Abstract
Pervaporation (PV) separation of water–isopropanol has been attempted using the blend membranes of poly (vinyl alcohol) (PVA) with poly (styrene-co-acrylonitrile) (PSA), and poly (vinyl alcohol) (PVA) with poly (styrene-co-methyl methacrylate) (PSM). Here, we described the fabrication of Hydrophilic–hydrophobic blend polymer membranes of PVA/PSM and PVA/PSA by solution casting method and cross-linked with glutaraldehyde (GA) in the presence of aqueous hydrochloric acid (HCl). These membranes were analyzed by Fourier transform (FTIR) infrared analysis, Field emission scanning electron microscopy (FESEM) analysis, Differential scanning calorimetry (DSC) and Thermo gravimetric analysis (TGA). Pervaporation experiments were conducted at 30 °C for 10, 12.5, and 15 wt.% of aqueous isopropanol feed mixtures and membrane performance was evaluated by calculating flux, selectivity and pervaporation separation index (PSI). Highest selectivity of 281 with flux of 0.076 kg/m2h has been observed PVA/PSM blend membrane when compared to pristine PVA membrane to dehydrate 12.5 wt.% water in feed mixture. Flux values were decreased from 0.112 to 0.076 kg/m2h to dehydrate 12.5 wt.% water in feed mixture for PVA/PSM blend membrane and plain PVA membrane. The developed blend membrane selectivity was improved dramatically whereas flux was decreased compared to the plain PVA membrane.
Similar content being viewed by others
1 Introduction
Isopropanol (IPA) is an essential alcoholic solvent in the chemical industry [1]. It has been extensively used for the removal of water in semiconductor and liquid crystal display industries, as a solvent in many pharmaceutical industries and also acts as a reaction reagent in organic reactions. IPA has been used in a large scale production of acetone through catalytic reduction [2]. IPA is widely used as a disinfectant and large amount of high purity IPA is always in demand for a cleansing agent in industries mainly paints and electronics. IPA used as a solvent, fuel additive and provides a comparatively non-toxic alternative to formaldehyde and also a pharmaceutical additive used in hand sanitizer and disinfecting pads [3]. However, isopropanol forms an azeotrope at 12.5 wt. % of water. It is not easy to break up the mixture at this stage by conventional distillation without adding a third component [4].
Pervaporation (PV) is a clean and energy-efficient separation process. Now-a-days, in the vision of the energy considerations and pollution concerns, this technology is considered to be a more environmentally friendly, effective and the convenient technique compared to conventional separation process in dehydrating organic solvents [5, 6], closer boiling point liquid mixtures and, azoetropes [7]. The basic principle regarding PV is the partial vapourization of the liquid separation mixture through a dense membrane barrier and the resulting component is in vapour form which is collected either by applying low pressure or by flowing inert medium on the permeate side [8]. The chemical potential gradient is the dynamic energy that helps in the passage of material through the membrane. The mechanism of separation in this process involves three stages: (i) sorption of the compounds in the membrane based on chemical affinity, (ii) concentration gradient dependent diffusion of the component through the membrane, (iii) desorption and evaporation of the substance on the membrane penetrating side [9, 10]. The first two steps are reliable for selective permeability. For industrial applications, the pervaporation may be seen as a new step in a classical separation/recovery process, coupled to another unit operation (distillation, liquid–liquid extraction) to enhance the overall process efficiency. In this sense, it is necessary to address the pervaporation in the scope of process intensification. A change of paradigm in pervaporation most probably will be achieved by joining these two fields of research.
In recent years, various polymeric membranes have been developed by many researchers for dehydration of organic solvents by PV separation applications. Several water attractive polymers such as poly (vinyl alcohol) (PVA), chitosan [11, 12], sodium alginate, gelatin, and their blends [13, 14] were used in the dehydration of organic solutions by pervaporation studies. Among different types of membranes studied, PVA is the most suitable and extensively studied polymer due its excellent hydrophilicity and good film forming ability. But, PVA membranes have shown low selectivity due to excessive swelling because of the presence of more abundant hydrophilic hydroxyl groups.
To improve its overall membrane performance in pervaporation separation, PVA needs to modify to achieve good mechanical stability and attain better selectivity in the dehydration of organic solvents. PVA has been subjected to modification through cross-linking [15], blending with other hydrophilic polymers [16], grafting [17], mixed matrix [18], zeolite filling [19], blending with hydrophobic polymer [20, 21], etc. Apart from these modifications, nano-hybrid [22], nanocomposite [23], hydrophobic composite membrane [24], sulphonated PVA membrane [25], multi-walled carbon nanotube incorporated PVA membrane [26], inorganic/organic hybrid nanocomposite membranes [27], different types of cross-linked PVA membrane [28], PVA ceramic membrane [29] and iron oxide nanocomposite [30] based on PVA were also reported in the literature for PV applications.
