Abstract
Geochemical evaluation of the species and potential sources of P in groundwater in and around Mataram City, Lombok Island, Indonesia can aid in the initial research on evaluating the fate of P when detailed geological information is unavailable. The results of ion chromatography and inductively coupled plasma-optical emission spectroscopy show that the concentrations of PO43– and total dissolved P (TDP) in groundwater range from approximately 0.1 to 8.5 mg l–1 and from 0.04 to 2.95 mg l–1, respectively. Dissolved inorganic P accounted for 86%, on average, of the TDP concentration, and PO43– represented the predominant P species in this groundwater. The potential sources for most of this dissolved PO43–, according to indices developed based on the PHREEQC software and groundwater quality data, could be the dissolution of hydroxyapatite and/or vivianite minerals. However, the potential sources of dissolved PO43– in groundwater with a TDP concentration of ≥ 1 mg l–1 is likely to be the reduction of Fe(III)–(hydro)oxides, the initial decomposition of organic matter, or the dissolution of carbonate-rich fluorapatite considering the Fe2+, dissolved organic carbon, Ca2+ and F− concentrations measured. In addition, as several groundwater samples had a TDP concentration of ≤ 1 mg l–1 and comparatively high concentration of NO3–, other potential sources of dissolved PO43– in this groundwater could be anthropogenic.
Similar content being viewed by others
1 Introduction
In recent years, extensive research has been conducted on submarine groundwater discharge in tropical developing regions such as Indonesia because such groundwater discharge can be an important contributor of nutrients to coastal marine ecosystems [1,2,3]. This is particularly important in Indonesia, which hosts one of the largest coastal ecosystems consisting predominantly of coral reefs [4]. Therefore, it is important to clarify the nutrient cycle of submarine groundwater discharge processes in the area where this occurs and understand the nutrient cycle of groundwater in coastal recharge areas in Indonesia.
The population and the levels of industrial and economic activities including tourism are increasing rapidly in Indonesia, and understanding the impacts these have on the natural environment can lead to increased awareness about groundwater quality. Many studies on groundwater pollution, especially from nitrogen and arsenic, have been conducted in Indonesian coastal cities such as Jakarta, Mataram, and Indramayu [5,6,7,8,9,10,11,12]. However, phosphorus cycling in groundwater ecosystems, which relates to nutrients present in groundwater in such cities, has not been extensively investigated.
Previous detailed research on the phosphorus cycle in groundwater has been conducted mainly on highly contaminated septic systems in shallow sand aquifers [13,14,15]. In the UK, although the phosphorus cycle of groundwater in sandstone, chalk, and limestone aquifers—among others—has been described, phosphorus is still less characterized than nitrogen in groundwater [16]. In the USA, the subsurface transport of orthophosphate in five agricultural watersheds composed of deposits of sand and clayey silt, alluvium, sand and gravel, glacial debris, and quartz sand and gravel has been considered in the studies of stream water eutrophication [17, 18]. In addition, Lewandoski et al. [19] extensively reviewed nutrient transport from groundwater to lake water. Given the adverse impacts of high nutrient concentrations in ecosystems, there is urgent need for detailed research to quantify the different sources of nutrients in groundwater and the likelihood of these being transported, transformed, and stored along flow paths [19]. More recently, there have been an increasing number of studies on the phosphorus cycle as it relates to arsenic contamination in groundwater in South and Southeast Asian floodplains and delta aquifers [20, 21]. However, the complete analysis of the phosphorus cycle in aquifers in volcanic deposits on a volcanic island has yet to be conducted.
Therefore, herein, the area around Mataram City, which is located on the coast of a volcanic island, is used as a study site. The main objectives of this study were to 1) study the species and distribution of phosphorus in the local groundwater and 2) evaluate potential sources of phosphorus and its species using groundwater quality data. Future research will focus on submarine groundwater in this area.
2 Study site
The island of Lombok is a part of the Lesser Sunda Islands chain, with the Lombok Strait dividing Bali to the west and Sumbawa to the east. It experiences consistent sunlight for several hours and a warm temperature throughout the year—a minimum of 24 °C and a maximum of 34 °C during hot months and 20 °C–31 °C during cooler months. There is a dry season that runs from April to September and a rainy season that stretches from October to March [22].
