particulate matter during feeding from its surrounding sediments [4].
Table 3 shows mean concentrations of trace metals in I. frater in comparison with other reported data for crab species from different parts of the world. The concentrations of most of the metals in I. frater are comparable with those recorded for Austruca sindensis [65] and Opusia indica [69] from coastal areas of Pakistan. The concentrations of some metals such as Fe and Cu in I. frater are comparable with those recorded for Uca tangeri [6] and Macropthalmus depressus [13]. Zn and Cd concentrations in I. frater were lower than M. depressus [13] and Ilyoplax deschampsi [29]. However, concentrations of Co, Ni, Cr, and Pb in I. frater were higher than the values stated for all other crab species (Table 3). The highest level of these metals in crabs was recorded probably due to the inclusion of whole crabs (with an exoskeleton) in the current study. The exoskeleton of crabs composed of chitin, calcium carbonates, and proteins. The acetamido groups of chitin are competent to act as non-specific chelators as they bind heavy metals through hydrogen bonding [3, 52]. Therefore, it is quite challenging to comprehend metal levels in small-sized organisms that cannot easily be partitioned into tissues and exoskeleton. As stated by ecological perspective, deposit-feeding crabs, like I. frater, characterize the link between detritus and consumers at higher trophic levels, which nourish on them [1, 60]. In marine sediments, the three main benthic components involved in energy flow are the microbial community, the meiobenthos, and the macro deposit feeders. Montagna [45] reported that both heterotrophic microbial and autotrophic standing stock (about 1 to 3%) is consumed by meiobenthos, which is the main food source for macro deposit feeders. Deposit-feeding crabs are an important link to higher trophic level in the food web of the intertidal environment. They transform detritus into secondary consumers (predators) such as terrestrial and aquatic organisms. This direct conversion of detritus to biomass is perhaps the main source of energy transfer to carnivore populations [59].
The accumulation factor (AF) for all studied metals in the crabs is shown in Fig. 3. Mean AF values of trace metals in crabs decreased in the order Cu (3.7) > Cd (3.2) > Co (3.1) > Zn (1.4) > Ni (1.0) > Pb (0.9) > Fe (0.4) > Cr (0.1, Fig. 3). AFs higher than 1.0 were found for Zn, Cu, Ni, Co, and Cd and indicated bioaccumulation of these metals. For decapod crustaceans, Cu and Zn are essential elements for hemocyanin and enzymatic activity; consequently, these metals are regulated to specific threshold [10, 54]. Once these edges are touched, the regulatory procedure of metals becomes saturated and accumulation begins to occur [54], Engel and Brouwer [25]. This could explain the low levels of Cu and Zn in the sediments, but high concentrations as well as their high AFs in the crabs. Ni, Co, and Cd are non-essential metals, but were also higher than 1.0. Non-essential metals are not regulated and accumulation can occur at all concentrations [9, 54]. According to Bondgaard and Bjerregaard [7], the molting of the crab (Carcinus maenas) did not reduce Cd levels in their body, which may also explain the high AF for non-essential and toxic metals in the current study. Moreover, high AFs of Zn, Ni, Cu, Co, and Cd also indicate the usefulness of I. frater, as an active accumulator of these metals.
3.5 Influence of environmental heterogeneities (hydrographical and sedimentological) on trace metal concentrations in the sediment and crabs
The environmental characteristics play a significant role in the trace metal distribution in sediment as well as their accumulation in biota; therefore, these associations were identified by Pearson’s correlation coefficient (Table 4). Firstly, we discussed the influence of hydrographical properties (temperature, salinity, and pH) and sedimentological traits (organics, sand, and mud) on trace metal levels in sediments. In the current study, temperature showed no correlation with any metal concentration in sediments. However, salinity was observed negative correlation with Ni levels in the sediments, indicating that it acts protectively toward the Ni toxicity, which is consistent with the previous studies [23, 34]. Generally, free ionic metal concentrations are reduced in high-salinity environments in comparison with low salinity because of the increased presence of complexing anions. For instance, the two anions (SO42− and Cl−) are noteworthy in seawater for nickel [34, 58]. Besides, at higher salinities, there should be increased competition with metal ions by protective cations such as Na+, Mg2+, and Ca2+ for binding to sites at the biotic ligand (Leonard et al. [34] and reference therein).