Susheelkumar et al. [21] studied hydrophilic–hydrophobic membrane poly(viny alochol) and poly (methyl methacrylate) (PVA/PMMA) for the separation of water–IPA azeotropic mixture using GA as a cross-linking agent. In this study, the observed flux and selectivity results were 0.075 and 400 respectively, for the 10 wt. % feed mixture. In another study, Bhat et al. [19] carried out PV studies using MCM-41-filled sodium alginate nanocomposite using GA as a cross-linking agent at 30 °C for the seperation of water–IPA mixture. In this study, flux for the plain sodium alginate membrane ranged between 0.067 and 0.340 kg/(m2 h), while for 20 mass % zeolite sodium alginate membrane, flux increased to 0.110 and 0.555 kg/(m2 h). Burshe et al. [31] conducted PV studies on PVA membrane using citric acid as a cross-linking agent for the separation of water–IPA. During this study, water flux and selectivities were 0.053 kg/(m2 h) and 291 respectively. In continues of research, Burshe et al. noticed flux and selectivity were 0.194 kg/(m2 h) and 116 using the PVA membrane with cross-linking agent citric acid [32].
Polymer membranes with enhanced selectivity and flux are all the time favored because an increase of flux, as well as selectivity at the same time, has been a major challenge in pervaporation studies. In any case, polymeric materials, as successful dewaterisation membranes, should keep a proper balance between swelling and selective separation of the water from organic molecules. One such way to maintain such balance is the introduction of hydrophobicity into a hydrophilic polymer matrix, which can control the swelling of the membranes in the aqueous organic solvents and also selective favors the separation of water from organic molecules. In the current work, in continuation of our previous work in developing polymer membranes for dehydration of aqueous organic liquids and to improve the overall PVA membrane performance, we account the fabrication of blend membrane of PVA with poly (styrene-co-acrylonitrile) (PSA) and poly (styrene-co-methyl methacrylate) (PSM) and their ability in dehydration of IPA by pervaporation. PSA exhibits the ease of processing of polystyrene combined with the rigidity and chemical resistance of polyacrylonitrile. Thus, PSA is broadly used in various applications due to excellent mechanical properties, chemical resistance and ease of processing. PSM has good mechanical and thermal properties, which is desirable for different kinds of applications.
2 Experimental
2.1 Materials
Poly (vinyl alcohol) 99% hydrolyzed (Mw = 85,000–124,000) and poly (styrene-co-acrylonitrile) (Mw = 650,000) and poly (styrene-co-methylmethacrylate) (Mw = 100,000–150,000) were obtained from Sigma Aldrich, USA. Isopropanol was obtained from Fisher Scientific, Mumbai. Glutaraldehyde (GA) ≥ 98% 25% aqueous solution, acetone, dimethyl sulfoxide (DMSO), hydrochloric acid (HCl) were obtained from Merck, Mumbai, India. Double distilled water was collected in our laboratory from the Distilon the pilot plant.
2.2 Blend membrane preparation
About 4 wt.% PVA polymer solution was prepared by solubilizing in 100 ml DMSO under controlled heating conditions at 90 °C on a hot plate magnetic stirrer until complete dissolution. Then, the heating was stopped, and stirring was continued for another 6 h. The homogeneous PVA solution was cast on a clean glass plate leveled horizontally using a spirit level. The membrane was allowed to dry for 1–2 weeks at ambient conditions. After that, the complete dried membrane was detached from the glass plate and cross-linked by dipping in 30:70 water–acetone mixture having 1 ml of GA and 2.5 ml of HCl for 10 h. Finally, the membrane was removed from the cross-linking bath and stored between filter papers for further use. Similarly, the Hydrophilic–hydrophobic blend membranes of PVA/PSA and PVA/PSM were fabricated by mixing the 4 wt.% solutions of hydrophobic polymers PSA and PSM with 4wt.% of PVA in 5:95 ml volume ratio. The membrane thickness as measured by a micrometer screw gauge was in the range of 35 µm. The schematic representation of membrane fabrication is shown in Scheme 1.
