Abstract
Essential trace metals are well known for their environmental toxicity and for being part of complex bio-chemical cycles. Their role as critical micronutrients, delivering vital health benefits, is less widely discussed and understood, holding back strategies for combatting malnutrition. Crops grown on many Indian soils suffer from deficiencies in essential metals, notably iron (Fe), zinc (Zn), and molybdenum (Mo). The list of deficient metals will likely grow due to increasing future crop demand. Geostatistical analysis of soils and farmyard manure (FYM), the predominant fertiliser, implies that residual oxide minerals carry high concentrations of the essential trace metals Fe, Zn, copper (Cu), chromium (Cr), nickel (Ni), cobalt (Co), manganese (Mn) not only in soil but also in FYM (especially Fe, Cr, Cu, Co and Ni). A geochemical survey across a road traverse of 600 km, encompassing an area of c. 15,000 km2, was conducted in Central India to evaluate reported essential metal deficiency in key agricultural topsoils. Importantly, our evaluation of the element cycling in this system reveals that despite high bulk concentrations, some key metals remain bio-unavailable. In effect, the existence of refractory (weathering-resistant) oxides is likely a significant factor for deficiency symptoms in the soil–plant-fertiliser cycle. Further, mass balance calculations of the bioavailable pool of metals imply that only Fe and Mn are present in sufficient quantities to combat deficiency problems. Notwithstanding this limitation of FYM, its high organic carbon content, as well as its importance for Zn, Cu and Fe, validates its traditional use to maintain the fertility and physical condition of Indian topsoils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Essential metals are often referred to as ‘heavy metals’ in the literature and there is ample evidence that high concentrations in soils cause risks to human health, plants, animals and ecosystems. Heavy metals can present risks to food security in a variety of ways, including direct ingestion, contact with contaminated soil, introduction into the food chain, contamination of groundwater, reduction in food quality via phytotoxicity (disruption of cell membrane functions, chlorosis, and necrosis) and harm to soil microorganisms [1]. However, in small quantities, essential metals are indispensable micronutrients for plants, animals and humans [2]. They are normally present in animal and plant cells as well as tissues, and are a necessary part of their nutrition and physiology and the evaluation of deficiency levels consequently not less important for food security plans. The natural availability of essential metals is not ubiquitous or uniform, so that different regions of the world can have deficiencies and/or surfeit of one or more of these elements. These variations are being mapped to understand current and future agricultural potential given predicted future rise of population. Presently, the World Health Organisation (WHO) recognises only Fe as a crucial micronutrient for global health. However, Zn deficiency in soils also has consequential effects upon crops and ultimately human populations that over-depend on local crops [3,4,5]. Likewise, Mn deficiency is emerging as a threat to sustaining high levels of food production [6].
Advances in analytical chemistry offer growing awareness that other metals are bio-essential nutrients, most only at trace levels [7]. For instance, Cu, Mo and Co sustain growth and health of plants, animals and humans [8]; plant growth and bacteria require Ni [9]; and Cr(III) is discussed as being bio-essential [7] as a co-factor for insulin [10].
In India, the importance of the soil-crop-human micronutrient deficiency chain is documented for Zn, Fe, Mn and Mo [11, 12] with direct knock-on effect on the health of those people who rely on local crops as their main diet [13]. To date, burgeoning population growth has been sustained with the “Green Revolution”, referring to the increase in agricultural production since 1950 (Fig. 1) [6], yet it is anticipated that by the year 2050, 1.5 billion people will live in India, with many relying on local food sources. To increase yield, farmers are now cultivating high yielding varieties (HYV) of crops to meet the exploding food demand [14], resulting in additional removal of micronutrients from soils. It is likely that further essential metals (e.g., Ni, Cu) will become deficient in Indian soils. The latest developments in biofortification (e.g., the ability of rice to uptake high concentrations of Fe and Zn [15]; will cause further stress on the essential metal status of farmed soils as there is very little scope for expanding the area of cultivated land [6].
Impact of “The Green Revolution” in the past, present and future (illustrated as blue beams) on the emergence of micronutrient deficiencies (including essential metals) in crops in India (modified after [6]). In addition, the percentage of Zn deficient soils in the different agro-ecological zones of India [89] are illustrated according to the classification of the [83]. The overview map in the lower, right corner of the figure shows the Indian state territory in red colour
Despite the large number of humans at risk, little is known about the origin of deficiency of most essential metals. This includes the mineralogical composition of the soil and its geological substrate, parameters influencing plans for improved sustainable land use in large parts of India. Two basic geological factors appear underappreciated within the Indian context. First, earlier aggressive chemical weathering has affected large areas under intensive agricultural cultivation. Most essential metals are lost during the formation of new secondary soil minerals [16,17,18] and the residual regolith becomes strongly depleted (Supplemental Figure 1). Second, large tracts of the subcontinent are underlain by cratonic crust that has remained tectonically stable for a long time, limiting removal of the depleted residual soils via erosion. Within the tropics, soils become vertically separated from active rock breakdown by up to 25–50 m. On such soils, crops struggle to access freshly released nutrients at depth [19].
Notwithstanding these gaps in knowledge, there is growing appreciation for the need of strategies to combat essential metal deficiencies. Balanced fertilisation practices are now being developed, including customised fertiliser products, complex fertiliser formulations and organic matter enrichment [6]. Most small-scale Indian farmers use traditional farming practices, however, and cannot afford bespoke fertilisers, relying instead on FYM. Therefore, uptake and release of essential metals by grazing livestock is of interest [20] but few data exist for the bulk composition of FYM, resultant cycling of multi-essential trace metals, and the role of organic carbon (Corg).
This study aims to help closing this knowledge gap through mineralogical/geochemical investigation, complementing insight from pedology and agricultural studies. The aim was to explore how geochemistry could distinguish between bio-available and immobile pools of essential metals in soils, thereby assessing whether FYM contains sufficient bio-essential trace metals to offset deficiencies in Central Indian soils and informing if application of FYM could help to convert refractory soil metals into bioavailable fractions, as previously suggested for Zn and Cu [21].
2 Sampling strategy
2.1 Selection of the lithology and soil types of the study area
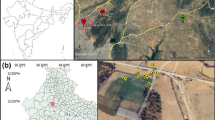
A reconnaissance-type sampling strategy achieved a representative and meaningful overview of the range of topsoils and cropping types over a large area in Central India. Twenty agricultural topsoils were sampled along a road traverse of c. 600 km, encompassing many lithological substrates (Fig. 1; Supplemental Figure 1), including: Deccan basalt (n = 11); clay-rich basaltic alluvium (n = 1); tonalite-trondhjemite-granodiorite (TTG) gneisses (n = 5); and grey shale (n = 3) (Table 1). Soils developed on Deccan basalt & alluvium, TTG, and grey shale classify as ‘vertisols’ (n = 17), whereas the lateritic materials developed over Deccan basalt are ‘ferralsols’ (n = 3). The investigated soils support a variety of crops (e.g., maize, rice, lentils, cotton, wheat, millet). At each GPS-recorded locality (Supplemental Table 1), 100–200 g of sample was taken from a depth of 5–15 cm and soil type, underlying geology and crop type (Table 1) were noted. It was anticipated that lithological control over metal depletion would be exposed by this sampling strategy. The studied samples cover four of the main Indian agro-ecological zones (Madhya-Pradesh, Maharashtra, Andhra Pradesh and Karnataka), all of which display some of the highest Zn deficiency (Fig. 1).
2.2 Chemical and mineralogical characteristics of vertisols and ferralsols
The study area is known for prolific (historic) cotton production, with vertisols locally called “black cotton soils”. The region of their main occurrence is the Deccan plateau between 15° and 25° north latitude and 73° and 80° east longitude [22]. Newbold [23] first described this soil type as chemically consisting of silica (SiO2) together with calcium (CaO), aluminium (Al2O3), iron (Fe2O3) and minor portions of vegetable and animal debris. Smectite is the dominant clay mineral in these vertisols. Non-expanding clay minerals (kaolinite, micas, chlorites, palygorskite and vermiculites) and carbonate (both pedogenic and non-pedogenic) also occur [24,25,26]. The typically dark colour of vertisols results from clay-organic complexes, whilst Fe–titanium (Ti)–Mn oxides also contribute to the pigmentation [27].