The pH showed significant positive correlations with Fe and Pb but negatively correlated with Cd indicating that the pH seems like a potential facilitator in the distribution of these metals. These results consistent with previous studies such as Elizabeth Botte et al. [24] also reported a significant positive correlation between pH and the Pb in sediments within the Bahia Blanca Estuary. These results were also consistent with previous reports by Sun et al. [73] and Tian et al. [74], as reported significant negative associations between pH and metal levels in sediments.
According to several studies, sedimentological properties can affect the distribution and circulation of trace metals in sediments [74] and references therein). In the current study, % organic matter was positively correlated with only Cu levels in sediments indicating that it is not the controlling factor for the distributions of metals in the tidal creek sediments. The concentrations of Zn and Pb were correlated significantly with sand proportion; contrariwise Cd showed a positive correlation with mud quantity in the sediments (Table 4), indicating that these metals were sensitive to variations in grain size composition due to the affinity through adsorption or complexation of the sedimentary matrix. Previous studies also showed a significant relationship between grain size and heavy metals [64, 82].
As I. frater has a close relationship with the sediment properties as stated in the previous research [60], Saher et al. [62]; therefore, it is worthy to assess the impacts of environmental heterogeneities on metal accumulation in this crab. In the current study, temperature and salinity exhibited no significant correlations with any metal accumulation. However, the pH showed significant positive correlations with Ni, Pb, and Cd accumulation in crab, but it established a significant negative correlation with Co, Zn, Fe, and Cu (Table 4). The results signify that most of the studied metals were sensitive to pH, and it acts as a controlling factor in the mobility of these metals in the coastal sediments. According to Ma [40], Cd and Zn levels in worms appeared to the pH-sensitive, and metal desorption was increased at low pH owing to the competition with H + ions. Perämäki et al. [51] also described that Cd accumulation increased at lower pH values in earthworm (Aporrectodea caliginosa), and they suggested that a slight increase in Pb accumulation exhibited in the most acidic treatments.
Among the sedimentological traits, organic matter presented only a strong positive correlation with Co accumulation in crab (Table 4). However, sediment grain size has a strong influence on metal accumulation in I. frater as compare to organic matter. The concentrations of Zn, Fe, Cu, and Cr in I. frater perceived considerable correspondence with mud proportions, but negative correlations appeared with sand. In contrast, Cd accumulation in crabs has a positive correlation with sand. On the other hand, it presented a negative correlation with mud. So that’s why the Cd has more vigorously accumulated by crabs in KC, where the sand was higher as compared to the other two locations. These animals can selectively ingest organic-rich fine-grained food particles, and the influx of contaminant will increase excessively because of the higher contaminant concentration associated with these fine particles. Metal bioaccumulation is determined by the feeding rate of an organism and metal concentration in ingested food particles; therefore, these factors are responsible for the relationship between metal bioaccumulation and organic content and particle size. Particle selectivity of deposit-feeding organisms can considerably affect the accumulation of contaminants (Wang et al. 1999). Furthermore, particle size and organic content established an inverse relationship on metal concentration in marine deposit feeders in field studies [12]. According to Rainbow (2002), trace metal accumulation depends on several features, for instance, substratum type, physical and chemical characteristics of the environment, the bioavailability of contaminants, feeding strategies, and physiology of an organism. Our results also highlighted that metals accumulation in I. frater were sensitive toward the pH of pore water and sediment grain size in the coastal areas.