2.3 Characterization analysis
2.3.1 Fourier transform (FTIR) infrared and FESEM analysis
Infrared spectrum was performed on FTIR spectrophotometer (Perkin Elmer, model two, UK). About 2 mg of the membrane was grounded well with KBr powder and then pellet was made using hydraulic press at a pressure of 600 kg/cm2. Finally, spectra were recorded between 4000 and 400 cm−1 range. Field emission scanning electron microscope (FESEM) photographs of blend membranes were taken on SUPRATM55 with gold sputtering.
2.3.2 Thermal studies
Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) analysis of the pristine PVA and PVA/PSM and PVA/PSA blend polymer membranes were analyzed using SDT Q 600 (UK). Thermograms were recorded from 30 °C to 600 °C at the rate of heating 10 °C per min in an inert dry nitrogen gas.
2.3.3 Sorption studies
Sorption method is useful to study the interactions between polymer membranes and liquid penetrates. Equilibrium swelling studies of cross-linked plain PVA, PVA/PSA and PVA/PSM blend membranes were carried out at 10, 12.5 and 15 wt % of aqueous isopropanol feed mixtures at 30 °C. In brief, initial weights of the circularly cut cross-linked membrane was measured on an electronic digital microbalance (model no ALC 210.4, sensitive to ± 0.01 mg, Sartorius AG, Germany). The circularly cut disc membranes were kept in inside the test bottles containing 30 ml of different wt.% of feed mixtures and placed in hot air oven maintained at 30 °C. To control the errors because of evaporation losses, this process was finished in less than 20 s. The swelled membrane's weight was measured by carefully wiping the surface-bound liquid droplets after 48 h. The % degree of swelling (DS) was calculated using the Eq. (1)
where w1 and w2 initial weight of dry membrane and equilibrium membrane weight respectively.
2.3.4 Pervaporation testing
PV separation study was done in 100 mL batch mode stainless steel body PV setup equipped with a overhead stirrer on feed side and subjected to operate under a vacuum level of 0.005 mmHg at the permeate line. The testing membrane was placed at the center of the PV cell, which gives the effective membrane area of 20 cm2. The feed mixture was poured to the feed side and membrane was equilibrated for about 2 h. Then, the permeate was collected at downstream side of the PV apparatus by evacuating with a vacuum pump (Toshniwal, Mumbai, India) at a pressure of 10 torr and collected the vapor permeate by cooling using liquid N2. The obtained permeate sample was weighed on a balance to assess flux and then analyzed by using Abbe digital refractometer (model AR4, KRUSS Optronic GmbH, Germany) to evaluate the membrane selectivity. From the pervaporation testing, total permeation flux (Jp), selectivity (α), pervaporation separation index (PSI) was determined by using the following equations.
Here \({W}_{\mathrm{P}}\) is the weight of collected permeate (g), A membrane surface area (m2), t is the time for permeation of liquid (h), P and F the fraction of weight of permeate and feed, respectively. Subscript w and ‘org’ correspond for water–isopropanol. PV studies were carried out in triplicate at 30 °C.
3 Results and discussion
3.1 Fourier transform infrared (FTIR) spectral and FESEM studies
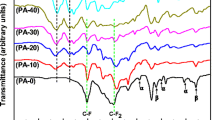
FTIR spectral results of plain PVA, PVA/PSA and PVA/PSM blend membrane are illustrated in Fig. 1. The effective cross linking of hydroxyl group of PVA with GA was confirmed by FTIR spectra. Spectra of all membranes shown strong and broad peak at high frequency region 3429 cm–1 indicated to –OH stretching vibrations [33]. The bands corresponding to the –CH2 asymmetric and the symmetric stretching were observed at 2923 cm−1 and 2854 cm–1. Two absorption bands at 1096 cm−1 and 1238 cm–1 observed correspond to ether linkage (C–O) and the acetal ring (C–O–C) created by the reaction between PVA with GA, which confirms that GA serves as a cross-linking agent. The peak at 1720 cm−1 with less intensity indicates that the –OH groups of PVA and the –CHO groups in GA participated in reaction entirely through acetal. In the spectrum of PVA/PSA, the bands between 2940 and 2900 cm−1 are due to C–H stretching vibration of alkyl groups of PVA chains [34]. The peak appeared at 2230 cm–1 indicated to stretching of –C≡N [35] and the characteristic peaks at 1597 cm−1 represent the aromatic C=C stretching of phenyl ring [36].