Most vertisols are inherently fertile and studies in India [28, 29] and elsewhere [30] have demonstrated that the high clay content exerts dominant control over essential pools of Fe, Mn, Zn, Cu, Co and Ni and their plant availability. The Corg content also correlates with extractable forms of essential metals (Zn, Cu, Mn, Fe) [29, 31], whereas soil pH (c. 7–8) and higher CaCO3 contents result in lower concentrations of Fe, Mn and Zn [30, 31]. In view of the high smectite content of the vertisols, it is surprising that the study area is among the most metal-deficient regions in Central India, indicating that only a small fraction of metals is bioavailable [31].
According to the UN Food and Agriculture Organization (FAO), ferralsols consist of highly weathered red- and yellow-coloured substrate arising from the accumulation of Fe- and Al oxides and oxy-hydroxides, with kaolinite a dominant mineral. These soils typically form on geologically old parent materials, which have lain exposed for long periods, and where the resulting intensive leaching has resulted in very low fertility [16, 18, 32, 33]. It is also likely that a fraction of essential metals redistributes into inert pedogenic or residual resistant original Fe oxides [33,34,35]. Therefore, while bulk composition may indicate otherwise, ferralsols often require addition of fertilisers to supply essential metals in bioavailable form.
Complementing the soil samples, we obtained FYM samples (dung; whole cowpats) at representative locations (Fig. 1; Supplemental Table 1). One single sample (P4) contained a high amount of hay bedding whereas the rest are considered to represent FYM from grazing stock (see Supplemental Table 1 for details).
3 Materials and methodology
3.1 Bulk soil elemental analyses
Soil samples were initially stored in plastic bags and allowed to air dry prior to being sealed. At Trinity College Dublin (TCD), samples were heated in an oven at 60 °C for 96 h to dryness. These were then sieved to collect the ≤ 2 mm soil fraction. From this subsample of ca. 40 g were pulverised in an agate mill in preparation for major and trace element analyses at ALS Minerals, Loughrea, Ireland. Measurements followed the standard procedures of ALS and method numbers (supplemental file). Major element [sodium (Na2O), potassium (K2O), (CaO), magnesium (MgO), Al2O3, Fe2O3, TiO2, MnO], Corg (wt%) and essential trace metal concentrations (Zn, Cu, Ni, Cr, Co, Mo) (µg/g) are listed in Table 1. The full dataset with 51 additional analyses (mainly trace elements considered; non-essential is listed in the supporting information (Supplemental Table 2).
The degree of weathering is listed in Table 1, calculated as the chemical index of alteration (CIA): Al2O3/(Al2O3 + CaO + Na2O + K2O) × 100, where CaO represents the molar proportion of Ca in silicate-bearing minerals only [37].
To determine the total Corg (TOC, wt%) of the soil samples 30–50 mg of the powdered sub-sample was acidified with HCl to remove the inorganic [36]. Analyses of the TOC were then undertaken using an Elementar Vario EL cube instrument at TCD (see Sect. 3.2 for more detail).
3.1.1 DTPA extraction on soils for Zn, Cu, Co, Ni, Mo, Cr, Fe
The DTPA extraction method of Lindsay and Norvell [37] was used on a subsample of 10 g weighed into flasks. To this was added 20 mL of the DTPA extracting solution, and samples were mounted on a shaker table and run for 2 h at 160 rpm. Next, the solution was filtered through a Whatman No. 2 filter paper and the filtrate collected in pre-cleaned flasks before decanting into test tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis using a Varian Liberty Series II. For the calibration of the instrument response a multi-element standard (Merck CertiPur ICP multi-element standard solution IV) and different dilution factors were used: 0.5, 1, 5 and 10 mg/L for Cu, Cr, Mo, Co, and Ni; and a single element standard for Fe at 50 and 100 mg/L (for instrument method parameters and analyte settings see Supplemental Table 3). A quality control (QC) multi-element standard at 1 mg/L (AccuStandard ICP multi-element standard solution IV) was used. Calibration, blank and QC solutions were prepared using the same DTPA extraction solution as the soil samples.
Calibration coefficients were greater than 0.999. The internal blank levels for all elements of interest were beyond detection limit. The mean concentration of two external blanks was ≤ 0.02 µg/g. We listed the concentration of all essential metals of the soil samples in Table 2, except for Cr and Mo, which were beyond detection limit (≤ 0.01 µg/g).
3.2 FYM—trace element and C analyses
All FYM samples were air-dried prior to sub-sampling and sterilisation. In the laboratory, samples were then divided, recombined and mixed with a spatula to minimise heterogeneity [38], then oven dried at 60 °C for 72 h. Two grams (2 g) of this material were then combusted in air at 500 °C for 8 h (heating steps = 5 °C/min) to remove C, N, sulphur (S) and residual water, and then weighted to determine the LOI for the conversion of the element concentrations.
For each ash sample, a 100 mg ash aliquot was next digested in thoroughly cleaned Savillex Teflon® beakers using a hydrofluoride-nitric (HF-HNO3) (4:1) acid mixture at ~ 160 °C for 72 h. After digestion, samples were dried down at 110 °C to drive off silicon tetrafluoride (SiF4). The fluoride residue was attacked twice with concentrated hydrochloric acid (HCl) (2 mL) at 160 °C to liberate any residual metals associated with organic material. After the evaporation, 2 mL of concentrated HNO3 were introduced to the residue for 24 h at 135 °C, and a double conversion with HNO3 followed. The converted residue was then taken up in 10 g of 20% HNO3 to yield a nominal 1:100 parts total dissolved solid stock solution and put on the hotplate for 96 h to ensure complete dissolution of the samples. Trace element composition was determined at TCD using the method of Eggins et al. [39], incorporating modifications described in Kamber [40] and Babechuk et al. [41].
Approximately 4% of this stock was gravimetrically diluted to produce a 2% HNO3 solution with an internal standard mixture containing 6Li, rhodium (Rh), rhenium (Re), bismuth (Bi), and uranium-235 (235U) for solution quadrupole ICP-MS (Q-ICP-MS) analysis on a Thermo Scientific iCap-Qs. During analytical runs, sample unknowns were preceded by blanks, calibration (USGS rock standard W-2a) and a quality control standard (BCR-2), and bracketed with monitor samples (one every 4–6 unknowns) for external drift correction [39]. The method reproducibility and accuracy were assessed using the long-term average of USGS and GSJ standards run as unknowns. The concentrations of the essential trace metals of interest reproduced better than 1% relative standard deviation (RSD) (1 sigma) for Cr, Co, Ni, Cu, at 2% RSD for Mo and 3% RSD for Zn. Furthermore, Fe reproduced at 0.5% and Ti at 0.8% RSD. The apparent reproducibility for Mo is poorer due to contamination of the second-generation USGS standards [42]. The highest blank contribution was for Zn (< 0.00004 µg/g). All other elements of interest had very low blank contributions, which we corrected, and did not affect the final sample concentrations.
For the analysis of the C content only very small amounts (10–20 mg) of unashed FYM were required, and prepared in a zirconium oxide ball mill at TCD. Aliquots were measured for TOC (%) with an Elementar Vario EL cube instrument at TCD. The method has excellent precision and accuracy (better than 2% for samples of the type analysed here) through high performance combustion, matrix-independent results, and long-term stability of calibration.