3.6 Relationship between metal loads in sediment and crab
Three different scenarios were reported among the interactions of trace metals in two matrixes, i.e., crabs (C) and sediments (S) (Fig. 4). Firstly, metals were found meaningfully (p < 0.05) higher in the creek sediments than Dotillid crabs (S > C) as seen in the cases of Fe and Cr. In the second pattern, a noticeably higher metal load was evaluated in crabs as compared to sediments (C > S). For instance, Cu, Co and Cd were assessed 80%, 70%, and 60% higher, respectively, in crabs than creek sediments (Fig. 4) indicative of the active bioaccumulation of these elements in I. frater. The bioavailability of contaminants in sediments may allow the accumulation of essential and non-essential elements in benthic organisms in higher amounts than the sediments and promote toxic effects in biota [21]. Lastly, no substantial variances was found between metal levels in sediments and crabs (S ≈ C or C ≈ S) as perceived in the cases of Zn, Ni, and Pb.
The interferences of trace metals between crabs and sediment were further highlighted by regression analysis (Table 5). The substantial inverse correlation between Fe (R2 = 0.451) and Ni (R2 = 0.541) levels in sediments and crabs implies lower accumulation in a higher exposure environment. However, a noteworthy linear correlation (R2 = 0.988) between Pb concentrations in sediments and crabs presented active accumulation in crabs in the high-exposure environment. By contrast, no substantial interactions were perceived for Cu, Zn, Co, Cr, and Cd in sediments and crabs. This may be attributed to complex relationships between sediments and crabs regarding metals.
The relationship between accumulation factors (AFs) of metals in crab correspondence with an exposure concentration in sediments was further evaluated. These results indicated strong relationships between AFs and metal levels in sediments as compared to the relationship based on metal concentrations in crabs and sediments discussed previously (Table 5). All metals presented substantial correlations (p < 0.05) between AFs and exposure metals concentrations in sediment, except Cu. Furthermore, all significant relationships were inverse associations, except Pb that showed a positive linear correlation. Inverse relationships between accumulation factor and exposure concentrations are discussed in few previous studies [19, 44, 47]. The authors mentioned that this relationship is a result of multiple mechanisms such as active regulation [80], the influence of natural background levels [8] or saturation of uptake kinetics at threshold [71]. A strong linear relationship was observed between Pb concentrations in sediments and crabs (Table 5). Lead is non-essential metal and tends to be detoxified via metallothioneins or phosphate granules and deposited in the muscles of an organism [41]. Similar accumulation strategies have been perceived in the Ocypodid crab, Mictyris longicarpus (solider crab) from Australian coastal areas, which exhibits Zn and Cu regulation and Pb accumulation [79]. The positive linear correlations indicated the bioavailability of Pb in the sediment as well as capacity of Dotillid crabs to integrate Pb in their body that made them potential indicator species toward Pb pollution monitoring programs.
4 Conclusion
Trace metal concentrations in sediments of three moderately contaminated tidal creeks were evaluated. Significant differences were observed in Zn, Ni, Cr, Pb, and Cd concentrations in sediments among creeks. Moderate contamination of Cr and considerable contamination of Pb and Cd were detected in sediments. Korangi Creek sediment showed the highest degree of contamination as compared to the other two creek areas. However, the low ecological risk was exhibited at all sites, but they all have a considerable risk of Cd. Biomonitoring studies were conducted through the deposit-feeding crab, I. frater, and crabs exhibited significant spatial variability in metal accumulation. The concentrations of Co, Cu, and Cd were significantly greater in crabs as compared to exposure concentrations of these metals in sediments. The AFs of Cu, Cd, Co, Zn, and Ni were > 1.0, which indicates that this crab species acts as a potential accumulator for these metals. The environmental heterogeneities revealed themselves as an influential factor in controlling the distribution of metals in the creek sediments as well as in I. frater. The relationship between metal levels in sediments and crabs indicated that only Pb concentrations in crabs showed a linear relationship with exposure concentration and revealed that it may be a possible indicator species for Pb contamination. Further biomonitoring approaches are indispensable to be conducted through various other species to comprehend and envisage the complex relationship between the biota and sediment in a contaminated environment.