In the spectrum of PVA/PSM blend membrane, peak at 1140 cm–1 may be due to –OCH3 stretching mode, peak at 1413 cm–1 corresponds to –CH3 symmetrical deformation mode and broad peak ranging from 1240–1000 cm–1 indicated to ester C–O stretching vibration. A low-intensity peak at 2960–2850 cm−1 due to aliphatic C–H stretching vibration and at 1720 cm−1 region, a high-intensity band that correspond to the ester C=O bond stretching. At low-to-medium-intensity signals in the region of 1450–1350 cm−1 correspond to C–H bending vibrations, and those at 810 and 750 cm−1 to the skeleton CH2 group vibration [37]. This analysis confirmed the presence of PSA and PSM in the respective blend membranes and the cross-linking of the blend and plain PVA membrane. FESEM images of blend membranes of (A) PVA/PSA, (B) PVA/PSA cross section, (C) PVA/PSM and (D) PVA/PSM cross section were presented in Fig. 2. No phase separation was observed in these SEM images suggest that the polymer–polymer compatibility of blend components.
3.2 Results of thermal studies of membranes
The thermal analysis of the blend membrane was analyzed by differential scanning electron microscopy (DSC) and thermogravimetric analysis (TGA). In the present study, the glass transition temperature (Tg) and the effect of hydrophobic polymer on the Tg values of the pristine PVA in the blend were studied by DSC. DSC curves of PVA, PSA and PVA/PSA blend was given in Fig. 3A. Tg of plain PVA and PSA is 61 °C and 106 °C, respectively [38, 39]. A single Tgwas identified for PVA/PSA blend around 85 °C suggested that the good miscibility of the blend polymers at this composition. For PVA, the onset of melting temperature (Tm) was observed at 225 °C [38], whereas for PSA the melting was around 409 °C, which shifted to lower temperature of 360 °C in the PVA/PSA blend membrane. As illustrated in Fig. 3B, Tg of PVA is around at 61 °C and 130 °C for pure PSM [39]. In the case of PVA/PSM blend membrane, a single Tg was observed at about 80 °C suggested the miscibility of PVA and PSM in the blend at this composition. The onset of Tm for PSM is around 395 °C, which is shifted to a lower degree temperature of 370 °C, when mixed with PVA in the blend membrane.
TGA was employed to determine the thermal stability of the membrane by observing the weight loss of the sample under a nitrogen atmosphere as a function of temperature. The TGA results of the cross-linked PVA, pure PSA and PVA/PSA blend membranes presented in Fig. 4A shows the three stage weight loss pattern. The weight loss observed at around 100 °C may be due the release of moisture from the membranes. As observed, the plain PVA membrane exhibited two degradation steps. The first step was observed between 205 and 238 °C, and the second step was between 305 and 460 °C, corresponding to the melting and complete decomposition, respectively for PVA [32]. It can be seen from Fig. 4A that PSA started to lose weight at 392 °C and loses weight completely at 448 °C [40] and PSM lose weight at 340 °C and completely loses weight at 380 °C [41] (Fig. 4B).
3.3 Results of membrane swelling
According to theory of Fory-Rehner [37], the membrane swelling will be controlled by the amount of cross-linking agent used for the membrane fabrication. As a consequence, water flux becomes a factor regulating the rate of PV separation. Although simultaneous improvement in flow and selectivity is difficult in PV experiments, the judicial choice of the membrane material with an appropriate balance between Hydrophilic–hydrophobic natures is important. In the present study, hydrophilic PVA was blended with hydrophobic PSA and PSM, which can hinders the free mobility of PVA chains and reduces the excess swelling.
It was well established that the sorption properties of membranes are useful and helpful in selecting the appropriate membranes for pervaporation separation applications. Sorption, namely membrane solubility, is caused by the interaction of the species with the membrane materials. Since, sorption alone does not relate to permeability, diffusion through the membrane also contributes extensively to the permeation process suggesting that swelling plays an important role in the pervaporation performance of polymer membranes. The calculated results of the degree of swelling of the membranes in aqueous IPA mixtures are presented in Fig. 5.
The degree of swelling of the membranes increased with an increasing amount of water in the feed in both the blend membranes. This may be due to improved solublization of PVA polymer at a higher amount of aqueous feed mixture. This resulted further increase of the free channels in the membrane matrix. Compared to the plain PVA membrane, the degree of swelling of PVA/PSA and PVA/PSM blend membranes was decreased considerably. The addition of small amounts of hydrophobic moieties of PSM or PSA to PVA decreases the overall blend matrix's solubilization, thereby decreasing the swelling of the membrane matrix in water–isopropanol mixtures.