One of the most striking features of the Lombok Island region is that most of its cities and densely populated settlements are located in valleys or sedimentary basins or on hills. It has a total area of 4,725 km2 and is a part of the West Nusa Tenggara Province of Indonesia. The provincial capital and largest city is Mataram, which is located on the western part of the island. The population of Mataram was 441,064 in 2015 [23].
The Lombok Island region experiences a very high level of seismic activity; the last known eruption was in September 2016 and the most recent earthquake with a magnitude of 6.9 occurred in September 2018. This is due to its position in the Indian Ocean, which places it adjacent to the zone of collision between the Eurasian and Indo-Australian plates [24]. Lombok Island is composed of relatively young Tertiary to Quaternary sediment. The lithology of Lombok Island is dominated by volcanic rocks such as volcanic breccia, various igneous rocks, and sandstone. The eastern part of Lombok Island is formed of breccia, igneous rock, and tuff, among others, from the Tertiary sediment; the western part of the island consists of alluvial or loose rock such as sand, gravel and mud; and the northern part of the island consists of Quaternary volcanic rock [24]. In Antareja, Mataram City, the first layer is composed of smoothly alternating of clay, sand, gravel, coarse grains of pumice, mud, and black sand, with an average thickness of 13 m, extending up to a depth of 28 m from the ground surface. The second layer consists of fine sand and black mud. The third layer is silt and fine sand saturated with water and occurs at an average depth of 39 m from the ground surface. The third layer, which began at a depth of 47 m from the ground surface, is interpreted as the bedrock consisting of sandstone and mudstone [25]. The rock that made up the layers of the bed of the volcanic deposits included clay, gravel, pumice sand, sand, rough pumice granules, silt, and fine black sand [25].
3 Hydrochemical sampling and analyses
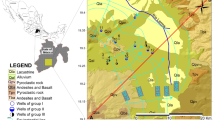
Groundwater samples were obtained from 19 wells in and around Mataram City and 1 natural spring (Fig. 1). The sampling points were located in an alluvial formation, dating from the Holocene period [26], and consists of pebbles, granule, sand, clay, peat, and fragments of coral (Fig. 2). It is a lowland surrounded by volcanoes (part of the Kalibabak and Pengulung formations). Standing water equivalent to at least one well volume was drawn from the wells before the groundwater samples were collected. The sampling campaign was conducted from September 4–7, 2017, i.e., during the dry season. Field measurements of the pH of the water at its surface were carried out using a pH meter (WM-32EP, DKK-TOA Co.).
Geology of Lombok Island (adapted from [26])
Water samples were typically collected over a period of 1–2 h. First, unfiltered splits were taken for the field measurements of pH and the laboratory measurements of alkalinity; samples for the latter were collected in 100 mL polyethylene bottles. Filtered splits (filter: polytetrafluoroethylene; DISMIC-25HP; pore size: 0.2 μm) were taken for the laboratory measurements of dissolved organic carbon (DOC) and cation and anion concentrations. Subsamples of the filtered water sample were transferred to 100 mL polyethylene bottles and acidified to pH < 2 using ultrapure nitric acid before the concentrations of Fe, Mn, Sr, Ba, B, Cu, Zn, Si, and P were measured. Unacidified splits needed for cation (Na+, NH4+, K+, Mg2+, and Ca2+) and anion (F–, Cl–, Br–, NO3–, PO43– and SO42–) analyses were kept in 100-mL polyethylene bottles, while those for DOC estimation were kept in 50 mL glass vials.
The elemental analyses were performed at the geothermal engineering laboratory at Hirosaki University, where the concentrations of Fe, Mn, Sr, Ba, B, Cu, Zn, Si, and P were determined using inductively coupled plasma-optical emission spectroscopy (Optima 7000DV, PerkinElmer Co. Ltd.). The concentrations of Na+, NH4+, K+, Mg2+, and Ca2+ were determined via ion chromatography with conductivity detection (ICS-1100, Thermo Fisher Scientific Inc.). The concentrations of F–, Cl–, Br–, NO3–, PO43–, and SO42– were determined via ion chromatography with conductivity detection (ICS-2100, Thermo Fisher Scientific Inc.). The DOC concentration was measured using a total organic carbon analyzer (TOC-VCPH, Shimadzu Co.). Alkalinity measurements were carried out in the field via titration, and the values were expressed in terms of the concentration of HCO3– (milligrams per liter of water). In terms of the error in the charge balance between the cations and anions, 16 samples were within ± 3%, 2 were within + 4%, and another 2 samples were within + 9%.