4 Results
4.1 The CIA and its relation to soil composition
For the analysed soils, the CIA ranged widely, increasing from 45 to 50 (unweathered) to 100 (complete breakdown of original silicates), reflecting the progressive removal of the labile cations (Ca2+, Na+, K+) from silicates and the concomitant relative enrichment in (Al3+). Other elements (Ti4+, Fe3+) are also enriched but these are not measured by the CIA calculation. Vertisols developed over Deccan basalt and alluvium show moderate CIA values (60–78), while those overlying TTG and grey shale vary between 59–66 and 67–95, respectively. Ferralsols overlying Deccan basalt have the expected very high CIA values (96–98). Compositions of relevant bedrocks close to the sample locations are reported in the literature [18, 43,44,45]. They include: Deccan basalt; TTG gneisses; and grey shale (Table 1). Most unaltered igneous rocks have CIA values between 40 and 50 [18], whereas average shale has higher CIA (65–70) [46]. In the study area, there is only one (grey) shale lithology with a documented CIA value of 75. One soil developed on grey shale (S19) has a lower CIA, suggesting that its bedrock was less weathered than the one literature analysis. The CIA values of all vertisol topsoils are consistent with saprolite containing substantial amounts of smectite [18] with the exception of one sample (S18) developed over grey shale (CIA = 95).
A robust way of quantifying absolute element loss and gain during local alteration/soil formation is calculation of tau values (τ), which determine enrichment-depletion of constituents relative to an immobile index element in the parent rock. Here, the concentration (C) of the immobile (i) element Nb was used to measure loss or gain of a more mobile element (j) relative to the concentration of the underlying parent rock (p) on which the soil (s) developed [47, 48]. Positive τ values indicate enrichment; negative values imply depletion:
The first order finding from tau values (Fig. 2; supporting information; Supplemental Table 4) is that vertisols forming over basalt and alluvium have substantial depletion of essential trace metals, excepting Mn and occasionally Cr and Ni. By contrast, vertisols developed over TTG are relatively enriched in essential trace metals. In only two TTG-hosted vertisols, from rice fields (S36, S37), do Cr, Co and Zn become depleted (Fig. 2a). Importantly, Cu and Ni in particular, exhibit extreme gains in all vertisols developed over TTG. Enrichment of Cu, Cr and Ni in vertisols that formed over shales is similarly evident (Fig. 2b), whereas Co and Zn show depletion. Ferralsols and vertisols overlying basaltic parent rock show the same trends, excepting Ni (depleted). Only two ferralsol topsoils have Mo concentrations exceeding the 1 µg/g detection limit and for this reason, Mo was not further considered.
Tau enrichment-depletion diagrams of agricultural vertisols and ferralsols developed over different lithologies for Co, Cu, Cr, Zn and Mn (a) and Cu, Cr and Ni (b). The horizontal, red dashed lines illustrate the parent rock composition. Note that literature data of Co from NY1 basalt and Mn from grey shale were missing
In summary, the tau calculations reveal that Co, Cu and Zn have the strongest tendency for depletion while Mn, Cr and Ni are often enriched. Importantly, there is no evidence for a characteristic weathering-controlled enrichment-depletion pattern that might relate to the underlying geology or soil type. From chemical data alone, it remains unclear why the most strongly weathered, major-oxide depleted vertisols and ferralsols contain high Zn, Cu, Ni and Cr concentrations, sometimes close to ecotoxicological limits that might even be a risk to crop and human health (Table 1) [49,50,51].
In view of this issue, mineralogy was next considered to test whether the presence of certain metals (Al, Ti, Fe, Mn) that tend to form minerals incorporating minor trace metals (Cr, Co, Cu, Ni, and Zn) result in observable strong metal concentrations in the associated derived soils. To evaluate this possibility, a Pearson’s correlation analysis was conducted with τ values of all samples (Table 3). This revealed that enrichments in Fe, Cr, Co, Cu, Ni and Zn are moderately to strongly correlated with elevated Ti. Moreover, Mn correlates moderately with Cr, Co, Al and Fe. However, Al is anti-correlated with elevated Co, Cu and Ni without any observable relationship with Ti or Fe. This suggests that Al (and CIA) play a less dominant role for trace metal concentrations. Strong correlations between Cu, Ni and Zn and moderate correlations of Cr with Co, Ni and Zn suggest a similar partitioning of essential trace metal concentrations into specific minerals in the vertisols and ferralsols. The key minerals are Fe-, Ti- and Cr-bearing phases into which minor and trace metals substitute, with additional roles for the substitution relationships between minor to trace metals.
The essence of the major element geochemistry can be illustrated with binary diagrams for all soils, regardless of parent rock or soil type. For example, a strong correlation (r2 = 0.771) exists between Fe2O3 (wt%) and TiO2 (wt%) (Fig. 3a). Furthermore, a moderate correlation between Fe2O3 (wt%) and Al2O3 (wt%) is evident (r2 = 0.556) (Fig. 3b). Behaviour of other major elements may be summarised as follows. There is a strong positive correlation between TiO2 (wt%) and Zn (r2 = 0.748), Cu (r2 = 0.882) and Co (r2 = 0.736) (Fig. 4a). Fe2O3 (wt%) correlates strongly with Cu (r2 = 0.882) and moderately with Zn (r2 = 0.628) (Fig. 4b) and Co (r2 = 0.620). Chromium and Ni concentrations moderately correlate with TiO2 (wt%) (Ni: r2 = 0.635; Cr: r2 = 0.449) and Fe2O3 (wt%) (Ni: r2 = 0.508; Cr: r2 = 0.584) (Fig. 4a, b). Manganese correlates moderately with TiO2 (r2 = 0.478; not shown) and Fe2O3 (wt%) (r2 = 0.498; Table 4). Moderate correlations (Fig. 4c) also exist between all essential trace metals and Al2O3 (wt%), except for Zn. In the more moderately weathered soils, this likely reflects the insoluble nature of both Al2O3 and Fe2O3 (Fig. 3b) when in the highly evolved, kaolinite-rich ferralsols and two vertisol rice field samples (Table 1; S36, S37), r2 values between Al2O3 (wt%) and metals remain moderate to low (Fig. 4d, e). These trends substantiate that the dominant association of essential metals is with Fe- and Ti-rich mineral phases in the vertisols and ferralsols. There is a minor relationship between Al phases and Zn, Cu, Ni and Co in most vertisols. Only Cr and Mn concentrations are clearly controlled by parent rock geology.
4.2 DTPA soil extractions of Zn, Cu, Ni, Cr, Co, Mo, Fe and their associations with bulk soil elements
The DTPA extraction data are listed in Table 2, including the minimum to maximum range. With few exceptions, the DTPA-essential metals display a similar range in all samples. Notable observations are the higher concentrations of DTPA-extractable Fe from two vertisols from rice fields on TTG (S36 and S37) and elevated Ni from two vertisols (S18 and S19) on grey shale (Fig. 5). Furthermore, extractable Co (S25) and Zn concentrations (S26 and S28) are elevated in ferralsols developed over basalt. Regardless, the analysis of the DTPA-extractable essential metal data reveals that no first order control is exerted by the underlying geology and soil type. Consequently, we compared bulk soil elements (Corg, Fe2O3, Al2O3, MnO) and the CIA to extractable metals in all soil samples in an attempt to identify relationships.
The Pearson’s correlation analysis showed that there are modest-moderate correlations (Table 4), suggesting that the DTPA metal concentrations are held in the soils within different minerals or complexation states. The most notable correlations are between Corg (wt%) and extractable Zn and Cu (p ≤ 0.05). Additionally, extractable Co and Zn correlate significantly with Al2O3 (wt%) (p < 0.01) (Table 4). Moreover, DTPA-extractable Cu correlates with Fe (p < 0.05), whereas inverse relationships between extractable Fe and Fe2O3 (wt%) (p ≤ 0.05) as well as the CIA (p ≤ 0.01) exist (Table 4). Extractable Co is the only metal associated with MnO (wt%) in the soil samples (p ≤ 0.01), and extractable Zn is the only essential metal that shows a relationship with Fe2O3 (wt%) (p < 0.01). Extractable Ni is not associated with any specific bulk soil element and Co (p < 0.01) shows only a moderate relationship with the CIA.