3.4 Membrane performance
Pervaporation transport concept is well interpreted in terms of solution-diffusion phenomenon [42, 43]. According to this phenomenon, the process involved in the transfer of mass from the early feed composition to the permeate zone via the membrane barrier is broken down into the following phases: transfer from the middle of the retentate flow to the membrane border line, adsorption of the components of the feed stream by the surface of the membrane, diffusion of components via the membrane, desorption of components into the permeate. Thus, preferential sorption characteristics of each membrane were explored in various feed mixtures. Sorption, namely solubilization of the membrane, is caused by the interaction of penetrating species with the membrane system. Since, the cross-linked plain PVA membrane has an uptake of > 70% for 15 wt. % of water containing feed mixture. However, for PVA/PSM and PVA/PSA membranes, the sorption values are lower than pristine PVA membrane. This may be due the plasticization effect [21]. This implies that when water molecules are come in contact with hydrophilic PVA membrane, more number of voids is generated between PVA chains due to excess swelling of the PVA membrane. The excess swelling was controlled by the addition of hydrophobic moieties. The sorption characteristics of membranes, are therefore, influenced by hydrophobic moiety in the PVA membrane matrix as well as amount of feed mixtures investigated. It can also be noticed that percentage of sorption capacities of the membranes increased with increasing water content of the feed mixture. In pervaporation, molecular transport takes place due to the existence of a concentration gradient on either side of the membrane. Generally, some amount of alcohol molecules can also diffuse through the PVA matrix along with water because of the polar nature of alcohol, which results in higher flux values. However, selectivity in dehydration of water from alcohol such as IPA is less. The permeation of alcohol can be reduced and water can be improved by modifying the PVA matrix [13,14,15,16,17,18,19]. The excessive swelling of PVA can be reduced by blending with hydrophobic PSA and PSM polymers, which can be expected to decrease the molecular transfer of alcohol molecules. Due to this, both free volume and the thermal mobility of the polymer chains in the membrane were controlled. This led to the diffusion of the water molecules over the IPA molecules due to the smaller molecular size of the water molecules. Pervaporation results of plain PVA, PVA/PSA, and PVA/PSM blend membranes in separating water–isopropanol mixtures were placed in Table 1, and flux and selectivity results are illustrated in Fig. 6a and b, respectively. Water flux of the plain PVA membrane increases from 0.098 to 0.143 (kg/m2h) with an increase in water content from 10 to 15 wt.% in feed mixtures due to the higher swelling of the membranes with increased content of water in feed mixtures. Similar behavior was noticed in the case of blend membranes also. Swelling curves could be the result of differences in the rate of molecular chain relaxation due to locally induced stresses in the polymer matrix and nature of liquid molecules transporting across the membrane. On the other hand, increasing the mass uptake was observed due to ingression of higher amount of water through the voids of the membrane matrix. However, the time required to attained equilibrium swelling varied depending upon the morphology of membrane.
For instance, flux enhanced from 0.062 to 0.091 kg/m2h and 0.059 to 0.106 kg/m2h for PVA/PSA and PVA/PSM blend membranes, respectively with increasing content of water in feed mixtures. However, these flux values for blend membranes are less when compared with the plain PVA membrane. This is because of the decreased swelling of the blend membranes due to the presence of hydrophobic PSA or PSM to the PVA compared to plain PVA membranes.
On the other case, plain PVA membrane showed selectivity of 80 for 10 wt.% of the water in the feed, which is reduced to 44 at 15 wt.% of water. But, blend membranes showed higher selectivity compared to the pristine PVA membrane system. PVA/PSA and PVA/PSM blend membranes have the highest selectivity of 455 and 475, respectively at 10 wt.% of water in the feed mixture. This may be due to reduced chain movement of PVA in the presence of hydrophobic PSA and PSM during solublization of membrane with feed mixtures during PV experiment, which controls the selective transport of more water molecules than IPA [20].
In PVA, due to the elastic nature of polymer chains, segments always exist in unsystematic movement, which allows feed molecules to diffuse through the void channels created due to fluctuations in transient gaps in the PVA polymer matrix. This phenomenon causes large scale swelling of the membrane, where moderately a quick diffusion could occur within the loose PVA matrix [21]. In addition, for glassy polymers such as PSA and PSM, comparatively, there exists less segmental motion, as a result controlling the diffusion of feed organic molecules through the membrane. This examination agrees with the swelling results of the blend membrane was lower when compared to the plain PVA membrane. However, with the increasing amount of wt.% of water in the feed, perm selectivity’s of blend membrane for feed mixtures decreased drastically, possibly due to the plasticization effect of hydrophobic polymers. However, the overall pervaporation separation can be explained based on the physical nature of the organic solvents, their attraction towards polymer membrane as well as the morphological set up of the membrane. In general, alcohol can also diffuse through the PVA matrix due to hydrophilic interactions between PVA and IPA causing excessive swelling. However, after adding hydrophobic moieties of PSA and PSM, the swelling of PVA is controlled, which will give a decrease in the diffusive transport of organic molecules since selectivity to water increases, The pervaporation separation index (PSI) values follow the same trends as in the case of selectivity, these values decrease with increasing water concentration in the feed mixture.
