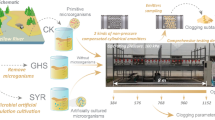
Abstract
The water shortage and soil salinization have become a great challenge for the Yellow River irrigation area. Mixing Yellow River water with brackish water for drip irrigation can be an effective solution because of the mixed water contents less sediment and salinity. However, the mixed water can still cause emitter’s clogging and significantly reduce drip irrigation system performance. In order to find out a proper method of relieving the emitter’s clogging in drip irrigation and increase the efficiency of irrigation water utilization, three different types of water including the Yellow River water, brackish water, and mixed water of Yellow River water and brackish water at the mixing ratio of 1:1 were used to cultivate the emitter’s clogging substance. The results showed that the clogging substance growth is a “S-curve.” The mixing of Yellow River water (YR) and brackish water (BW) can mitigate the formation of the clogging substance as the dry weight on mixed water was 6.07 mg/cm2, which was 47.38–65.71% less than YR and 26.53–47.40% less than BW. Moreover, the Si deposit had significant effect on the clogging substance in the condition of YR and the Ca/Mg deposit had significant effect on the clogging substance in the condition of BW. There was no deposit that had significant effect on the clogging substance in the mixed water. The contents of sediment and ions were reduced significantly after mixing Yellow River water and brackish water. The result can be helpful to facilitate utilization of brackish water in Yellow River irrigation area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Yellow River, Mother River of China, is getting the name from the perennial color and the high sediment concentration, which is about 35 kg/m3 over years [1]. Due to the sediment concentration and a lot of organic and microorganisms attached on the surface of sediment particles [2, 3], direct use of Yellow River water (YR) for irrigation requires frequent maintenance on the irrigation system and poses the risks for clogging. On the other hand, Chen et al. [4] reported about 13 billion m3 of brackish water (BW) is available in Yellow River irrigation area and can be an alternative water source for drip irrigation, which is the most effective and safe way to irrigate using saline water [5, 6]. However, the crop growth might be inhibited after long-term use of brackish water irrigation due to high salt concentration, which could increase salt content of the soil, change soil structure, and make a potentially threat to the environmental health of soil [7]. To address the challenges listed above, mixing Yellow River water and brackish water could be a solution. Mixed water not only reduces the sediment concentration in the Yellow River water but also decreases the salt content in brackish water. The water with appropriate range of salt has many advantages including improving soil structure, increasing soil permeability, reducing soil bulk density, enhancing soil saturated water content, and increasing water absorption capacity of crop root [8].
Although mixed water could be a promising irrigation water source, there are still several key bottlenecks limiting the use of mixed water for drip irrigation. The clogging risk of the irrigation system still exists as the sediment and salt in the mixed water still lead to the clogging substance formation. The emitter, which is the most important key part in the whole drip irrigation system, is clogged by the impurities including the sediment, salt, and organics because the flow path size of the emitter is only 0.5–1.2 mm for the hydraulic energy dissipation [9, 10]. Irrigation uniformity will be decreased significantly due to the emitter’s clogging. And the decreasing irrigation uniformity coefficient could affect the crop’s growth and hinder the large-scale development of the drip irrigation. Some previous studies suggested that various mechanisms of the emitter’s clogging occurred in different irrigation water including Yellow River water and brackish water. The formation process and components of clogging substance for Yellow River water become more complex than conventional sandy water. It is because the sediment in YR has enormous specific surface area and leads to the formation of excess biofilm, which has micro-dynamics behaviors response to the microorganism communities, nutrients, and organics in the Yellow River water [2, 3]. Zhangzhong et al. [11] established a model to describe the clogging substance growth kinetics using brackish water suggested that the development of clogging substance was progressive because the cations (Ca2+, Mg2+, Fe3+, etc.) and anions (CO32−, HCO3−, SO42−, etc.) react chemically to form insoluble substances and lead to clogging problem. There was a linear negative correlation between the saline water salinity and the emitter clogging parameters including relative average discharge and uniformity coefficient (Cu) [11]. Hills et al. [12] found that decreasing the pH of irrigation water could reduce chemical clogging according to the effect of 4 different operation managements on the chemical clogging of emitters. Some other studies suggested that the emitter’s clogging could be reduced after decreasing the particles concentration in the flow path [13, 14]. According to the literature reviewed, mixing YW and BW can resolve the emitter’s clogging problem because of less sediment and lower salinity. However, previous studies about the emitter’s clogging of drip irrigation in Yellow River irrigation area mainly focused on the condition of Yellow River water or saline water suing alone. No literature or reports about the formation process and characteristic of clogging substance in mixed water are published so far.