References
Ashton EC, Macintosh DJ, Hogarth PJ (2003) A baseline study of the diversity and community ecology of crab and molluscan macrofauna of the Sematan mangrove forest, Sarawak, Malaysia. J Trop Ecol 19:127–142
Barkati S, Rahman S (2005) Species composition and faunal diversity at three sites of Sindh mangroves. Pakistan J Zool 37:17–31
Barriada JL, Herrero R, Prada-Rodriguez D, Sastre ME (2007) Waste spider crab shell and derived chitin as low-cost materials for cadmium and lead removal. J Chem Tech Biotech 82:39–46
Bastami AA, Khoei JK, Esmailian M (2012) Bioaccumulation of heavy metals in sediment and crab, Portunus pelagicus From Persian Gulf. Iran Middle-East J Sci Res 12(6):886–892
Besser JM, Brumbaugh WG, Allert AL, Poulton BC, Schmitt CJ, Ingersoll CG (2009) Ecological impacts of lead mining on Ozark streams: toxicity of sediment and pore water. Ecotoxicol Environ Saf 72:516–526
Blasco J, Arias AM, Sáenz V (1999) Heavy metals in organisms of the River Guadalquivir estuary: possible incidence of the Aznalcόllar disaster. Sci Tot Environ 242:249–259
Bondgaard M, Bjerregaard P (2005) Association between cadmium and calcium uptake and distribution during the moult cycle of female shore crabs, Carcinus maenas: an in vivo study. Aquat Toxicol 71:17–28
Borgmann U, Norwood WP (1995) Kinetics of excess (above background) copper and zinc in Hyalella azteca and their relationship to chronic toxicity. Can J Fish Aquat Sci 52:864–874
Brouwer M, Lee RF (2007) Responses to toxic chemicals at the molecular, cellular, tissue, and organismal level. In: Kennedy VS, Cronin E (eds) The Blue Crab, Callinectes sapidus. Maryland Sea Grant College, College Park, pp 565–654
Bryan GW (1971) The effects of heavy metals (other than mercury) on marine and estuarine organisms. Proc R Soc Lond Ser B Biol Sci 177:389–410
Bryan GW (1979) Bioaccumulation in marine organisms. Philos Trans R Soc Lond Ser B Biol Sci 286:483–505
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries—a review. Environ Pollut 76:89–131
Bu-Olayan AH, Subrahmanyam MNV (1998) Trace metal concentrations in the crab Macrophthalmus depressus and sediments on the Kuwait Coast. Environ Monit Assess 53:297–304
Cammen LM (1980) Ingestion rate: an empirical model for aquatic deposit feeders and detritivores. Oecologia 44:303–310
Chapman PM, Wang F, Janssen C, Persoone G, Allen HE (1998) Ecotoxicology of metal in aquatic sediment: binding and release, bioavailability, risk assessment and remediation. Can J Fish Aquat Sci 55:2221–2243
Chapman PM, Wang FY (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Chaudhary MZ, Ahmad N, Mashiatullah A, Ahmad N, Ghaffar A (2013) Geochemical assessment of metal concentrations in sediment core of Korangi Creek along Karachi Coast, Pakistan. Environ Monit Assess 185:6677–6691
Chen M, Chen C, Chou H, Wen T (2005) Gender and size effects of metal bioaccumulation on the rock crab, Thalamita crenata, in Dapeng Bay, southwestern Taiwan. Mar Pollut Bull 50:463–484
DeForest DK, Brix KV, Adams WJ (2007) Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat Toxicol 84:236–246
Dixit SS, Witcomb D (1983) Heavy metal burdens in water, substrate and macro-invertebrate body tissue of a polluted River Irwell (England). Environ Pollut 6B:161–172
Eça GF, Pedreira RMA, Hatje V (2013) Trace and major elements distribution and transfer within a benthic system: Polychaete Chaetopterus variopedatus, commensal crab Polyonyx gibbesi, worm tube, and sediments. Mar Pollut Bull 74:32–41
Eisler R (1981) Trace metal concentrations in marine organisms. Pergamon Press, New York
Eisler R (1998) Nickel Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. 1998–0001. Biological Science Report. United States Geological Survey, Biological Resources Division, Washington, D.C.