4 Conclusions
In this work, a successful attempt was made in fabrication of Hydrophilic–hydrophobic membranes of PVA/PSA and PVA/PSM. Highest selectivity of 281 with flux of 0.076 kg/m2h has been observed for PVA/PSM blend membrane when compared to pristine PVA membrane with flux and selectivity of 59 and 0.112 kg/m2h to dehydrate 12.5 wt.% water in feed mixture. The cross-linked plain PVA membrane has an uptake of > 70% for 15 wt.% of water containing feed composition. However, for PVA/PSM and PVA/PSA sorption values are lower than pristine PVA membrane. However, the lower values of flux are of some concern for successful practical utilization of these membranes. It was further demonstrated that this kind of membranes are effective in separating water from the water–IPA feed mixtures at their azeotropic composition.
Abbreviations
- PVA:
-
Poly (vinyl alcohol)
- PSA:
-
Poly (styrene-co-acrylonitrile)
- PSM:
-
Poly (styrene-co-methyl methacrylate)
- FTIR:
-
Fourier transform infrared
- FESEM:
-
Field emission scanning electron microscopy
- DSC:
-
Differential scanning calorimetry
- TGA:
-
Thermogravimetric analysis
- PSI:
-
Pervaporation separation index
- IPA:
-
Isopropanol
- PV:
-
Pervaporation
- GA:
-
Glutaraldehyde
- DMSO:
-
Dimethyl sulfoxide
- HCl:
-
Hydrochloric acid
- DS:
-
Degree of swelling
- T g :
-
Glass transition temperature
- T m :
-
Melting temperature
- WP :
-
Weight of collected permeate (g)
- A:
-
Membrane surface area (m2)
- t:
-
Time for permeation of liquid (h)
- P:
-
Weight of permeate
- F:
-
Weight of permeate feed
- W:
-
Water
- Org:
-
Isopropanol
- J p :
-
Flux
- Α :
-
Selectivity
References
Yilgor I, Wanxin Li, Ali Farajtabar, Rong Xing, Yiting Zhu, Hongkun Zhao, Rongguan Lv (2020) Thermodynamic solubility, solvent effect and preferential solvation analysis of rebamipide in aqueous co-solvent mixtures of propylene glycol, n-propanol, isopropanol and ethanol. J Chem Thermodyn 143:106045
Pato AH, Balouch A, Talpur FN, Abdullah AM, Mahar MT, Shah AK, Fahad SQ, Gabole AA (2020) Synthesis and catalytic practicality of titania@ITO-grown nanofakes: an excellent candidate for isopropanol conversion to acetone. Appl Nanosci 10:739–749
Golin AP, Dexter C, Aziz G (2020) Hand sanitizers: a review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am J Infect Control 48:1062–1067
Elenitsa B, Elpianna D (2018) Epaminondas Voutsas Separation of the isopropanol-water azeotropic mixture using ionic liquids. Fluid Phase Equilib 456:77–83
Amirilargani M, Sadatnia B (2014) Poly (vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J Memb Sci 469:1–10.
Eng Toon S, Kun Liang A, Wei H, Xuecheng D, Seeram R (2019a) Molecular sieve ceramic pervaporation membranes in solvent recovery: a comprehensive review. J Environ Chem Eng 7:103367
Jyothi MS, Kakarla Raghava R, Soontarapa K, Naveenc S, Anjanapura VR, Raghavendra VK, Suhas DP, Nagaraj PS, Mallikarjuna NN, Tejraj MA (2019) Membranes for dehydration of alcohols via pervaporation. J Environ Manage 242:415–429
Jing Z, Wanqin J (2017) Manipulation of confined structure in alcohol-perm selective pervaporation membrane. Chin J Chem Eng 25:1616–1626
Eng TS, Kun LA, Wei H, Xuecheng D, Seeram R (2019) Molecular sieve ceramic pervaporation membranes in solvent recovery: a comprehensive review. J Environ Chem Eng 7:103367
Xie Z, Ng D, Hoang M, Zhang J, Gray S (2018) Study of hybrid PVA/MA/TEOS pervaporation membrane and evaluation of energy requirement for desalination by pervaporation. Int J Environ Res Public Health 15(9):1913
Kumar BV, Sairam M, Raju KVSN, Aminabhavi TM (2005) Pervaporation separation of water + isopropanol mixtures using novel nanocomposite membranes of poly (vinyl alcohol) and polyaniline. J Memb Sci 260:142–155.