4.3 FYM—element (C, Fe, Ti, Mn) and essential trace metal (Cr, Co, Ni, Cu, Zn, Mo) concentrations
Predictably, C content in the FYM samples is high (ca. 30–40 wt%), except for sample (P4) that contained hay bedding (< 15 wt%) (Table 5). Metals show a general pattern of concentration from high: Fe (10,000–35,000 µg/g), Ti (1500–5000 µg/g) and Mn (600–1100 µg/g); to moderate: Zn (30–300 µg/g), Cu (30–100 µg/g) and Cr (10–50 µg/g); to low: Co (5–20 µg/g) and Mo (c. 1–5 µg/g). Iron, Co, Ni, Cu, and Cr concentrations are strongly to moderately correlated with Ti (r2 = 0.983, 0.924, 0.724, 0.664 and 0.559, respectively) as shown in Fig. 6. This is an important observation because Ti has earlier been identified as a strong controlling phase for the association with essential metals in the bulk soils (Sect. 4.1; Figs. 3, 4). Manganese (r2 = 0.02; not shown), Mo (r2 = 0.084) (Fig. 6b) and Zn (r2 = 0.031) (Fig. 6c) are not correlated to Ti, and their concentrations in FYM must therefore relate to a non-Ti-bearing phase. As with the bulk soil- and extractable chemistry, a first order relationship to the geology is absent. This can be illustrated, for example, with FYM samples from TTG regions that have the lowest but also the second highest Ti concentrations (Table 5).
5 Discussion
5.1 Bulk soil compositions—primary silicates and clays
Under most environmental conditions—Al typically remains immobile in soils with 5 < pH < 7.5 because it is a significant constituent of clay minerals and hydroxides. Only in (sub)-tropical regions covered by forest (e.g., podsols) [52] Al solubilises due to high contents of organic ligands [53,54,55,56]. Secondary phases in typical soils initially form through breakdown of primary silicates [32, 57, 58]. At more advanced weathering intensity, the clays and any remaining primary silicates transform into kaolinite and finally into Al-hydroxide (gibbsite), which is extremely depleted in trace elements [16, 32]. Primary minerals (e.g., plagioclase and pyroxene) have CIA of 40–50, initial secondary minerals such as smectite or illite have CIA values of 70–85, and more advanced weathering produces kaolinite with CIA values of c. 100 [18]. The soil CIA may therefore be a useful guide for the presence or absence of clays with appreciable metal content.
The CIA values in the vertisols (59–80) are similar to those in the uncultivated saprolitic subsoils described by Babechuk et al. (2014), indicating that they still contain fertile rock debris and nutrient-rich clay minerals. The ferralsol topsoils (CIA 93–96) are kaolinite-rich (incidentally, the vertisol developed over grey shale, S18; CIA = 95 shows similar behaviour), indicating that the clay of these soils is typically poorer in essential metals, and thus likely problematic for agricultural fertility [16].
In terms of element behaviour, Al2O3 (wt%) displays modest correlations with Zn, Ni, Cu and Co concentrations in most vertisol topsoils of Central India (Fig. 6c). This implies that silicates and/or complex clay minerals are an important reservoir of Zn, Ni, Cu and Co, however, without being the main host of essential metals in Central Indian topsoils.
5.2 Bulk soil compositions—weathering resistant mineral phases
Refractory primary mineral phases may persist even in soils with elevated CIA, and thus significantly influence bulk elemental compositions. The presence of these type of refractory phases may be tracked using the behaviour of the least mobile elements.
5.2.1 Titanium and molybdenum
In parent rock, most Ti is not hosted in silicates but occurs in ilmenite (FeTiO3) and rutile (TiO2) and as impurity in the Fe-oxide magnetite (Fe3O4). This is especially the case in mafic igneous and metamorphic parent rocks. All these phases, along with other heavy inert minerals, often form the residual, undecomposed content in soils [59]. By contrast, only a small proportion of parent rock Ti occurs in primary silicates such as pyroxene, amphibole, biotite mica and garnet [60]. During oxidative chemical weathering of primary silicates, liberated Ti and Fe are rapidly redistributed into newly formed Fe–Ti hydrous oxides. This is also reflected in the low content of TiO2 in dominant clay mineral groups ranging from 0–0.3 wt% (kaolinite), 0–0.5 wt% (smectite), 0–0.6 wt% (vermiculite), 0–0.8 wt% (illite) to 0–0.2 wt% (chlorite) [61]. The original primary mineral association of Fe and Ti and the secondary formation of Fe–Ti hydrous oxides thus explain the strong correlations between Fe and Ti in fine fractions of soils [62]. Molybdenum can also be associated with refractory minerals but in our dataset displays no relationship to the Fe–Ti mineral except in the two ferralsols (S25 and S26). In the other soils, Mo was likely originally hosted in sulphides but because concentrations are mainly below the detection limit of 1 µg/g, the inorganic soil constituents are clearly not a source of Mo.
5.2.2 Titanium as a potential vector towards deficiency status of soil Fe, Zn, Cu, Ni, Co and Cr
The global average and median of Ti in soils lie around 0.33% and 0.7%, respectively [60]. The vertisol and ferralsol samples from Central India display TiO2 (wt%) much higher concentrations (Fig. 7a) (ca. 1–3.5%; n = 18 of 20; Table 1), which points to high quantities of inert residual oxides. Since Ti is not a bio-essential metal (Fig. 7b), its high abundance is not critical to agricultural practice on these soils. However, of relevance is the correlation of bio-essential metals Zn and Cu with TiO2 (wt%), Fe2O3 (wt%) and τZn,Cu,Ni,s with τFe,Ti,s (Table 3; Fig. 4a, b). These relationships suggest that most Zn, Cu and Ni are bound in weathering resistant oxides. Similarly, the correlation of Co with TiO2 (wt%), Zn, Cu, Ni, Cr, Mn and τCo,s with τFe,Ti,Cu, Ni,Zn,Cr,Mn,s further illustrates the dominance of oxide phases as hosts of metals in the cultivated vertisol- and ferralsol topsoils. Because Ti phases and other refractory minerals (e.g. zirconium-silicate) are dense, their occurrence in the topsoil horizons may not only reflect mass loss due to weathering but also loss of clays to erosion and transport by water [59] and winnowing wind [63].
5.3 Plant available essential metal contents in topsoils
5.3.1 Zinc, Cu and Fe and the importance of organic carbon
The Pearson’s correlation analysis reveals statistical relationships between DTPA metal extractions, elements (Al2O3, Fe2O3, MnO) and the CIA, the latter two reflecting bulk soil mineralogy (Table 4). However, the bio-availability of Zn and Cu is also controlled by TOC and both essential metals are commonly complexed by organic matter in weathered substrate in India [33, 64].
Since DTPA-extractable Fe and Cu moderately correlate, it is reasonable to infer that a substantial proportion of the bio-available Fe is associated with TOC. By contrast, Fe-oxides and clays are not the main source of bio-available Fe from the soil because of the moderate inverse correlation of DTPA extracted Fe and Fe2O3 (wt%) as well as the CIA. This finding agrees with studies on alluvial soils elsewhere in India, demonstrating that only a small fraction of the total Fe reserve is plant available [6], with the dominant Fe pool unavailable from crystalline, oxide/oxyhydroxide-bound and residual Fe fractions [6, 65].
Release of Zn to crops appears to be controlled by clay minerals, minor amounts of fertile rock debris with pyroxene [33], and possible exchange reactions on the surface sites of pedogenic oxides, as Zn concentration correlates with Al2O3 (wt%) and Fe2O3 (wt%). This agrees with the observation that complex clay minerals and residual primary phases contain modest amounts of essential metals like Zn that seem to be bio-available (Fig. 4d).