Elizabeth Botte S, Hugo Freije R, Eduardo Marcovecchio J (2010) Distribution of several heavy metals in tidal flats sediments within Bahia Blanca Estuary (Argentina). Water Air Soil Pollut 210:371–388
Engel DW, Brouwer M (1991) Short metallothionein and copper changes in blue crabs at Ecdysis. Biol Bull 180:447–452
Folk RL, Ward WC (1957) Brazos river bar: a study in the significance of grain size parameters. J Sediment Petrol 27:3–27
Gbaruko BC, Friday OU (2007) Bioaccumulation of heavy metals in some fauna and flora. Int J Environ Sci Techol 4(2):197–202
Hakanson L (1980) An ecological risk index for aquatic pollution control A sedimentological approach. Water Res 14:975–1001
He W, Lu J (2001) Distribution of Cd and Pb in a wetland ecosystem. Sci China 44:178–184
Horowitz E, Elrick K (1987) The relation of stream sediment surface area, grain size and surface area to trace element chemistry. Appl Geochem 2:437–451
Ismail A, Badri MA, Ramlan MN (1991) Heavy metal contamination in fiddler crabs (Uca annulipes) and hermit crabs (Clibanarius sp.) in a coastal area of northern peninsular Malaysia. Environ Technol 12(10):923–926
Karthe D (2017) Environmental changes in central and East Asian Drylands and their effects on major river-lake systems. Quat Int. https://doi.org/10.1016/j.quaint.2017.01.041
Kitaura J, Wada K (2006) New species of Ilyoplax (Brachyura: Ocypodidae: Dotillinae) from the Philippines and Indonesia: behavioral, molecular and morphological evidence. Bull Raffle Mus 4:373–379
Leonard EM, Barcarolli I, Silva KR, Wasielesky W, Wood CM, Bianchini A (2019) The effects of salinity on acute and chronic nickel toxicity and bioaccumulation in two euryhaline crustaceans: Litopenaeus vannamei and Excirolana armata. Comp Biochem Physiol C: Toxicol Pharm 154(4):409–419
Leung KMY, Furness RW (1999) Effect of animal size on concentrations of metallothionein and metals in periwinkles Lithorina litorea collected from the fifth of Clyde, Scotland. Mar Pollut Bull 39:126–136
Lim BL (1997) Preliminary results on the effects of salinity and settling condition on megalopal metamorphosis of fiddler crab Ilyoplax pusilla. Hydrobiol 358:297–299
Lin Y, Chang-Chien G, Chiang P, Chen W, Lin Y (2013) Multivariate analysis of heavy metal contaminations in seawater and sediments from a heavily industrialized harbor in Southern Taiwan. Mar Pollut Bull 76:266–275
Luoma SN (1990) Processes affecting metal concentrations in estuarine and coastal marine sediments. In: Furness RW, Rainbow PS (eds) Heavy Metals in the Marine Environment. CRC Press, Inc., Boca Raton
Luoma SN, Rainbow PS (2005) Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Tecnol 39:1921–1931
Ma W-C (1982) The influence of soil properties and worm-related factors on the concentration of heavy metals in earthworms. Pedobiologia 24:109–119
MacFarlane GR, Booth DJ, Brown KR (2000) The Semaphore crab, Heloecius cordiformis: bio-indication potential for heavy metals in estuarine systems. Aquat Toxicol 50:153–166
Marin V, Moreno M, Vassallo P, Vezzulli L, Fabiano M (2008) Development of a multistep indicator-based approach (MIBA) for the assessment of environmental quality of harbors. ICES J Mar Sci 65:1436–1441
Mashiatullah A, Chaudhary MZ, Ahmad N, Javed T, Ghaffar A (2013) Metal pollution and ecological risk assessment in marine sediments of Karachi Coast, Pakistan. Environ Monit Assess 185:1555–1565
McGeer JC, Brix KV, Skeaf JM, DeForest DK, Brigham SI, Adams WJ, Green A (2003) Inverse relationship between bioconcentration factor and exposure concentration for metals: implications for hazard assessment of metals in the aquatic environment. Environ Toxicol Chem 22:1017–1037
Montagna PA (1984) In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Mar Ecol Prog Ser 18:119–130
