Abstract
This paper reports the synthesis of Fe3O4 ferrite nanoparticles and ferrofluid via a coprecipitation method. Structural studies were investigated using X-ray diffraction technique that confirmed the formation of single-phase cubic spinel structure. Morphology and particle size were elucidated using high-resolution transmission electron microscopy. Magnetic characteristics were investigated using a vibrating sample magnetometer, and saturation magnetization of nanoparticles was found to be 23.72 emu/gm with negligible coercivity and retentivity showing superparamagnetic behaviour. A functional self-cooling design employing ferrofluid (as a coolant) was demonstrated in which the system uses heat from the heat source and a permanent magnet to maintain the fluid flow that transfers heat to heat sink. The system is self-regulating and requires no power or pump for flowing of the fluid in the loop. The performance of the device on various parameters like volume fraction of nanoparticles, the temperature of heat source and strength of the magnetic field has also been studied. It was found that cooling by ~ 20 °C was achieved when temperature of heat source was maintained at 85 °C. Cooling performance was enhanced by ~ 7% when the concentration of nanoparticles in ferrofluid was increased from 2 to 6% and by ~ 4% upon the application of magnetic field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The cooling operation of many industrial systems is restricted by using traditional heat transfer fluids such as water, kerosene and ethylene glycol, which have poor thermal conductivity. By substituting these with higher thermal conductivity fluids, the performance of such devices can be improved. Choi [1], by adding nanoparticles to the fluids, presented an innovative way to improve their thermal conductivity. Suspending nanoparticles in a suitable carrier liquid alters the flow and heat transfer properties of the base fluid. Li et al. investigated the thermophysical properties of nanofluids of silver and single-walled carbon nanotubes by dispersing them in distilled water. They suggested that some nanofluids with suitable thermophysical properties can be used in the refrigeration system as the secondary working fluids in condenser [2]. Sarafraz et al. conducted a study to evaluate the efficiency and thermal performance of solar collector employing graphene-methanol nanofluids in heat pipes. It was found that graphene nanoplatelets enhanced the thermal conductivity of methanol by 20% [3]. Goodarzi et al. carried out a numerical study on the free convection of water-based nanofluids with Al2O3, Cu and multi-walled carbon nanotubes nanoparticles inside a closed cavity. They showed that density difference due to temperature gradient has a great effect on fluid circulation and hence heat transfer in the cavity [4]. Jalali et al. presented a numerical study of laminar flow and nanofluid jet injection of oil-based multi-walled carbon nanotube nanofluid in a microchannel. It was found that dispersing the solid nanoparticles in oil with a higher concentration and using nanofluid jet injection increased the rate of heat transfer by disturbing the thermal boundary layer [5]. Hassan carried out a comparative study on a series of nanofluid with magnetic and non-magnetic particles. They showed that although nanofluid with non-magnetic particles has a higher thermal conductivity than nanofluids with magnetic nanoparticles, the heat transfer rate is faster in magnetic nanofluid due to the applied magnetic field which can control the rate of heat transfer. Further, the heat transfer rate is enhanced by increasing the nanoparticles concentration and reducing the particle size [6]. Marin et al. examined the hydromagnetic transport of Fe nanoparticles suspended in water. They showed that the rate of heat transfer can be enhanced by increasing the concentration of nanoparticles in the back bone. However, increasing the dead-end particles improved the wall shear stress and decreased the rate of heat transfer [7]. Hassan et al. investigated the flow of ferrofluid over a rotating disc in the oscillating magnetic field. They found that when compared to zero-field condition, the particles rotate faster and the viscosity of ferrofluid is reduced, leading to enhanced heat transfer rate under an oscillating magnetic field [8]. Many other studies have been carried out investigating the role of nanofluids in the heat transfer rate [9,10,11,12,13].
Ferrofluids are colloidal suspension of magnetic nanoparticles in the magnetically passive carrier liquid. The nanoparticles are layered with a surfactant to maintain a stable state. However, due to dipole–dipole interactions and van der Waals forces between the magnetic nanoparticles, they may combine to form agglomerations at a high concentration. Without magnetic field, nanoparticles are oriented randomly, whereas, with magnetic field, they align in the applied field direction [14].