Jennifer Runhong Du, Hsu LH, Xiao ES, Guo X, Zhang Y, Feng X (2020) Using genipin as a “green” crosslinker to fabricate chitosan membranes for pervaporative dehydration of isopropanol. Sep Purif Technol 244(1):116843
Vijaya B, Naidu K, Aminabhavi TM (2005) Pervaporation separation of water/2-propanol mixtures by use of the blend membranes of sodium alginate and (Hydroxyethyl) cellulose: roles of permeate–membrane interactions. Zeolite Filling Memb Swell 44(19):7481–7489
Sajjan AM, Premakshi HG, Kariduraganavar MY (2018) Synthesis and characterization of polyelectrolyte complex membranes for the pervaporation separation of water–isopropanol mixtures using sodium alginate and gelatin. Polym Bull 75:851–875
Singha NR, Kar S, Ray S, Ray SK (2009) Chemical engineering and processing: process intensification separation of isopropyl alcohol–water mixtures by pervaporation using crosslin. IPN membranes 48:1020–1029
Yang J, Wang N, Chiu H (2014) Preparation and characterization of poly (vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J Memb Sci 457:139–148
Sajjan AM, Kumar BKJ, Kittur AA, Kariduraganavar MY (2013) Journal of Industrial and Engineering Chemistry Development of novel grafted hybrid PVA membranes using glycidyltrimethylammonium chloride for pervaporation separation of water–isopropanol mixtures. J Ind Eng Chem 19(2):427–437
Li T, Yu P, Luo Y (2014) Preparation and properties of hydrophobic Poly (vinylidene fluoride)–SiO 2 mixed matrix membranes for dissolved oxygen removal from waterr. J Applied Sci 40430:1–8
Bhat SD, Naidu BVK, Shanbhag GV, Halligudi SB, Sairam M, Aminabhavi TM (2006) Mesoporous molecular sieve (MCM-41)-filled sodium alginate hybrid nanocomposite membranes for pervaporation separation of water–isopropanol mixtures. Sep and Purif Techn 49:56–63
Omidali M, Raisi A, Aroujalian A (2014) Chemical engineering and processing: process intensification separation and purification of isobutanol from dilute aqueous solutions by a hybrid hydrophobic/hydrophilic pervaporation process. Chem Eng Process Process Intensif 77:22–29
Adoor SG, Manjeshwar LS, Naidu BVK, Sairam M, Aminabhavi TM (2006) Poly (vinyl alcohol)/poly (methyl methacrylate) blend membranes for pervaporation separation of water + isopropanol and water + 1, 4-dioxane mixtures. J Mem Sci 280:594–602
Premakshi HG, Sajjan AM, Kittur AA, Kariduraganavar MY (2015) Enhancement of pervaporation performance of composite membranes through in situ generation of silver nanoparticles in Poly (vinyl alcohol) matrix. J Appl Polym Sci 132:1–11
Zohreh R, Ahmad M, Morteza S, Amir A, Mehrdad A (2019) Titanate nanotubes–incorporated poly (vinyl alcohol) mixed matrix membranes for pervaporation separation of water-isopropanol mixtures. Chem Eng Res Des 145:99–111
Fu Y, Lai C, Chen J, Liu C, Huang S (2014) Hydrophobic composite membranes for separating of water–alcohol mixture by pervaporation at high temperature. Chem Eng Sci 111:203–210
Rachipudi PS, Kariduraganavar MY, Kittur AA, Sajjan AM (2011) Synthesis and characterization of sulfonated-poly (vinyl alcohol) membranes for the pervaporation dehydration of isopropanol. J Memb Sci 383(1–2):224–234
Mariia D, Anna K, Andrey Z, Sergey E, Denis R, Anastasia P (2020) Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. Appl IPA Dehydration Polymers 12:14
Magalad VT, Gokavi GS, Nadagouda MN, Aminabhavi TM (2011) Pervaporation separation of water–ethanol mixtures using organic–inorganic nanocomposite membranes. J Phys Chem C 115(30):14731–14744
Yu J, Haeng C, Hi W (2002) Performances of crosslinked asymmetric poly (vinyl alcohol) membranes for isopropanol dehydration by pervaporation. Chem Eng Process 41:693–698
Eng Toon S, Kun Liang A, Wei H, Xuecheng D, Seeram R (2019b) Molecular sieve ceramic pervaporation membranes in solvent recovery: a comprehensive review. J Environ Chem Eng 7(5):103367