Mountouris A, Voutsas E, Tassios D (2002) Bioconcentration of heavy metals in aquatic environments: the importance of bioavailability. Mar Pollut Bull 44:1136–1141
Na C-K, Park H-J (2012) Distribution of heavy metals in tidal flat sediments and their bioaccumulation in the crab Macrophthalmus japonicas in the coastal areas of Korea. Geosci 16:153–164
Naz F, Qureshi NA, Saher NU (2012) Seasonal variation in hydrographic parameters from the coastal waters of Pakistan. Pakistan J Mar Sci 21:37–50
Ololade IA, Lajide L, Olumekun VO, Ololade OO, Ejelonu BC (2011) Influence of diffuse and chronic metal pollution in water and sediments on edible seafood within Ondo oil-polluted coastal region, Nigeria. J Environ Sci Health Part A 46(8):898–908
Pakzad HR, Pasandi M, Soleimani M, Kamali M (2014) Distribution and origin of heavy metals in the sand sediments in a sector of the Oman Sea (the Sistan and Baluchestan province, Iran). Quat Int 345:138–147
Peramaki P, Ithamies J, Kopltunen V, Lajunen LHJ (1992) Influence of pH on the accumulation of cadmium and lead in earthworms (Aporrectodea caliginosa) under controlled conditions. Ann Zool Fenn 29:105–111
Pinto PX, Al-Abed SR, Reisman DJ (2011) Biosorption of heavy metals from mining influenced water onto chitin products. Chem Eng J 166:1002–1009
Proshad R, Kormoker T, Islam MS (2019) Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa River, Bangladesh. Toxin Rev. https://doi.org/10.1080/15569543.2018.1564143
Rainbow PS (1985) Accumulation of Zn, Cu, and Cd by crabs and barnacles. Estuar Coast Shelf Sci 21:669–686
Rainbow PS (1990) Heavy metal levels in marine invertebrates. In: Furness RW, Rainbow PS (eds) Heavy metals in the marine environment. CRC Press, Boca Raton
Rizvi SHN (1997) Status of Marine Pollution in the context of coastal zone management of Pakistan. In: Haq BU, Haq SM, Kullenberg G, Stel JM (eds) Coastal zone management Imperative for maritime developing Nations. Kluwer Academic Press, Amsterdam
Rizvi SHN, Saleem M, Baquer J (1988) Steel mill effluents influence on the Bakran Creek environment. In: Thompson MF, Tirmizi NM (eds) Proceedings of marine science of the Arabian Sea. American Institutes of Biological Sciences, Washington
Sadiq M (1989) Nickel sorption and speciation in marine environment. Hydrobiologia 176(177):225–232
Saher NU (2008) Population dynamics and biology of fiddler crab in the mangrove area of Karachi coast (PhD. thesis) University of Karachi, Karachi, Pakistan.
Saher NU, Qureshi NA (2010) Zonal distribution and population biology of Ilyoplax frater (Brachyura: Ocypodoidea: Dotillidae) in a Coastal Mudflat of Pakistan. Curr Zool 56:244–251
Saher NU, Qureshi NA (2011) Relative growth and morphological sexual maturity of Ilyoplax frater (Brachyura: Ocypodoidea: Dotillidae) from mangrove area of Korangi Creek. Pak J Zool 43:133–140
Saher NU, Qureshi NA, Aziz U (2014) Spatial distribution and substrate preference pattern of Ilyoplax frater along intertidal areas of Pakistan. Pakistan J Mar Sci 23(1&2):33–43
Saher NU, Qureshi NA, Siddiqui AS (2018) Influence of sediment characteristics on density and distribution of Ocypodoid crab burrows (superfamily: ocypodoidea) along the coastal areas of Pakistan. Acta Ecol Sin 38:234–241
Saher NU, Siddiqui AS (2016) Comparison of heavy metal contamination during the last decade along the coastal sediment of Pakistan: Multiple pollution indices approach. Mar Pollut Bull 105:403–410
Saher NU, Siddiqui AS (2017) Evaluation of heavy metals contamination in mangrove sediments and their allied fiddler crab species (Austruca sindensis Alcock 1900) from Hawks Bay, Karachi. Pak Int J Biol Biotechnol 14(3):411–417