5.3.2 Chromium, Mo, Ni and Co
In soils, chromium occurs mainly (> 80%) in the chemically immobile residual fraction [59], such as in pedogenic Fe-oxides [34]. It is thus unsurprising that our measured Cr concentrations in the DTPA-extraction were below detection limit (˂ 0.01 µg/g), similar to simulated plant uptake of Cr with DTPA [66]. The very low DTPA-extraction concentrations ≤ 0.01 µg/g of Mo could reflect the low total content of Mo in the original bulk soil samples or its very early loss due to sulphide oxidation in the subsoil [67]. The concentration of extractable Ni is not relatable to a specific phase on a statistically significant level, regardless of the fact that clays do contain Ni (Fig. 4c, e). The correlation of extractable Co with Al2O3 (wt%) suggests that primary silicates and clay minerals determine the release of Co to plants. Additionally, Mn-oxides also play a role for Co release, which is best explained by co-precipitation and consequent enrichment of Co under oxidising, near neutral conditions and potential mobility under acidic and reducing conditions [68, 69].
5.4 Essential metal deficiency status of topsoils
Accurate assessment of critical metal deficiency limits also depends on crop type and various growth factors controlled by the environmental conditions. Therefore, different DTPA values have been proposed as critical deficiency limits for micronutrients [70]. For example, Lindsay, Norvell [37] suggested soils with > 4.5 µg/g of Fe in the DTPA-extract solution would not show Fe deficiency, yet such cut-off values are problematic because the DTPA-extraction cannot discern between different forms of Fe [71]. Regardless of the fact that the Fe concentration in the DTPA-extract solution likely overestimates the total value available to the plant, the generally high concentrations found in this study (Table 2) suggest that Fe deficiency is currently not a problem in the central Indian vertisol and ferralsol topsoils.
By contrast to Fe, the critical deficiency level for Zn is well established [70] and DTPA extractable Zn values of ca. 0.5 µg/g represent the critical lower limit for several crops but it is noted that suggested ‘low’ values of up to 2 µg/g can still lead to deficiency symptoms [72]. In previous Indian soil studies, average concentrations of DTPA-extractable Zn varied widely from 0.1 to 6.92 µg/g, with a mean of 0.87 µg/g. Soils in Central India apparently have even lower mean DTPA-Zn concentrations of 0.65 µg/g with the highest levels of deficiency in red loam (entisol) and black clayey soils (swell-shrink vertisol) of the central Deccan plateau [6]. Based on the defined critical level of Zn, two vertisol samples in this study (S6 derived from basaltic alluvium; S21 derived from grey shale) are clearly Zn deficient (< 0.5 µg/g), while most other topsoils reveal critically low levels (< 2 µg/g). Accordingly, based on the limited current data, vertisols and even ferralsols could be at risk of Zn deficiency in Central India, indicating that Zn supply from fertile rock debris and clays (see also Sect. 5.1) is insufficient for modern crops.
Extractable Cu concentrations are above the various proposed critical limits of 0.2 µg/g [37], 0.6 µg/g [71] and 2.5–3.0 µg/g [70]. The critical levels of Ni are not yet established. Globally, typical Ni values in food plants range between ca. 0.2–2.0 µg/g [59]. These values are comparable to the DTPA extractable Ni concentrations found in this study, and we conclude Ni deficiency is unlikely to be a problem in the vertisol- and ferralsol topsoils. Similarly, a critical deficiency level for DTPA extractable Co has not yet been defined. Regardless, Co levels in plants rarely exceed concentrations above 1–2 µg/g [73], suggesting that the Co levels in the topsoil samples remain above critical level. Since the tentative critical level of Mo deficiency is ˂0.01–0.02 µg/g [70], it is not possible to determine bio-availability of Mo from our data.
5.5 Challenges and opportunities in the application of FYM as an essential metal fertiliser on farmland in Central India
The key finding of our agricultural vertisol and ferralsol study on Indian farmland is that Ti concentration could be an easy and inexpensive tracer to identify possible bio-unavailable essential metal pools in soil substrate. Previous studies demonstrated that Ti has an extremely low bioaccumulation index (i.e. the ratio between trace element concentrations in plants relative to soils) [59]. All other essential metals show higher indices, typically the order of Ti < Fe < Cr < Ni < Mn < Co < Cu < Mo < Zn (Fig. 7b). Even though cattle may digest metals differently, the Indian FYM mainly revealed low concentrations of measured essential metals, except for Fe, Mn, Zn and Cu. Strikingly, except for Zn, Mo and Mn, most essential metals (Fe, Cr, Co, Ni, Cu) show a strong correlation with Ti (Fig. 6). The first order relationship between Ti with Fe, Cr, Co, Ni and Cu suggest that this represents material (including weathering-resistant minerals) from the topsoil itself, and that Ti concentration is thus a proxy for soil ingestion and thus “contamination” of the FYM. Thornton, Abrahams [74] and Green et al. [75] showed that the relatively high concentrations of Ti in soils together with very small amounts in herbage are conditions which permit usage of Ti concentration in livestock faeces as a stable marker of soil ingestion (see equation below). However, this assumes a herbage digestibility factor of 72% in monthly rotational pasture grass [75]. We performed a similar calculation with respect to the ten soils sampled at the same location as FYM.
Equation:
where Dh = digestibility of herbage, Tif = titanium in faeces, Tis = titanium in soil.
The results suggest that the amount of soil ingested by cattle ranges between 2 and 25% (Supplemental Table 6). Considering the daily mineral availability for essential metals (Zn, Cu, Co, Mo, Fe, Mn) from traditional foddering in India, as well as a dietary intake of 11.5 kg per day for Indian cattle [76], the contribution of oxides to Mo intake is insignificant and low to moderate for Zn (5–40%). By contrast, the intake of Cu (45–500%) and Mn (30–130%) varies between moderate to high values, but is extreme for Co (400–1800%) and Fe (350–2200%).
The C-content of the studied FYM is ca. 30–40% (dry weight), and comparable to previous studies [77]. The Indian FYM samples have concentration levels of Zn, Cu and Mo comparable to the cow manure reported by McBride, Spiers [78] (Fig. 8a). Since the DTPA availability of these metals in the Indian soils is limited by factors previously described, and because there is no first order relationship between Ti, Zn, Mo and Mn in FYM, it thus appears likely that a mixture of digested fodder and food additives dominates the budget of Zn, Mo, Mn and possibly Cu in FYM (Supplemental Table 5; Supplemental Table 6). By contrast, the total Fe and Co intake of cattle is significantly related to soil, which can explain the strong r2 correlation between Ti with Fe and Co (Fig. 6a, b). Importantly, Co, Cr, Ni and Fe show positive correlation to calculated soil ingestion (%) on a statistically significant level (Cr, Fe: p < 0.01; Ni, Co: p < 0.05) (Supplemental Table 7). However, Cu is also correlated with Co and Ni (p < 0.01) that are likely bound in weathering resistant Fe–Ti oxides in the Central Indian topsoils (Table 3; Fig. 4a, b). Refractory oxides could therefore also represent a metal contamination agent in FYM. In summary, our analysis demonstrates that essential metal concentrations in FYM are primarily associated with organic (fooder/plants) and inorganic (especially bio-unavailable refractory Fe–Ti minerals) pools that need careful distinction. Without assessment of these pools, FYM cannot be reliably evaluated as a natural metal fertiliser because otherwise it remains unclear which metals serve as bio-available crop micronutrients.
Comparison of average essential metal concentrations in FYM from this study to FYM from the USA [79] (a). Illustration of essential metal concentrations in FYM based on average-, minimum- and maximum- soil/oxide ingestion. Additionally, concentrations of Co, Zn, Cu, Mo, Fe and Mn from daily fodder intake (undigested organic sources) [72] are shown. Note the control of absolute Ti concentrations on essential metals (b)
The absolute concentrations of essential metals in FYM and fodder sources can be illustrated with a multi-element histogram (Fig. 8b). This illustrates that absolute concentrations are much higher in FYM where the amount of oxide ingestion is elevated. By contrast, in examples of FYM with minor ingestion of oxides/soil, it is obvious that essential metals are not significantly biased by the inorganic, bio-unavailable pools. Furthermore, the digested fodder residue in such FYM is much lower in metal concentrations after the intake by cattle in comparison to the fodder sources. Considering its lack of bioactive role in the plant-soil cycle (Fig. 7b), the lowest Ti concentration in FYM can serve as a new vector towards the readily bio-available metal pool/residue in this natural fertiliser. Sample P18 (Table 5) is a most useful representative example for a mass balance calculation to estimate the potential of FYM as an essential metal fertiliser in India. This may be achieved by using the tons (t) that are annually available as FYM by cattle and the required fertiliser amount on Indian farmland.