Studies show the forces involved the clogging process mainly include diffusion, gravity, momentum and water shear forces [15]. In addition, clogging substance is the result of simultaneous physical, chemical, and biological processes [16]. Physical processes are mainly hydrodynamics [17,18,19,20]; water shear forces and flow rates play a crucial role in clogging substance formation and development. First, the flow rate of water significantly affects the material transport mechanism [21]. Second, hydrodynamics also produces water shear forces that have a direct effect on the structure, deformation and detachment of biofilms. Van Loosdrecht et al. [22] argue that the phenomenon that clogging substance structures depend on the balance between substrate load and shear force is a balance between water shear forces and clogging substance growth [23]. This phenomenon leads to the water shear forces control threshold value on clogging process. Moreover, most of clogging substance was attached in the water-side dedendum zone and water-reverse-side dedendum zone [24], and the hydrodynamic shear force (τ) near wall in emitter’s flow path can be calculated by computational fluid dynamics (CFD). That makes it possible that the shear force index represents the location point where most of clogging substance was attached. It will become easier to study the mechanism of clogging and help to find the proper method of relieving the emitter’s clogging in drip irrigation.
Hence, this study analyzed the effects of mixed water on the emitter’s clogging substance and solid particles using clogging substance cultivation system designed by our group. Three different types of water including the Yellow River water, brackish water, mixed water of Yellow River water and brackish water with the mix ratios of 1:1 were used to cultivate the emitter’s clogging substance. X-ray diffractometer was used to determine the mineral components of clogging particles. Clarifying the emitter’s clogging mechanism could provide a theoretical reference for clogging model and establishing control approaches for the whole drip irrigation system. It is also helpful to facilitate utilization of brackish water in Yellow River irrigation area.
2 Materials and methods
2.1 Clogging substance cultivation system
2.1.1 Irrigation water
Three different types of water including the Yellow River water (YR), brackish water (BW), mixed water (MW) were used in this experiment. The YR was collected from the second canal of Yellow River located in Linhe District, Bayannaoer City, Inner Mongolia Province. The sediment content of YR was 36.5 kg/m3. The BW was collected from the local underground brackish water, and the total dissolved solid (TDS) was 3.77 g/L. The MW was mixed the YR and BW in a mix ratio of 1:1. And the mineral components of sediment are shown in Fig. 1 (X-ray diffraction and its Topas software were used to analyze the inorganic mineral compositions). Meanwhile, the water quality test was based on water and exhausted water monitoring analysis method [25]. Each 500 ml water sample was kept at − 4 °C constant temperature (Table 1).
2.1.2 Clogging substance cultivation system
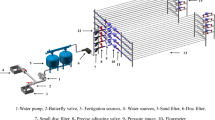
The clogging substance cultivation system designed by our group was integrated by a water tank (400 L), peristaltic pump (BT100L; Baoding, China), and cultivation simulator. The three components were connected by latex tube. Four simulators were set up in parallel for each water type in a constant temperature light incubator (GZH-500B; Hangzhou, China). Figures 2 and 3 show the drawings of the clogging substance cultivation system and a cultivation simulator. Table 2 shows the parameters of the cultivation simulator. The background and introduction of clogging substance cultivation system designed by our group can be found in the document of Supporting Information.
Overall drawing of cultivation simulator. Note: 1—motor, 2—flanges, 3—gasket, 4—connect bearing, 5—motor shaft, 6—fixing steel sheet, 7—outer cylinder, 8—inner cylinder, 9—sampling rack, 10—water outlet, 11—water inlet, 12—fixing screws, 13—fixing bearing, 14—transformer, 15—rotating speed controller, 16—power distribution box, 17—vents, 18—wire, 19—sample groove
2.1.3 Hydrodynamic shear force of study site
Figure 4a shows a cross-sectional drawing of the emitter’s labyrinth flow path with rectangular straight tooth. The length, depth, and width of the flow path are 450.00 mm, 0.78 mm, and 1.68 mm. The height and width of the rectangular straight tooth are 1.5 mm and 0.50 mm. All sizes of the flow path were measured by a digital caliper (Mitutoyo, Japan; 0.01 mm). Figure 4b shows the computational fluid dynamics (CFD) calculation result of hydrodynamic shear force (τ) near wall in emitter’s flow path. The boundary conditions were pressure inlet (0.1 MPa) and free outflow (0 MPa), and the calculation model was k–ε in Ansys15.0 [26]. The result showed the τ in Site a (water-side tooth-tip zone), Site b (water-reverse-side tooth-tip zone), and Site c (main flow deformation zone) is 2–26 Pa, 0.3–2 Pa, and 2.4–6.5 Pa. The τ in Site d (water-side dedendum zone) and Site e (water-reverse-side dedendum zone) is 0.3–0.8 Pa. According to the calculation formula of shear stress in microfluidic channel [27], the τ in main flow deformation zone of this emitter’s labyrinth flow path is 4.8 Pa, which is consistent with the CFD calculation result (2.4–6.5 Pa). The simulation result of this study can be proved. And Site d and Site e were chosen as the studying object because most of clogging substance was attached in the two sites [24]. So, hydrodynamic shear force of this study is 0–0.8 Pa.