The saturation indices (SI = log (ion activity product/solubility product)) of the groundwater samples were calculated using the thermodynamic database (phreeqc.dat) for a mineral phase that might serve as a sink or source for PO43–, as provided with the aqueous speciation software PHREEQC ver.3 [27]. An annual average air temperature of 27 °C was used as the temperature of analysis because the actual temperature of the groundwater in situ was unknown.
4 Results and discussion
4.1 Phosphorus species and distribution
The dissolved phosphorus species in 19 samples of groundwater and 1 sample of spring water taken in and around Mataram City were analyzed and the concentrations of total dissolved phosphorus (TDP; or total filterable phosphorus [28]) and PO43– were determined. The concentrations of TDP and dissolved PO4–P, as measured using inductively coupled plasma-optical emission spectroscopy and ion chromatography, ranged from 0.04 mg l–1 to 2.95 mg l–1 and from 0.028 to 2.74 mg l–1, respectively (Table 1). The concentration of dissolved PO4–P of groundwater from parts of Lombok Island that were outside the study area, measured using standard photochemical methods, ranged from 0.018 to 0.384 mg l–1 (i.e., ranged from 0.58 to 12.4 μmol l–1 as PO4) according to Oehler et al. [3]. The ratio of dissolved PO4–P that was related to TDP ranged from 67 to 96%; exceptions were values of 14%, 35%, and 172%, respectively, in wells LM9, LM15, and LM4 (Table 1). The reason for the high value from well LM4 is unknown; however, the very low values (14% and 35%) from wells LM9 and LM15 could possibly be due to the fact that two groundwater samples with a high Fe2+ concentration (1.70 and 2.71 mg l–1) were precipitated before the ion chromatography analysis, while PO43– in the groundwater samples was co-precipitated with Fe(III)–(hydr)oxides [21, 29]. Dissolved inorganic P accounted for 86%, on average, of the TDP concentrations, while PO43– represented the predominant ion species in this groundwater. The latter result is similar to that obtained in the analyses of groundwater in Asian floodplain and delta aquifers [21]. Figure 3 shows the relationship between TDP concentration and well depth; if the latter was unknown, it is described in the caption of Fig. 3. It was found that the groundwater from wells LM1-2, LM14, and LM18 sampled from wells with a depth of 50 m contained more P compared with samples taken from wells of other depths (Fig. 3). The TDP concentration of these samples was also relatively high, compared to the others.
4.2 Potential sources of dissolved PO4 3–
One possible source of dissolved PO43– is the dissolution of minerals (e.g., apatite) that may contain P in some phases [21, 30, 31]. Volcanic rocks and deposits contain P [32, 33], as does lava on Lombok Island; for the latter, the levels of P detected were reported as percentages of P2O5 ranging from 0.15 to 0.47 [34] and 0.19 to 0.27 [35]. The presence of apatite was also reported [35]. In addition, the microbial degradation of organic carbon (sedimentary and dissolved) and the concomitant reductive dissolution of Fe(III)–(hydro)oxides represent another potential source of P that could have released PO43– into groundwater [21]. Other potential sources include leachate from arable land and leaking septic tanks or sewer pipes [13,14,15, 17, 18, 36].
Taking the first potential source into consideration, the contribution of the dissolution of hydroxyapatite and vivianite was evaluated on the basis of the calculated SI (Figs. 4 and 5). Only groundwater sampled from well LM15 appeared to be over-saturated with hydroxyapatite and vivianite. The groundwater sampled from wells LM1–2, LM14, and LM18 were over-saturated with hydroxyapatite only (Fig. 4). In addition, the groundwater sampled from wells LM17 and LM22 was nearly saturated with hydroxyapatite (Fig. 4), while that sampled from well LM18 was nearly saturated with vivianite (Fig. 5). Thus, the dissolved PO43– in groundwater from all the samples could potentially be sourced through the dissolution of hydroxyapatite and/or vivianite. Notably, the presence of apatite in volcanic lava deposits from Mount Rinjani has already been reported [35]. However, only well LM15 may have other sources.