Tandon [79] estimated that in 2010, 119 million t of livestock dung was potentially available as fertiliser in India, and approximately 37% of Indian livestock were cattle in 2014 [80]. Using these data as a first-order estimate for the year 2020, ca. 44 million t of FYM are available annually for manure production of which ca. 20% remain after drying. By 2025 approximately 324,000 t Zn, 130,000 t Fe, 11,000 t Cu and 22,000 t Mn are required to ameliorate recognised essential metal deficiencies in Indian soils [81]. Fertiliser requirements for other essential metals are, to our knowledge, not published. Following this estimate approach using the least “soil/oxide contaminated” FYM sample (P18), the metal concentrations determined from this study indicate that the amount of Fe (ca. 72%) and Mn (155%) is sufficiently high to effectively counterbalance the net output of bio-available essential metals. By contrast, the levels of Cu (ca. 5%) and Zn (ca. 2%) are very clearly insufficient to correct the metal deficiency in agricultural soils in India, at least from an agro-industrial perspective (Fig. 9).
A biogeochemical perspective on the fertiliser potential of FYM in India to ameliorate deficiencies of Zn, Fe, Cu and Mn until 2025, using a mass balance with the least oxide contaminated FYM. Additionally, the annually required t of potash-, phosphate-, sulphate-, and EDTA-chelated fertilisers are illustrated that complement FYM
The above first-order calculation is likely to underestimate the potential of FYM as natural essential metal fertiliser due to the role of TOC. For instance, the application of FYM in a long-term experiment [21], involving 37 cycles and rotational cropping practices on Indian surface and sub-surface soils has proven to increase the concentrations of bio-available Zn and Cu in all soil fractions (exchangeable, carbonate-bound, oxide bound, organically bound and residual). Further, considering that TOC in the vertisols and ferralsols exerts control on the mobilisation of Zn, Cu and Fe to soil solutions (Table 4), it appears that the high Corg of ca. 35% in FYM (Table 5) is indispensable to maintain the fertility status of Indian soils of the type studied here, as well as also being an excellent source for Fe and Mn. This finding reinforces the important status of organic matter for the improvement of the soil structure, water retention, prevention of soil erosion as well as cycling and storage of plant nutrients. Since climatic conditions in India accelerate organic matter decomposition within soils, any significant net off-take of FYM would likely impoverish crop productivity [82] and further exacerbate the bio-availability of essential metals on farmland. This would be especially the case in vertisols whose physical conditions make them very difficult to cultivate and manage [83].
The importance of co-application of chemical fertilisers with manure for the essential metal availability from soils was highlighted in a review by Dhaliwal et al. [84]. The analysis of our data suggests that C-rich FYM could usefully be supplemented with sulphate salts of Zn, Fe and Cu. These are inexpensive and readily available [6]. To counterbalance the limited bio-available Zn in FYM, our calculations show that c. 318,000 t are additionally needed on Indian farmland per annum. Therefore, the cost of required sulphate salts for Zn would lie around 850 million US$ for c. 1.5 million t. This is far more affordable than EDTA-chelated Zn, which would cost c. 24 billion US$ for c. 2.7 million t [85] to complement the bio-available pool of Zn from FYM (Supplemental Table 8). By contrast, the quantities of potash- or phosphate fertilisers required to address Zn and other essential trace metal deficits would be in the order of billions of t, costing trillions of US$ [86], making them from the applied quantities on soil and cost an impossible essential trace metal fertiliser (Fig. 9; Supplemental Fig. 2). The disadvantage of the more affordable sulphate fertilisers is that in long-term experiments they have caused severe acidification of soil and harmed agricultural productivity and ecosystem functions. Therefore, Kidd et al. [87] proposed that long-term monitoring is required to establish if the co-application with FYM could counteract these adverse effects of sulphate salts. This is because FYM can buffer against soil acidification (> pH 5). Alternatively, the application of sulphate salts, FYM and lime could be a solution to achieve less acidic and more nutrient-rich soils in India and other developing countries to help meeting the future demands of multi-micronutrients in the soil, plant, animal and human continuum.
6 Summary and conclusion
Chemical and associated data analysis of Central Indian agricultural topsoils and FYM reveals the following:
-
(1)
Geochemistry can contribute to food security plans by helping to better quantify the inventory of bio-essential minerals (e.g., primary silicates, smectite, etc.) from those that “immobilise” key bio-available nutrients in the natural cycling of soil-crop-FYM-fertiliser application. Primary and secondary oxides in agricultural topsoils contain high concentrations of essential trace metals in bio-unavailable form. Only moderately weathered vertisols contain sufficient bio-essential minerals that can act as a reservoir of several essential metals (Zn, Cu, Co, possibly Ni). By contrast, Cr (held in oxide phases) and Mo (very low total content) are not bio-available from the inorganic soil constituents. With advanced weathering and enrichment of oxides in the soil reservoirs, any original expected differences in concentrations of bio-available essential metals in different parent rocks or soil types will diminish significantly. Bulk analysis may indicate usable concentrations, but ‘lock-up’ of essential elements in highly residual Fe–Ti-rich (oxidative weathering-resistant) phases contributes significantly to metal-deficiency problems in this region. This observation implies that ecotoxicity levels of ‘heavy metals’ in oxidative environment require a careful evaluation of the mineralogical inventory to assess risks in the soil-crop-human health cycle, particularly in India where heavy metal enrichment in soils is accompanied by strong Fe-enrichment [88].
-
2)
High quantities of Fe–Ti-rich phases are ingested by cattle and remain undigested. Hence, excepting Fe (c. 70%) and Mn (c. 150%), bio-available trace metal concentrations in FYM alone are likely insufficient to sustain the anticipated productivity of this land. This is exemplified by Zn and Cu that can only contribute 2% and 5% to the total fertiliser requirements annually. Regardless, the low levels of extractable Zn, Co, Mo and Cr are critical and the relationship between Zn and Cu with TOC and Cu with Fe provides evidence that organo-metallic complexes play an essential role in the availability of these micronutrients to plants. These findings underline the importance of traditional farming practices in India with C-rich FYM. We propose that the combination of C-rich FYM with affordable and widely available metal fertilisers could represent a successful way to combat multi-essential metal deficiencies in soils and crops and help to diminish the deficiency problems in the human diet in India to sustain food security, once potential risks have been evaluated in long-term studies.