Many thermal management systems employing liquid cooling technology have been successfully used in various electronic devices. However, most of these techniques have drawbacks such as vibrating moving parts, noise problem, leakage, high maintenance and large power consumption due to driving pump [15,16,17]. From the last several decades, researchers have proposed liquid transport systems without pumps to overcome the aforementioned problems. Membrane structure-based actuators have been proposed like piezoelectric, magnetic, shape-memory alloy and thermo-pneumatic actuators [18,19,20,21]. However, considering the fluctuations in temperature and pulsating flow, they are not considered reliable.
In recent years, ferrofluid has fascinated many researchers in cooling application due to enhanced heat transfer characteristics. Experimental investigations show that the enhancement in heat transfer properties mainly depends on various parameters like volume fraction, size, chemical composition of nanoparticles, temperature of heat source, strength of magnetic field and base fluid. Many researchers in the past have investigated on heat transfer characteristics of ferrofluid in natural convection and reported the enhancement in heat transfer, making them suitable for cooling applications [22, 23]. Lajvardi et al. built an experimental set-up to study the heat transfer characteristics of ferrofluid in a heated copper tube. It was found that heat transfer is significantly enhanced by applying the magnetic field and by increasing the concentration of nanoparticles in ferrofluid. This enhancement was attributed to the improved thermophysical properties of ferrofluid [24]. Bahiraei et al. carried out a numerical investigation on water-based Fe3O4 ferrofluid in a square channel under the applied magnetic field. They studied the effect of various factors like concentration and diameter of nanoparticles, strength of magnetic field and Reynolds number. It was established that increasing the strength of magnetic field and using ferrofluid with a higher nanoparticle concentration and diameter lead to the enhancement in the heat transfer rate [25]. Wen et al. constructed an experimental system to study the heat transfer properties of nanofluids and asserted that the use of nanofluids significantly improved the heat transfer in the laminar flow regime, which was due to the migration of particles, resulting in higher thermal conductivity, and reduced the thickness of thermal boundary layer [26]. Li et al. established a miniature cooling device using ferrofluid and investigated its operational characteristics. They showed that the flow velocity of the ferrofluid increases on increasing the temperature of heat load, applying stronger magnetic field which enhances the performance of cooling device [27].
In this paper, Fe3O4 ferrite/ferrofluid has been prepared by a coprecipitation method. Structural, morphological and magnetic properties of developed nanoparticles have been studied using XRD, HRTEM and VSM. An automatic cooling device is constructed employing Fe3O4–kerosene ferrofluid, owing to their long-term stability. By analysing the experiments performed, the effect of concentration of nanoparticles in ferrofluid, magnetic field (external) and temperature of heat source on the efficiency of the cooling device is investigated and discussed.
2 Materials and methods
2.1 Synthesis
The synthesis of Fe3O4–kerosene-based ferrofluid involves mainly two steps: synthesis of Fe3O4 nanoparticles and dispersing them in a suitable carrier liquid, i.e. kerosene to get a stable ferrofluid. Fe3O4 nanoparticles were prepared by the chemical coprecipitation technique using analytical-grade chemical reagents (Sigma-Aldrich). In the process, iron(II) chloride (FeCl2) and iron(III) chloride (FeCl3) were chosen as initial precursors in the ratio (Fe2+/Fe3+) 1:2. Each of these was mixed separately with distilled water in appropriate molar quantities to get a homogeneous solution. These salt solutions were then mixed and stirred, while oleic acid was added dropwise to the solution to prevent their agglomeration. Later, under vigorous stirring and constant heating at 80 °C, analytical-grade ammonia (NH3) solution was put dropwise for immediate precipitation. The pH of the reaction mixture was monitored constantly, and ammonia was added until its pH reached 11. The nanoparticles obtained were washed several times with distilled water. Then, these samples were dispersed in suitable liquid (kerosene) and decanted to get a stable ferrofluid. The developed nanoparticles were spherical in shape with the diameter of 15–18 nm. Ferrofluid with different concentrations of nanoparticles, i.e. 2%, 4% and 6%, was prepared to carry out the study.