Saturation index for a potential phosphate-bearing phase of vivianite, as calculated by PHREEQC. For the PHREEQC analysis of the groundwater sampled from wells LM9 and LM15, the concentrations of total dissolved phosphate, instead of PO43 − , were used as the input value for P. All Fe concentrations measured using inductively coupled plasma-optical emission spectroscopy were input to PHREEQC as Fe2+. When the Fe concentration measured using this method was less than the lower limit of quantification, the lower limit of quantification was entered in PHREEQC
The groundwater sampled from well LM15 contained 1.31 mg l–1 of TDP and high concentrations of Fe2+, DOC, and NH4+ (Figs. 6, 7, and 8). Thus, the reduction of Fe(III)–(hydro)oxides or the initial decomposition of organic matter are considered potential key mechanisms underlying the enrichment of PO43– in groundwater from this well. Furthermore, one characteristic of the groundwater sampled from wells LM1-2 and LM18 is that the Ca2+ concentrations are very low, at 1.57 mg l–1 and 5.8 mg l–1, respectively, while the concentrations of F– are high at 1.14 mg l–1 and 1.76 mg l–1, respectively (Figs. 9 and 10). In addition, the concentrations of DOC, Fe2+, NO3–, and NH4+ in these samples were low (Figs. 6, 7, 8, and 11). These results indicate that the origin of dissolved PO43– in these wells is neither the microbial degradation of organic carbon nor the concomitant reductive dissolution of Fe(III)–(hydro)oxides. Furthermore, these results show that there is no anthropogenic influence here as these samples contain no N components. Thus, the dissolved PO43– in these samples may be considered to have a geological origin—the dissolution of carbonate-rich fluorapatite [31]. The low Ca2+ concentrations are most likely a result of several mechanisms, including (1) mostly cation exchange reactions and (2) to a lesser degree, the formation of the hydrated di-basic calcium phosphate surface and sorption [31].
Finally, the groundwater sampled from well LM14 had high DOC and NH4+ concentrations (Figs. 7 and 8). The decomposition of organic matter is considered the potential source of dissolved PO43– in these groundwater samples, particularly given that the latter may have a very low concentration of Fe(III)–(hydro)oxides. In addition, because the groundwater sampled from wells LM12, LM17, LM21, LM22, and LM23 have a relatively high concentration of NO3– (Fig. 11), it is also likely that anthropogenic sources may be considered as having a potential impact on the levels of dissolved PO43– measured therein.
5 Conclusion
The chemical composition of groundwater in and around Mataram City, Lombok Island, Indonesia was investigated to identify the species and potential sources of P in groundwater. The concentration of TDP in the groundwater samples ranged from approximately 0.04 to 2.95 mg l–1, with PO43– representing the predominant ion therein. The potential source of dissolved PO43– in most of the groundwater samples is the dissolution of hydroxyapatite and/or vivianite. However, the potential source of dissolved PO43– in groundwater with a TDP concentration of ≥ mg l–1 is more likely to be the reduction of Fe(III)–(hydro)oxides, the decomposition of organic matter, or the dissolution of carbonate-rich fluorapatite. The geochemical approach used in this study was effective, even in the absence of detailed geological data, for the evaluation of the potential sources of P in groundwater. The geochemical findings could aid researchers working on coastal marine ecosystems.
References
Adyasari D, Oehler T, Afiati N, Moosdorf N (2018) Groundwater nutrient inputs into an urbanized tropical estuary system in Indonesia. Sci Total Environ 627:1066–1079. https://doi.org/10.1016/j.scitotenv.2018.01.281
Oehler T, Eiche E, Putra D, Adyasari D, Hennig H, Mallast U, Moosdorf N (2018) Seasonal variability of land-ocean groundwater nutrient fluxes from a tropical karstic region (southern Java, Indonesia). J Hydrol 565:662–671. https://doi.org/10.1016/j.jhydrol.2018.08.077