References
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. https://doi.org/10.5402/2011/402647
Aggett P, Nordberg GF, Nordberg M (2015) Essential metals: assessing risks from deficiency and toxicity. In: Handbook on the toxicology of metals. Elsevier, pp 281–297
World Health Organization (1996) Trace elements in human nutrition and health. World Health Organization
Bhan MK, Sommerfelt H (2001) Micronutrients, maternal and child health. CABI Publishing
Andersen P (2007) A review of micronutrient problems in the cultivated soil of Nepal. Mt Res Dev 27(4):331–335
Singh MV (2008) Micronutrient deficiencies in crops and soils in India. In: Micronutrient deficiencies in global crop production. Springer, pp 93–125
Maret W (2016) The metals in the biological periodic system of the elements: concepts and conjectures. Int J Mol Sci 17(1):66
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19(2–3):125–140
Brown PH, Welch RM, Cary EE (1987) Nickel: a micronutrient essential for higher plants. Plant Physiol 85(3):801–803
Deckers J, Steinnes E (2004) State of the art on soil-related geo-medical issues in the world. Adv Agronomy 84:1–35
Singh M (2001) Importance of sulphur in balanced fertilizer use in India. Fertiliser News 46(10):13–36
Singh M (2004) Micronutrients seed treatment to nourish the crop plants at critical stages of growth. Res Bull, IISS, Bhopal 2:1–87
Welch RM, Graham RD (1999) A new paradigm for world agriculture: meeting human needs: productive, sustainable, nutritious. Field Crops Res 60(1–2):1–10
Hazell PB (2009) The Asian green revolution, vol 911. Intl Food Policy Res Inst
Singh SP, Gruissem W, Bhullar NK (2017) Single genetic locus improvement of iron, zinc and β-carotene content in rice grains. Sci Rep 7(1):1–11
Fyfe W, Kronberg B, Leonardos O, Olorunfemi N (1983) Global tectonics and agriculture: a geochemical perspective. Agric Ecosyst Environ 9(4):383–399
White AF, Blum AE, Schulz MS, Vivit DV, Stonestrom DA, Larsen M, Murphy SF, Eberl D (1998) Chemical weathering in a tropical watershed, Luquillo Mountains, Puerto Rico: I. Long-term versus short-term weathering fluxes. Geochimica et Cosmochimica Acta 62(2):209–226
Babechuk M, Widdowson M, Kamber B (2014) Quantifying chemical weathering intensity and trace element release from two contrasting basalt profiles, Deccan Traps, India. Chem Geol 363:56–75
Suhr N, Widdowson M, McDermott F, Kamber B (2018) Th/U and U series systematics of saprolite: importance for the oceanic U-234 excess. Geochem Perspect Lett 6:17–22
Kao P, Darch T, McGrath S, Kendall N, Buss H, Warren H, Lee M (2020) Factors influencing elemental micronutrient supply from pasture systems for grazing ruminants. In: Sparks DL (ed) Advances in agronomy. Elsevier, pp 161–229
Narwal BR, Kumar AR, Antil AR (2010) Long-term effect of farmyard manure and N on the distribution of zinc and copper in soil fractions under pearl millet–wheat cropping system. In: 19th World congress of soil science
Simonson RW (1954) Morphology and classification of the Regur soils of India. J Soil Sci 5(2):275–288
Newbold TJ On the régar or black cotton soil of India (1843). In: Abstracts of the papers printed in the philosophical transactions of the Royal Society of London, The Royal Society London, pp 53–54
Pal D, Dasog G, Vadivelu S, Ahuja R, Bhattacharyya T (2000) Secondary calcium carbonate in soils of arid and semi-arid regions of India. Global climate change and pedogenic carbonates 149–185
Pal D, Bhattacharyya T, Chandran P, Ray S, Satyavathi P, Durge S, Raja P, Maurya U (2009) Vertisols (cracking clay soils) in a climosequence of Peninsular India: evidence for Holocene climate changes. Quatern Int 209(1–2):6–21
Pal D, Wani S, Sahrawat K (2012) Vertisols of tropical Indian environments: pedology and edaphology. Geoderma 189:28–49
Coulombe CE, Wilding LP, Dixon JB (1996) Overview of Vertisols: characteristics and impacts on society. In: Sparks DL (ed) Advances in agronomy. Elsevier, pp 289–375
Srinivasarao C, Gayatri SR, Venkateswarlu B, Jakkula V, Wani S, Kundu S, Sahrawat K, Rao BR, Marimuthu S, Krishna GG (2014) Heavy metals concentration in soils under rainfed agro-ecosystems and their relationship with soil properties and management practices. Int J Environ Sci Technol 11(7):1959–1972
Sharma B, Mukhopadhyay S, Katyal J (2006) Distribution of total and DTPA-extractable zinc, copper, manganese, and iron in Vertisols of India. Commun Soil Sci Plant Anal 37(05–06):653–672
Yerima B, Van Ranst E, Sertsu S, Verdoodt A (2013) Pedogenic impacts on the distribution of total and available Fe, Mn, Cu, Zn, Cd, Pb and Co contents of vertisols and vertic inceptisols of the Bale Mountain area of Ethiopia. Afr J Agric Res 8(44):5429–5439
Katyal J, Sharma B (1991) DTPA-extractable and total Zn, Cu, Mn, and Fe in Indian soils and their association with some soil properties. Geoderma 49(1–2):165–179
Widdowsow M, Gunnell Y (2009) Lateritization, geomorphology and geodynamics of a passive continental margin: the Konltan and Kanara coastal lowlands of western peninsular India. Palaeowea, Palaeosurf and Rel Cont Deposits 73:245
Suhr N, Schoenberg R, Chew D, Rosca C, Widdowson M, Kamber BS (2018) Elemental and isotopic behaviour of Zn in Deccan basalt weathering profiles: chemical weathering from bedrock to laterite and links to Zn deficiency in tropical soils. Sci Total Environ 619:1451–1463
Wille M, Babechuk MG, Kleinhanns IC, Stegmaier J, Suhr N, Widdowson M, Kamber BS, Schoenberg R (2018) Silicon and chromium stable isotopic systematics during basalt weathering and lateritisation: a comparison of variably weathered basalt profiles in the Deccan Traps, India. Geoderma 314:190–204
Nesbitt H, Wilson R (1992) Recent chemical weathering of basalts. Am J Sci 292(10):740–777
Harris D, Horwáth WR, Van Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci Soc Am J 65(6):1853–1856
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42(3):421–428
Peters J, Combs S, Hoskins B, Jarman J, Kovar J, Watson M, Wolf A, Wolf N (2003) Recommended methods of manure analysis. University of Wisconsin Cooperative Extension Publishing, Madison
Eggins SM, Woodhead JD, Kinsley LPJ, Mortimer GE, Sylvester P, McCulloch MT, Hergt JM, Handler MR (1997) A simple method for the precise determination of ≥ 40 trace elements in geological samples by ICPMS using enriched isotope internal standardisation. Chem Geol 134(4):311–326
Kamber BS (2009) Geochemical fingerprinting: 40 years of analytical development and real world applications. Appl Geochem 24(6):1074–1086
Babechuk MG, Kamber BS, Greig A, Canil D, Kodolányi J (2010) The behaviour of tungsten during mantle melting revisited with implications for planetary differentiation time scales. Geochim Cosmochim Acta 74(4):1448–1470
Babechuk M, Widdowson M, Murphy M, Kamber B (2015) A combined Y/Ho, high field strength element (HFSE) and Nd isotope perspective on basalt weathering, Deccan Traps, India. Chem Geol 396:25–41
Sano T, Fujii T, Deshmukh S, Fukuoka T, Aramaki S (2001) Differentiation processes of Deccan Trap basalts: contribution from geochemistry and experimental petrology. J Petrol 42(12):2175–2195
Nagarajan R, Madhavaraju J, Nagendra R, Altrin JSA, Moutte J (2007) Geochemistry of Neoproterozoic shales of the Rabanpalli Formation, Bhima Basin, Northern Karnataka, southern India: implications for provenance and paleoredox conditions. Revista Mexicana de Ciencias Geológicas 24(2):150–160
Prabhakar B, Jayananda M, Shareef M, Kano T (2009) Petrology and geochemistry of late archaean granitoids in the northern part of Eastern Dharwar Craton, Southern India: implications for transitional geodynamic setting. J Geol Soc India 74(3):299
McLennan SM (1993) Weathering and global denudation. J Geol 101(2):295–303
Brimhall GH, Dietrich WE (1987) Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: results on weathering and pedogenesis. Geochim Cosmochim Acta 51(3):567–587
Anderson SP, Dietrich WE, Brimhall GH Jr (2002) Weathering profiles, mass-balance analysis, and rates of solute loss: linkages between weathering and erosion in a small, steep catchment. Geol Soc Am Bull 114(9):1143–1158