Synthesized nanoparticles were characterized on Rigaku Ultima-IV X-ray diffraction (XRD) having CuKα radiations at a scan rate of 2°/min for structural studies. Morphological studies were carried out using a high-resolution transmission electron microscopy (HRTEM) of Tecnai G2 20 (FEI) S-Twin at 200 kV. Magnetic studies were performed using Lakeshore VSM Model 7410 in the field range of ± 15,000 Gauss.
2.2 Experimental set-up for cooling
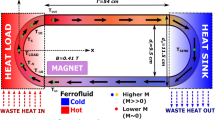
A self-powered cooling device was designed that may use the unwanted heat from electronic devices/some other sources to drive the fluid flow inside the loop and transfer the heat from source to sink. This device was designed by assembling a glass loop through which fluid flows, heat sink, heat source and a magnet (NdFeB). The fluid used has uniformly dispersed nanoparticles of average size 15–18 nm and saturation magnetization of ~ 23.72 emu/gm. A rectangular glass loop of diameter 7 mm and dimensions 250 mm × 140 mm was used as a fluid channel. Heat source and sink were kept in opposite directions. Heating chamber of dimensions 180 mm × 40 mm was built-in to provide a constant adjustable temperature with the help of high-temperature water bath with a good control on temperature and pressure. Iced water was taken as a heat sink, while a permanent magnet (NdFeB—0.3 T) was carefully adjusted near the loop and placed along the axial direction to the loop. The device was thermally insulated to avoid any kind of heat loss (Fig. 1).
Here, when ferrofluid in the loop comes in the thermal field of heat source maintained at a constant temperature, fluid heats up, rises (some thermal energy gets converted to kinetic energy), move towards the sink and consequently transfers the heat from source to sink. After losing the heat to sink, this cold ferrofluid progresses towards heat source and the cycle goes on without employing any pump. The permanent magnet induces a driving force that allows a higher convective heat transfer rate. So, the driving force, which is responsible for circulating the fluid, depends upon magnetic field and temperature. After some time, if all the conditions are kept unchanged, then loop temperature stops increasing and eventually become constant, when it attains the thermal stability. This device is self-regulating, is easy to handle and requires no maintenance using the heat from heat source (e.g. boiling water, heat-dissipated electronic devices, etc.) as a driving force for liquids and reliable in the field of cooling. The cooling efficiency of the device is affected by the temperature of the source, concentration of nanoparticles and external magnetic field as discussed below.
3 Results and discussion
3.1 XRD analysis
XRD of the synthesized samples is shown in Fig. 2, and the characteristic peaks are observed corresponding to the planes (220), (311), (400), (422), (511) and (440). The diffraction pattern obtained at room temperature indicated single-phase cubic spinel structure and was found to be consistent with the JCPDS Card No. 85-1436. The crystallite size (D) was calculated about the most intense peak at (311) plane from XRD data using the Debye–Scherrer formula [28] as given by:
where D is the crystallite size, k = 0.89 is the dimensionless shape factor, λ = 1.54056 Å is the incident X-ray wavelength of CuKα radiation, β is the line broadening at half the maximum intensity and θ is the glancing angle. The crystallite size was determined as 15.86 nm.
The interplanar spacing and lattice parameter for the synthesized sample was calculated as 2.52 Å and 8.35 Å, respectively, which are in good agreement with the standard data. The sample showed broad peaks, signifying the fine and small crystallite size; therefore, ferrofluids can be easily synthesized by making use of the small size of the particles.
3.2 HRTEM analysis
HRTEM image and electron diffraction pattern indicated the shape, size and morphology of the synthesized Fe3O4 nanoparticles as shown in Fig. 3. It showed that the examined particles have a spherical shape with a narrow size distribution. It can also be seen from the micrograph that the average particle size is about 15–18 nm and that the position of bright spots in electron diffraction pattern confirmed the spinel structure of synthesized nanoparticles.