Saher NU, Siddiqui AS (2019) Occurrence of heavy metals in sediment and their bioaccumulation in sentinel crab (Macrophthalmus depressus) from highly impacted coastal zone. Chemosphere 221:89–98
Sheaves M (2009) Consequences of ecological connectivity: The coastal ecosystem mosaic. Mar Ecol Progr Ser 391:107–115
Siddique A, Mumtaz M, Zaigham NA, Mallick KA, Saied S, Zahir E, Khwaja HA (2009) Heavy metal toxicity levels in the coastal sediments of the Arabian Sea along the urban Karachi (Pakistan) region. Mar Pollut Bull 58:1406–1419
Siddiqui AS, Saher NU (2016) Assessment of heavy metal accumulation in Opusia indica (Alcock, 1900) (Ocypodoidea: Camptandriidae) with Reference to sediment contamination from Coastal areas of Pakistan. Int J Biol Res 4(2):56–61
Silva JD, Srinivasalu S, Roy PD, Jonathan MP (2014) Environmental conditions inferred from multi-element concentrations in sediments off Cauvery delta, Southeast India. Environ Earth Sci 71:2043–2058
Simkiss K, Taylor MG (1989) Metal fluxes across membranes of aquatic organisms. Reviews in Aquat Sci 1:173–188
Sultana R, Mustaquim J (2003) Some physical parameters of the Sandspit backwaters, Karachi coast. Pak J Sci Ind Res 46(5):333–343
Sun X, Fan D, Liu M, Tian Y, Pang Y, Liao H (2018) Source identification, geochemical normalization and influence factors of heavy metals in Yangtze River Estuary sediment. Environ Pollut 241:938–949
Tian K, Wu Q, Liu P, Hu W, Huang B, Shi B, Zhou Y, Kwon B, Choi K, Ryu J, Khim JS, Wang T (2020) Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ Inter 136:105512
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth's crust. Geol Soc Am Bull 72: 175–192
Vermeiren P, Abrantes K, Sheaves M (2015) Generalist and specialist feeding crabs maintain discrete trophic niches within and among estuarine locations. Estuar Coast 38:2070–2082
Vermeiren P, Sheaves M (2015) Predictable habitat associations of four crab species across the low intertidal landscape of a tropical estuary over time. Estuar Coast 38:285–295
Vermeiren P, Sheaves M (2015) Modeling intertidal crab distribution patterns using photographic mapping among tropical Australian estuaries. Estuar Coast 38:1545–1556
Weimin Y, Batley GE, Ahsanullah M (1994) Metal bioavailability to the soldier crab, Mictyris longicarpus. Sci Tot Environ 141:27–44
White SL, Rainbow PS (1982) Regulation and accumulation of copper, zinc and cadmium by the shrimp Palaemon elegans. Mar Ecol Progr Ser 8:95–101
Zheng S, Wang P, Wang C, Hou J, Qian J (2013) Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quat Int 286:94–102
Zhuang W, Gao X (2014) Integrated assessment of heavy metal pollution in the surface sediments of the Laizhou Bay and the coastal waters of the Zhangzi Island, China: comparison among typical marine sediment quality indices. PLoS ONE 9(4):1–16
Acknowledgements
The current study was supported by the World Wildlife Fund for Nature, Pakistan, under the project, WWF/PF/HA (11) /86 to Noor Us Saher, which we gratefully acknowledge. We are grateful to the Centralized Science Laboratory (CSL), University of Karachi, for heavy metals analysis through atomic absorption spectrometer (Perkin Elmer (USA), model A Analyst 700). We are thankful to Peter Vermeiren (Department of Environmental Science, Radboud University, Netherlands) for smoothing language. The authors also appreciate the contribution of Mustafa Kamal (M.Phil. Zoology) for the production of the map of study sites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siddiqui, A.S., Saher, N.U. Interferences of trace metals between sediment and Dotillid crab (Ilyoplax frater) from three tidal creeks, Karachi, Pakistan. SN Appl. Sci. 3, 109 (2021). https://doi.org/10.1007/s42452-020-04041-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-04041-x