Oehler T, Bakti H, Lubis RF, Purwoarminta A, Delinom R, Moosdorf N (2019) Nutrient dynamics in submarine groundwater discharge through a coral reef (western Lombok, Indonesia). Limnol Oceanogr 64:2646–2661. https://doi.org/10.1002/lno.11240
Burke L, Selig L, Spalding M (2002) Reefs at risk in Southeast Asia. World Resources Institute. https://www.wri.org/publication/reefs-risk-southeast-asia. Accessed 31 July 2020
Smith GD, Wetselaar R, Fox JJ, Hidayati D, Yogasawara H (2000) Ingestion of nitrate from well water by village people in Indonesia. AMBIO J Human EnviroN 29:525–527. https://doi.org/10.1579/0044-7447-29.8.525
Onodera S, Saito M, Sawano M, Hosono T, Taniguchi M, Shimada J, Umezawa Y, Lubis RF, Buapeng S, Delinom R (2008) Effects of intensive urbanization on the intrusion of shallow groundwater into deep groundwater: examples from Bangkok and Jakarta. Sci Total Environ 404:401–410. https://doi.org/10.1016/j.scitotenv.2008.08.003
Umezawa U, Hosono T, Onodera S, Siringan F, Buapeng S, Delinom R, Yoshimizu C, Tayasu I, Nagata T, Taniguchi M (2008) Source of nitrate and ammonium contamination in groundwater under developing Asian megacities. Sci Total Environ 404:361–376. https://doi.org/10.1016/j.scitotenv.2008.04.021
Delinom RM, Assegaf A, Abidin HZ, Taniguchi M, Suherman D, Lubis RF, Yulianto E (2009) The contribution of human activities to subsurface environment degradation in Greater Jakarta Area, Indonesia. Sci Total Environ 407:3129–3141. https://doi.org/10.1016/j.scitotenv.2008.10.003
Kagabu M, Shimda J, Delinom R, Tsuijimura M, Taniguchi M (2011) Groundwater flow system under a rapidly urbanizing coastal city as determined by hydrogeochemistry. J Asian Earth Sci 40:226–239. https://doi.org/10.1016/j.jseaes.2010.07.012
Hosono T, Nakano T, Shimizu Y, Onodera S, Taniguchi M (2011) Hydrological constraint on nitrate and arsenic contamination in Asian metropolitan groundwater. Hydrol Processes 25:27422754. https://doi.org/10.1002/hyp.8015
Suryono CA (2015) The toxic metal arsenic contamination of the coastal aquifers in the north coast of Java Indonesia. Tropical Mar J 18:76–81. https://doi.org/10.14710/jkt.v18i2.518
Rusydi AF, Saito M, Ioka S, Maria R, Onodera S (2019) Estimation of ammonium sources in Indonesian coastal alluvial groundwater using Cl– and GIS. Int J Geomate. 17:53–58. https://doi.org/10.21660/2019.62.4749
Robertson WD, Cherry JA, Sudicky EA (1991) Ground-water contamination from two small septic systems on sand aquifers. Groundwater 29:82–92. https://doi.org/10.1111/j.1745-6584.1991.tb00500.x
Robertson WD, Schiff SL, Ptacek CJ (1998) Review of phosphate mobility and persistence in 10 septic system plumes. Groundwater 36:1000–1010. https://doi.org/10.1111/j.1745-6584.1998.tb02107.x
Robertson WD, Van Stempvoort DR, Schiff SL (2019) Review of phosphate attenuation in groundwater plumes from 24 septic systems. Sci Total Environ 692:640–652. https://doi.org/10.1016/j.scitotenv.2019.07.198
Stuart ME, Lapworth DJ (2016) Macronutrient status of UK groundwater: nitrogen, phosphorus and organic carbon. Sci Total Environ 572:1543–1560. https://doi.org/10.1016/j.scitotenv.2016.02.181
Domagalski J, Johnson HM (2011) Subsurface transport of orthophosphate in five agricultural watersheds, USA. J Hydrol 409:157–171. https://doi.org/10.1016/j.jhydrol.2011.08.014
Puckett LJ, Zamora C, Essaid H, Wilson JT, Johnson HM, Brayton MJ, Vogel JR (2008) Transport and fate of nitrate at the ground-water/surface-water interface. J Environ Qual 37:1034–1050. https://doi.org/10.2134/jeq2006.0550
Lewandowski J, Meinikmann K, Nützmann G, Rosenberry DO (2015) Groundwater-the disregarded component in lake water and nutrient budgets. Part 2: effects of groundwater on nutrients. Hydrol Processes 29:2922–12955. https://doi.org/10.1002/hyp.10384