3.3 VSM analysis
The variation of magnetization versus magnetic field at room temperature in the range ± 15,000 G for synthesized Fe3O4 nanoparticles is shown in Fig. 4. It can be seen that magnetization initially increased with magnetic field and then at higher fields it started to saturate. The values for coercive field and remanence magnetization were approximately zero which indicated the superparamagnetic behaviour of the tested nanoparticles. The curve revealed that the saturation magnetization of developed Fe3O4 nanoparticles was 23.72 emu/gm. Liu et al. [29] and Ghandoor et al. [30] reported saturation magnetization of 50.27 emu/gm and 46.7 emu/gm, which is significantly higher than obtained in the current research. The low saturation magnetization of the developed nanoparticles can be attributed to the superparamagnetic behaviour of the samples, spin canting effect and random distribution of particle size.
The saturation magnetization value for Fe3O4 nanoparticles depends upon superexchange interaction and particle size. The smaller the size is, the lesser the magnetization of the particle will be due to prominent surface effects in the nanoparticles due to spin canting disorder on the surface, since the surface-to-volume ratio increases significantly in nanoparticles and their surface is composed of some canted spins that prevent the core spins to align in the direction of magnetic field, hence decreasing their saturation magnetization [31,32,33].
4 Experimental set-up
4.1 Role of temperature of the heat source
By keeping the other conditions constant, experiments were performed to investigate the impact of heat source on cooling efficiency of the device in the presence and absence of the magnet. Figure 5 shows that enhanced cooling (ΔT) can be achieved when the temperature of source was changed from 63 °C to 85 °C. This can be understood based on the relation between flow velocity of fluid and the difference in temperature of source and sink. Random motion of nanoparticles plays an important part in the heat transfer process in the system where the velocity of the motion is directly proportional to the square root of the temperature. So, as the temperature of heat source rises, the flow velocity of the fluid inside the loop ascends, resulting in faster heat transfer. However, this cooling ability of the device is limited to below the boiling temperature of carrier fluid [34, 35].
4.2 Role of concentration of nanoparticles
To investigate the impact of nanoparticles concentration on cooling, a stable ferrofluid with 2%, 4% and 6% of nanoparticles in kerosene was prepared. Heat source temperature was fixed at 75 °C. Figure 6 shows that the efficiency of the cooling device improved as the concentration of particles was increased. This enhancement in the rate of heat transfer can be ascribed to Brownian motion, thermoconvection and development of chainlike structure of nanoparticles.
At low concentration of nanoparticles (2%), enhancement observed in heat transfer may be attributed to the following effects: (a) thermoconvection of fluid and (b) Brownian motion caused by the random motion of dispersed nanoparticles due to collision among themselves and with molecules of the base fluid. In thermoconvection, when the fluid enters the thermal field of source, it begins to rise towards the sink. Further, Brownian motion contributes to the enhancement in the rate of heat transfer in further two ways: first, by direct influence of diffused nanoparticles that transport heat and second, by indirect influence by microconvection of the base fluid [36].
At higher concentration, properties of the ferrofluid are influenced by interparticle interactions, as a result of chainlike structures formed due to dipole interactions among magnetic nanoparticles; however, Brownian motion becomes negligible. So, when the concentration of nanoparticles in ferrofluid increases, dipolar interaction becomes stronger and starts to form chainlike aggregates of nanoparticles which transport heat more effectively [37]. The formation of these chains in such a stable ferrofluid leads to highly conductive and lengthened path for the flow of heat that results in an increase in the rate of heat transfer, since solid particles conduct heat at a faster rate than the base fluid [38].
So, it can be noted from Fig. 6 that the cooling performance of the device is enhanced as the concentration of nanoparticles is increased from 2 to 6%. Asymptotic behaviour of the temperature in Fig. 6 can be understood as: here, the temperature of heat load was maintained at 75 °C, and when ferrofluid comes in its thermal vicinity, the temperature of the fluid in the system starts rising. Further, non-uniform temperature distribution and high temperature of heat load induce ferrofluid to circulate in the loop and transfer the heat from heat load to sink. The temperature of the system stops rising when thermal steady state is achieved. The temperature follows the same trend for all the volume fractions. Cooling performance is enhanced by 7% when the concentration of ferrofluid was increased from 2 to 6% due to increased interparticle interaction forming chainlike structures, which leads to highly conducting path for heat flow.