Awashthi S (2000) Central and state rules as amended for 1999: prevention of food adulteration Act no. 37 of 1954. Ashoka Law House, New Delhi
McGrath S (1997) Behaviour of trace elements in terrestrial ecosystems. COLLOQUES-INRA 35–56
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop Ecol 51(2):375–387
Van Hees P, Lundström US, Giesler R (2000) Low molecular weight organic acids and their Al-complexes in soil solution—composition, distribution and seasonal variation in three podzolized soils. Geoderma 94(2–4):173–200
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166(2):247–257
Drever J, Stillings L (1997) The role of organic acids in mineral weathering. Colloids Surf A Physicochem Eng Asp 120(1–3):167–181
Cama J, Ganor J (2006) The effects of organic acids on the dissolution of silicate minerals: a case study of oxalate catalysis of kaolinite dissolution. Geochim Cosmochim Acta 70(9):2191–2209
Perez-Fodich A, Derry LA (2019) Organic acids and high soil CO2 drive intense chemical weathering of Hawaiian basalts: insights from reactive transport models. Geochim Cosmochim Acta 249:173–198
Anand RR (2005) Weathering history, landscape evolution and implications for exploration. In: Anand RR, de Broekert P (eds) Regolith landscape evolution across Australia: a compilation of Regolith landscape case studies with Regolith landscape evolution models, pp 2–40
Dahlgren R, Boettinger J, Huntington G, Amundson R (1997) Soil development along an elevational transect in the western Sierra Nevada, California. Geoderma 78(3–4):207–236
Kabata-Pendias A (2010) Trace elements in soils and plants. CRC Press
Reeder S, Taylor H, Shaw R, Demetriades A (2006) Introduction to the chemistry and geochemistry of the elements. In: Tarvainen T, de Vos M (eds) Geochemical Atlas of Europe. Part 2. Interpretation of geochemical maps, additional tables, figures, maps, and related publications. Geological Survey of Finland, Espoo, pp 48–429
Blume H-P et al (2016) Scheffer/Schachtschabel Lehrbuch der Bodenkunde. Springer-Verlag
Taboada T, Cortizas AM, García C, García-Rodeja E (2006) Particle-size fractionation of titanium and zirconium during weathering and pedogenesis of granitic rocks in NW Spain. Geoderma 131(1–2):218–236
Marx SK, Kamber BS, McGowan HA, Petherick LM, McTainsh GH, Stromsoe N, Hooper JN, May J-H (2018) Palaeo-dust records: a window to understanding past environments. Glob Planet Change 165:13–43
Little SH, Munson S, Prytulak J, Coles BJ, Hammond SJ, Widdowson M (2019) Cu and Zn isotope fractionation during extreme chemical weathering. Geochim Cosmochim Acta 263:85–107
Sakal R, Singh A, Sinha R, Bhogal N (1996) Twenty five years of research on micro and secondary nutrients in soils and crops of Bihar. RAU, Pusa, Bihar 1–208
Penney D (2004) The micronutrient and trace element status of forty-three soil quality benchmark sites in Alberta. AESA Soil Quality Monitoring Program
Smedley PL, Kinniburgh DG (2017) Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl Geochem 84:387–432
Cicchella D, Giaccio L, Dinelli E, Albanese S, Lima A, Zuzolo D, Valera P, De Vivo B (2015) GEMAS: spatial distribution of chemical elements in agricultural and grazing land soil of Italy. J Geochem Explor 154:129–142
Zuzolo D, Cicchella D, Lima A, Guagliardi I, Cerino P, Pizzolante A, Thiombane M, De Vivo B, Albanese S (2020) Potentially toxic elements in soils of Campania region (Southern Italy): Combining raw and compositional data. J Geochem Explor 106524
Sillanpää M (1982) Micronutrients and the nutrient status of soils: a global study, FAO Soil Bull 48, Rome
Horneck DA, Sullivan DM, Owen JS, Hart JM (2011) Soil test interpretation guide
Kuldeep S (2009) The critical zinc deficiency levels in Indian soils and cereal crops. The Proceedings of the International Plant Nutrition Colloquium XVI. UC Davis. https://escholarship.org/uc/item/4h0788h5. Accessed 17 Dec 2020
Perez-Espinosa A, Moral R, Moreno-Caselles J, Cortes A, Perez-Murcia M, Gomez I (2005) Co phytoavailability for tomato in amended calcareous soils. Biores Technol 96(6):649–655
Thornton I, Abrahams P (1983) Soil ingestion—a major pathway of heavy metals into livestock grazing contaminated land. Sci Total Environ 28(1–3):287–294
Green N, Johnson D, Wilkins B (1996) Factors affecting the transfer of radionuclides to sheep grazing on pastures reclaimed from the sea. J Environ Radioact 30(2):173–183
Bhanderi B, Goswami A, Garg M, Samanta S (2016) Study on minerals status of dairy cows and their supplementation through area specific mineral mixture in the state of Jharkhand. J Anim Sci Technol 58(1):42
Pettygrove G, Heinrich A, Eagle A (2010) Dairy manure nutrient content and forms. Manure Technical Guide Series Davis: University of California Cooperative Extension
McBride MB, Spiers G (2001) Trace element content of selected fertilizers and dairy manures as determined by ICP–MS. Commun Soil Sci Plant Anal 32(1–2):139–156
Tandon H (1997) Organic resources: an assessment of potential supplies, their contribution to agricultural productivity and policy issues for Indian agriculture for 2000–2025. National Academy of Agricultural Sciences, New Delhi (Plant Needs Supply, Efficiency and Policy Issues) 15–28
Husbandry (2014) Basic animal, and fisheries statistics. Ministry of Agriculture Department of Animal Husbandry, Dairying and Fisheries, Krishi Bhawan, GOI, New Delhi
Takkar P, Singh M, Ganeshmurthy A (2000) Micronutrient deficiency and fertilizer requirement by 2025. In: Proceedings of national symposium on plant nutrient needs, supply, efficiency and policy issues for 2000–2025, 1997. NAAS, New Dehli, pp 218–224
Katyal J, Rao N, Reddy M (2001) Critical aspects of organic matter management in the tropics: the example of India. Nutr Cycl Agroecosyst 61(1–2):77–88
FAO (2005) Fertilizer use by crop in India. FAO, Rome. http://www.fao.org/3/a0257e/A0257E00.htm. Accessed 17 Dec 2020
Dhaliwal SS, Naresh R, Mandal A, Walia M, Gupta RK, Singh R, Dhaliwal M (2019) Effect of manures and fertilizers on soil physical properties, build-up of macro and micronutrients and uptake in soil under different cropping systems: a review. J Plant Nutr 42(20):2873–2900
Singh A, Shivay YS (2016) Effect of summer green manuring crops and zinc fertilizer sources on productivity, Zn-uptake and economics of basmati rice. J Plant Nutr 39(2):204–218
Franklin R, Duis L, Brown R, Kemp T (2005) Trace element content of selected fertilizers and micronutrient source materials. Commun Soil Sci Plant Anal 36(11–12):1591–1609
Kidd J, Manning P, Simkin J, Peacock S, Stockdale E (2017) Impacts of 120 years of fertilizer addition on a temperate grassland ecosystem. PLoS ONE 12(3):e0174632
Kumar V, Sharma A, Kaur P, Singh Sidhu GP, Bali AS, Bhardwaj R, Thukral AK, Cerda A (2019) Pollution assessment of heavy metals in soils of India and ecological risk assessment: a state-of-the-art. Chemosphere 216:449–462
Singh MV (2001) Evaluation of current micronutrient stocks in different agro-ecological zones of India for sustainable crop production. Fertiliser News 46(2):25–42
Acknowledgements
This work is a contribution of the Marie Curie Initial Training Network IsoNose (www.IsoNose.eu) funded by the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007–2013/under REA Grant Agreement No. [608069]. We thank Cora McKenna for assistance of the trace metal analysis and Mark Kavanagh for the organic carbon analysis and assistance with DTPA-extractions and analysis. We are indebted to Dhanajay Mohabey and Bandana Samant (Rashtrasant Tukadoji Maharaj Nagpur University) who provided invaluable assistance and expertise both in the field, and with wider institutional support. We thank two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suhr, N., Widdowson, M. & Kamber, B.S. The role of pedogenesis and natural fertiliser as vectors for essential metal content in agricultural topsoils, Central India. SN Appl. Sci. 3, 40 (2021). https://doi.org/10.1007/s42452-020-03982-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03982-7