Sairam M, Vijaya B, Naidu K, Kotrappanavar S, Sreedhar B, Aminabhavi TM (2006) Poly (vinyl alcohol)-iron oxide nanocomposite membranes for pervaporation dehydration of isopropanol, 1, 4-dioxane and tetrahydrofuran. J Mem Sci 283:65–73
Hui B, Zhang Y, Ye L (2014) Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem Eng J 235:207–214
Choi YS, Xu M, Chung IJ (2003) Synthesis of exfoliated poly (styrene-co-acrylonitrile) copolymer/silicate nanocomposite by emulsion polymerization; monomer composition effect on morphology. Polymer 44:6989–6994
Sachdeva S, Kumar A (2008) Synthesis and modeling of composite poly (styrene-co-acrylonitrile) membrane for the separation of chromic acid. J Appl Polym Sci 307:37–52
Burshe MC, Sawant SB, Joshi JB, Pangarkar VG (1997) Separation sorption and permeation of binary water-alcohol systems through PVA membranes crosslinked with multifunctional crosslinking agents. Sep purif Techn 12:145–156
Burshe MC, Netke SA, Sawant SB, Joshi JB, Pangarkar VG (1997) Separation science and technology pervaporative dehydration of organic Sslvents. Sep Sci Techn 32:1335–1349
Miura K, Kimura N, Suzuki H, Miyashita Y (1999) Thermal and viscoelastic properties of alginate/poly (vinyl alcohol) blends cross-linked with calcium tetraborate. Carbo polym 39:139–144
Wang E, Batra S, Cakmak M (2015) A real time study on drying and the mechano-optical behavior of polyvinyl alcohol films in solid and swollen state. Polymer 67:200–207
Hui B, Zhang Y, Ye L (2013) Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem Eng J 235:207–214
Asmaa S, Andras Jozsef T, Eniko H, Daniel F, Agnes S, Nora H, Mizsey P (2019) Preparation and characterization of PVA/GA/Laponite membranes to enhance pervaporation desalination performance. Sep Purif Techn 221:201–210
Martin FB, Diego FA, Denise L, Heidi RP, Cesar AB, Andrés FL (2015) Rapid fabrication of periodic patterns on Poly(styrene-co-acrylonitrile) surfaces using drect laser interference patterning. Int J Polymer Sci 2015:1–8
Zvonimir M, Marko R, Juraj S (2009) Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym Degrad Stab 94:95–101
Cai Y, Yuan Hu, Xiao J, Song L, Fan W, Deng H, Gong X, Chen Z (2007) Morphology, thermal and mechanical properties of poly (Styrene-Acrylonitrile) (SAN)/clay nanocomposites from organic-modified montmorillonite. Polymer-Plastics Technol Eng 46:541–548
Zubair M, Jose J, Al-harthi MA (2015) Evaluation of mechanical and thermal properties of microwave irradiated poly (styrene-co-methyl methacrylate)/graphene nanocomposites. Compos Interfaces 22:595–610
Siyang Mu, Guoa J, Chunfang Yu, Liua Y, Gonga Y, Zhanga S, Yang L, Qi S (2015) A novel solid-solid phase change material based on poly (styrene-co-acrylonitrile) grafting with Palmitic acid copolymers a novel solid-solid phase change material based on poly (styrene-co-acrylonitrile) grafting with palmitic acid copolymers. J Macro Sci 52:617–624
Jyoti L, Das D, Dolui SK (2010) Development of core–shell nano composite of poly (styrene-co-methyl acrylate) and bentonite clay by ultra sonic assisted mini-emulsion polymerization. Mater Chem Phys 124(2–3):1182–1187
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca
Binning RC, Lee RJ, Jennings JF, Martin EC (1961) Separation of liquid mixtures. Ind Eng Chem 53(1):45–50
Wijmans JG, Baker RW (1995) The solution-diffusion model : a review. J Memb Sci 107:121
Acknowledgements
The authors (BVKN and SSS) acknowledge the Department of Science and Technology (DST), New Delhi for releasing grant (SR/FT/CS-70/2010). We thankful to Dr. Krishna Rao, Dept. of Chemistry, Y. V. University, Kadapa for providing FT-IR facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sana, S.S., Badineni, V.R., Arla, S.K. et al. Hydrophilic–hydrophobic polymer based blend membrane for separation of water–isopropanol mixtures by pervaporation. SN Appl. Sci. 2, 1848 (2020). https://doi.org/10.1007/s42452-020-03700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03700-3