4.3 Role of magnetic field
Experiments were performed to investigate the impact of magnetic field on the device performance. Figure 7 shows that cooling is enhanced when the external magnetic field (0.3 T) was incorporated by 4% compared to when no field was applied. Heat conduction is the key mode of transport of energy inside the loop carrying ferrofluid in the absence of magnetic field, and their magnetic moments are randomly aligned. On the other hand, in the presence of the applied magnetic field, the driving force that is responsible for circulating the fluid inside the loop is the result of thermal and magnetic fields. In the absence of field, magnetic moments of the particles are randomly oriented, whereas when field is applied, they start to align themselves in the field direction. Here, the enhanced cooling can be ascribed to the formation of chainlike structures formed under the influence of magnetic field that conducts heat effectively [34, 39]. It should also be noted that the chainlike structures formed are highly ordered and fractal in nature. Once the magnetic field is removed, the magnetic moments are again randomly aligned, and there are no aggregates left due to superparamagnetic nature of magnetic nanoparticles. Moreover, magnetic field attracts the ferrofluid and also governs their direction of flow towards the heat source. Thus, the efficiency of the cooling device can be controlled by adjusting the aforementioned parameters.
Yarahmadi et al. [40] found out that the application of a constant magnetic field exerts an inverse effect on the convective heat transfer and causes the heat transfer to decline. Lajvardi et al. [24] found out that the use of Fe3O4 magnetic nanoparticles as the dispersed phase in water cannot enhance the convective heat transfer in the laminar flow regime in the absence of magnetic field. However, using our present device design, we have achieved cooling effects even in the absence of magnetic field, which increases even on the application of constant magnetic field. So, the drawbacks observed in earlier ferrofluid-based cooling device designs are removed in the present device. The studies carried by other researchers are on the role of device dependence on parameters like temperature gradient, heat load/sink temperature, role of AC or DC magnetic fields, etc., and their mathematical simulations [35, 41, 42]. Their studies are based on different experimental results and our present device design, and the results of measurements will surely add up for reaching to a good automatically cooling device using ferrofluids.
5 Conclusion
Fe3O4 ferrite/ferrofluid was prepared via a coprecipitation method. Structural and morphological studies were carried out using XRD and HRTEM. Lattice parameter and crystallite size were found to be 8.35 Å and 15.86 nm, respectively, and the particle size estimated by HRTEM was 15–18 nm. Magnetic studies revealed that saturation magnetization of the sample was 23.72 emu/gm with negligible retentivity and coercivity indicating superparamagnetic behaviour. A self-powered cooling device was presented that comprises sink chamber, heat source, a permanent magnet (NdFeB), ferrofluid and a thermally insulated glass tube. Various experiments were performed to study the effect of parameters like volume fraction of nanoparticles, temperature of heat source and magnetic field on the cooling efficiency of device. It was found that cooling by ~ 20 °C was achieved when the temperature of heat source was 85 °C. When the concentration of nanoparticles was increased from 2 to 6%, the cooling was enhanced by 7% and by 4% under the influence of magnetic field (0.3 T). This device also has many other advantages like:
-
1.
It is a self-powered, self-regulating device.
-
2.
It does not require any pump or moving parts.
-
3.
A variety of base fluids may be used as per requirement.
-
4.
Extent of cooling can be controlled.
-
5.
It is low cost.
-
6.
It is easy to maintain and a reliable system.
However, with the advancement in nanotechnology and nanomaterials, such a cooling device proposed may find a better application where waste heat from electronic devices may be utilized.