Winkel L, Berg M, Stengel C, Rosenberg T (2008) Hydrogeological survey assessing arsenic and other groundwater contaminants in the lowlands of Sumatra, Indonesia. Appl Geochem 23:3019–3028. https://doi.org/10.1016/j.apgeochem.2008.06.021
Neidhardt H, Schoeckle D, Schleinitz A, Eiche E, Berner Z, Tram PTK, Lan VM, Viet PH, Biswas A, Majumder S, Chatterjee D, Oelmann Y, Berg M (2018) Biogeochemical phosphorus cycling in groundwater ecosystems-Insights from South and Southeast Asian floodplain and delta aquifer. Sci Total Environ 644:1357–1370. https://doi.org/10.1016/j.scitotenv.2018.07.056
Danish Energy Agency, Ea Energy Analyses (2019) Lombok energy outlook. https://www.ea-energianalyse.dk/en/publications/1858-the-lombok-energy-outlook-2030/. Accessed 31 July 2020
Widayanti BH, Hirsan FP, Kurniawan A (2017) Effectiveness of waste management in Mataram City. IOP Conf Ser Earth Environ Sci. 70:01204
Yasin S, Brotopuspito KS, Sartohadi J, Suryanto W, Adhi MA (2014) Local seismic hazard assessment of the Mataram City, Indonesia based in single station microtremor measurement. https://www.researchgate.net/publication/285299292_LOCAL_SEISMIC_HAZARD_ASSESSMENT_OF_THE_MATARAM_CITY_INDONESIA_BASED_ON_SINGLE_STATION_MICROTREMOR_MEASUREMENT. Accessed 3 June 2020
Hiden H, Brotopuspito KS, Hadmoko DS, Lavigne F, Airaksinen KB, Mutaqin BW, Hananto ND, Handayani L, Sudrajat Y, Suryanto W (2017) The isopach mapping of volcanic deposits of Mount Samalas 1257 AD based on the values of resistivity and physical properties. Geosciences 7:67. https://doi.org/10.3390/geosciences7030067
Mangga SA, Atmawinata S, Hermanto B, Setyogroho B, Amin TC (1994) Geological map of the Lombok sheet, Nusatenggara Barat Scale: 250,000. Geological Research and Development Center, Banding, Indonesia
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In: U.S. geological survey techniques and methods, book 6, chap. A43, 497 p. https://pubs.usgs.gov/tm/06/a43/
Worsfold P, McKelvie I, Monbet P (2016) Determination of phosphorus in natural waters: a historical review. Anal Chim Acta 918:8–20. https://doi.org/10.1016/j.aca.2016.02.047
Crompton TR (1996) Determination of anions: a guide for the analytical chemist. Springer-Verlag, Berlin Heidelberg, Berlin
Edmunds WM, Smedley P (2013) Fluoride in natural waters. In: Selinus O (ed) Essentials of Medical Geology. Springer, Dordrecht, pp 311–336
Schafer D, Donn M, Atteia O, Sun J, MacRae C, Raven M, Pejcic B, Prommer H (2018) Fluoride and phosphate release from carbonate-rich fluorapatite during managed aquifer recharge. J Hydrol 562:809–820. https://doi.org/10.1016/j.jhydrol.2018.05.043
Felitsyn SB (2002) The redistribution of phosphorus in basic volcanic rocks. Lithol Miner Resour 37:94–96. https://doi.org/10.1023/A:1013695708085
Nanzyo M, Ebuchi Y, Kanno H (2003) Apatite in the pyroclastic flow deposit (1990–1995) of the Unsen volcano, Japan, and its utilization by buckwheat. Phosphorus Res Bull 16:1–10. https://doi.org/10.3363/prb1992.16.0_1
Foden JD (1983) The petrology of the calcalkaline lavas of Rindjani volcano, East Sunda: a model for Island Arc petrogenesis. J Petrol 24:98–130. https://doi.org/10.1093/petrology/24.1.98
Rachamat H, Rosana MF, Wirakusumah AD, Jabbar GA (2016) Petrogenesis of Rindjani Post-1257-Caldera-Forming-Eruption Lava Flows. Indones J on Geosci 3:107–126. https://doi.org/10.17014/ijog.3.2.107-126
Nyenje PM, Foppen JW, Kulabako R, Muwanga A, Uhlenbrook S (2013) Nutrient pollution in shallow aquifers underlying pit latrines and domestic solid waste dumps in urban slums. J Environ Manage 122:15–24. https://doi.org/10.1016/j.jenvman.2013.02.040
Funding
This work was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research B) Grant Number 17H04494.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ioka, S., Onodera, Si., Saito, M. et al. Species and potential sources of phosphorus in groundwater in and around Mataram City, Lombok Island, Indonesia. SN Appl. Sci. 3, 27 (2021). https://doi.org/10.1007/s42452-020-03975-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03975-6