References
Choi SUS, Eastman JA (1995) Enhancing thermal conductivity of fluids with nanoparticles. Dev Appl Non-Newton Flows 231:99–105
Li ZX, Renault FL, Gómez AOC, Sarafraz MM, Khan H, Safaei MR, Filho EPB (2019) Nanofluids as secondary fluid in the refrigeration system: experimental data, regression, ANFIS, and NN modeling. Int J Heat Mass Transf 144:118635
Sarafraz MM, Safaei MR (2019) Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew Energy 142:364–372
Goodarzi H, Akbari OA, Sarafraz MM, Karchegani MM, Safaei MR, Shabani GAS (2019) Numerical simulation of natural convection heat transfer of nanofluid with Cu, MWCNT, and Al2O3 nanoparticles in a cavity with different aspect ratios. J Therm Sci Eng Appl 11:061020
Jalali E, Akbari OA, Sarafraz MM, Abbas T, Safaei MR (2019) Heat transfer of oil/MWCNT nanofluid jet injection inside a rectangular microchannel. Symmetry 11(6):757
Hassan M, Ellahi R, Bhatti MM, Zeeshan A (2019) A comparative study on magnetic and non-magnetic particles in nanofluid propagating over a wedge. Can J Phys 97:277–285
Marin M, Maskeen MM, Zeeshan A, Mehmood OU, Hassan M (2019) Hydromagnetic transport of iron nanoparticle aggregates suspended in water. Indian J Phys 93:53–59
Hassan M (2018) Impact of iron oxide particles concentration under a highly oscillating magnetic field on ferrofluid flow. Eur Phys J Plus 133(6):230
Sarafraz MM, Shadloo MS, Tian Z, Tlili I, Alkanhal TA, Safaei MR, Goodarzi M, Arjomandi M (2019) Convective bubbly flow of water in an annular pipe: role of total dissolved solids on heat transfer characteristics and bubble formation. Water 11(8):1566
Sarafraz MM, Tlili I, Tian Z, Bakouri M, Safaei MR, Goodarzi M (2019) Thermal evaluation of graphene nanoplatelets nanofluid in a fast-responding HP with the potential use in solar systems in smart cities. Appl Sci 9:2101
Sarafraz MM, Safaei MR, Tian Z, Goodarzi M, Filho EPB, Arjomandi M (2019) Thermal assessment of nano-particulate graphene-water/ethylene glycol (WEG 60:40) nano-suspension in a compact heat exchanger. Energies 12(10):1929
Nazari S, Ellahi R, Sarafraz MM, Safaei MR, Asgari A, Akbari OA (2019) Numerical study on mixed convection of a non-Newtonian nanofluid with porous media in a two lid-driven square cavity. J Therm Anal Calorim. https://doi.org/10.1007/s10973-019-08841-1
Hassan M, Fetecau C, Majeed A, Zeeshan A (2018) Effects of iron nanoparticles’ shape on convective flow of ferrofluid under highly oscillating magnetic field over stretchable rotating disk. J Magn Magn Mater 460:531–539
Rosensweig RE (1997) Ferrohydrodynamics. Dover Publications, New York
Zhang Y, Pang LP, Xie YQ, Jin SC, Liu M, Ji YB (2015) Experimental investigation of spray cooling heat transfer on straight fin surface under acceleration conditions. Exp Heat Transf 28:564–579
Fabbri M, Jiang S, Dhir VK (2005) A comparative study of cooling of high power density electronics using sprays and microjets. J Heat Transf 127:105–217
Yong H, Boon L, Xiaowu Z (2015) Package-level microjets-based hotspot cooling solution for microelectronic devices. IEEE Electron Device Lett 36:502–504
Shinozawa Y, Abe T, Kondo T (1997) A proportional microvalve using a bi-stable magnetic actuator. In: Proceedings of the 10th IEEE international workshop on MEMS, pp 233–237
Wang B, Chu X, Li E, Li L (2006) Simulations and analysis of a piezoelectric micropump. Ultrasonics 44:643–646
Wego A, Glock H, Pagel L, Richter S (2001) Investigations on thermo-pneumatic volume actuators based on PCB technology. Sens Actuators Part A 93:95–102
Benard WL, Kahn HA, Heuer H, Huff MA (1998) Thin-film shape-memory alloy actuated micropumps. J Microelectromech Syst 7:245–251
Zablotsky D, Mezulis A, Blums E (2009) Surface cooling based on the thermomagnetic convection: numerical simulation and experiment. Int J Heat Mass Transf 52:5302–5308
Jafari A, Tynjala T, Mousavi SM, Sarkomaa P (2008) Simulation of heat transfer in a ferrofluid using computational fluid dynamics technique. Int J Heat Fluid Flow 29:1197–1202
Lajvardi M, Moghimi-Rad J, Hadi I, Gavili A, Dallali Isfahani T, Zabihi F, Sabbaghzadeh J (2010) Experimental investigation for enhanced ferrofluid heat transfer under magnetic field effect. J Magn Magn Mater 322(21):3508–3513
Bahiraei M, Hangi M, Rahbari A (2019) A two-phase simulation of convective heat transfer characteristics of water-Fe3O4 ferrofluid in a square channel under the effect of permanent magnet. Appl Therm Eng 147:991–997
Wen D, Ding Y (2004) Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int J Heat Mass Transf 47(24):5181–5188. https://doi.org/10.1016/j.ijheatmasstransfer.2004.07.012
Li Q, Lian W, Sun H, Xuan Y (2008) Investigation on operational characteristics of a miniature automatic cooling device. Int J Heat Mass Transf 51(21–22):5033–5039
Cullity BD (1978) Elements of X-ray diffraction. Addison-Wesley, Reading
Liu ZL, Liu YJ, Yao KL, Ding ZH, Tao J, Wang X (2002) Synthesis and magnetic properties of Fe3O4 nanoparticles. J Mater Synth Process 10:83–87
El Ghandoor H, Zidan HM, Khalil M, Ismail MIM (2012) Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int J Electrochem Sci 7:5734–5745
Kumar V, Rana A, Yadav MS, Pant RP (2008) Size-induced effect on nano-crystalline CoFe2O4. J Magn Magn Mater 320:1729–1734
Calero-DdelC V, Rinaldi C (2007) Synthesis and magnetic characterization of cobalt-substituted ferrite (CoxFe3–xO4) nanoparticles. J Magn Magn Mater 314:60–67
Zhang Y, Yang Z, Yin D, Liu Y, Fei C, Xiong R, Shi J, Yan G (2010) Composition and magnetic properties of cobalt ferrite nano-particle prepared by the co-precipitation method. J Magn Magn Mater 322:3470
Phor L, Kumar V (2019) Self- cooling device based on thermomagnetic effect of MnxZn1-xFe2O4 (x = 0.3, 0.4, 0.5, 0.6, 0.7)/ferrofluid. J Mater Sci Mater Electron 30:9322–9333
Lian W, Xuan Y, Li Q (2009) Characterization of miniature automatic energy transport devices based on the thermomagnetic effect. Energy Convers Manag 50:35–42
Eapen J, Rusconi R, Piazza R, Yip S (2010) The classical nature of thermal conduction in nanofluids. J Heat Transf 132:102402
Genc S (2017) Heat transfer of ferrofluids. Nanofluid Heat Mass Transf Eng Probl. https://doi.org/10.5772/65912
Nkurikiyimfura I, Wang Y, Pan Z (2013) Heat transfer enhancement by magnetic nanofluids: a review. Renew Sustain Energy Rev 21:548–561
Philips J, Shima PD, Raj B (2007) Enhancement of thermal conductivity in magnetite based nanofluid due to chainlike structures. Appl Phys Lett 91:203108
Yarahmadi M, Goudarzi H, Shafii MB (2015) Experimental investigation into laminar forced convective heat transfer of ferrofluids under constant and oscillating magnetic field with different magnetic field arrangements and oscillation modes. Exp Therm Fluid Sci 68:601–611
Goharkhah M, Ashjaee M, Shahabadi M (2016) Experimental investigation on convective heat transfer and hydrodynamic characteristics of magnetite nanofluid under the influence of an alternating magnetic field. Int J Therm Sci 99:113–124
Lian W, Xuan Y, Li Q (2009) Design method of automatic energy transport devices based on the thermomagnetic effect of ferrofluids. Int J Heat Mass Transf 52:5451–5458
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phor, L., Kumar, V. Self-cooling by ferrofluid in magnetic field. SN Appl. Sci. 1, 1696 (2019). https://doi.org/10.1007/s42452-019-1738-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1738-z