2.1.4 Hydrodynamic shear force (τ) of cultivation simulator
The suggested hydraulic retention time (HRT) of cultivation simulator is 2 h because water enters from the bottom and flows out from the top. The volume and water intake of cultivation simulator are 1210 mL and 10.08 mL/min. The hydrodynamic shear force (τ) between coaxial rotating cylinders is calculated by Eq. 1 because of the small water intake [28].
Note τ is the hydrodynamic shear force in the site with radius r (N/m2), R1 and R2 are the radius of inner cylinder and outer cylinder, ω1 is the rotating angular velocity of inner cylinder (rad/s), ω1 is the rotating angular velocity of outer cylinder (rad/s), r2 means a radius of a certain cylindrical section between outer cylinder and inner cylinder (m), and μ is the liquid viscosity coefficient (N s/m2).
Equation 1 could be simplified to Eq. 2 because the condition of this experiment was ω2 = 0 rad/s, and r2 = R2.
So,
Note The liquid viscosity coefficient (μ) of water in 20 °C is 1.005 × 10−3 (N s/m2)
According to Table 2, R1 = 65 mm, R2 = 55 mm, the τ of cultivation simulator in this experiment could be calculated by Eq. 4,
2.2 Treatment and reference
A sampling rack (9) with slices of polyethylene (PE) hose tube was fixed on inner wall of outer cylinder by fixing screws. In order to simulate more real wall surface of emitter’s flow path, the slices were collected from Φ16 drip irrigation tubes. And the size of slices was 19 cm × 1 cm. Hydrodynamic shear force would be occurred on slice’s surface as the inner cylinder rotates to drive the water flow. The τ in simulating sites of Site d and Site e could be calculated by Eq. 4. Table 3 is the experiment condition of different water type and τ. Three systems for YR, BW, and MW were all in three constant temperature light incubators (GZH-500B; Hangzhou, China) with 30 °C and no light intensity due to the avoidance of the interference of light. Two slices of PE samples were collected every 80 h over the course of 800-h operation.
2.3 Clogging substance constituent analyses
2.3.1 Dry weight (DW)
Sample slices were weighted by an electronic balance (HZT-A, Suzhou) and put in a valve bag with 50 mL of deionized water. Then, the bag with sample was stripped in an ultrasonic cleaner (GVS-10L, Qingdao) for 60 min at 60 Hz to obtain the slices without clogging substance and suspension with clogging substances. The slices without clogging substance were dried in an oven (60 °C, 30 min) and then weighed. The total dry weight (DW) of clogging substance was the difference between the initial weight and the weight after stripping and drying.
2.3.2 Mineral components
After the stripping treatment, the suspension was centrifuged to obtain the solid clogging substances. The solid substances were dried (60–80 °C) and grinded. Finally, a polycrystalline diffraction map was obtained by scanning the grinded samples using a D8-Advance X-ray diffraction system (Bruker, Germany). The primary conditions of scanning were voltage of 40 kV, current of 40 mA, Cu target, and wavelength λ of 1.5406 A. The map was then analyzed using Topas software, which works with X-ray diffraction to determine the solid substance mineral components. Some XDR maps of clogging substance can be found in the document of Supporting Information.
3 Results
3.1 Dynamic changing process of DW
Dry weight (DW), which is the most important factor that can indicate the growth of clogging substances, is shown in Fig. 5. The result indicated that the clogging substance growth typically follows “S shape” curve, which could be divided into three phases, namely growing adaptive phase, rapid growing phase, and dynamic balance phase. There was a consistent demarcation point for three kinds of water sources. For the first 320 h of the system operation, clogging substance attached on the surface of PE slice was in growing adaptive phase as the DW increased slowly. And clogging substance DW of MW increased slower than that of other water, and the DW of MW reached 1.83 mg/cm2 at the end of the period, in which the DW of MW was 50.42% less than that of YR and 35.18% less than that of BW. Then the clogging substance entered the rapid growing phase, and clogging substance DW of different water grew to 5.30–10.39 mg/cm2 till 640 h of the system operation, while DW of MW increased slower than that of other water. DW of MW reached 5.30 mg/cm2 at the end of the period, in which the DW of MW was 49.03% less than that of YR and 37.90% less than that of BW. After these, clogging substance DW tended to be in a dynamic balance phase, as they increased quite slowly or even decreased slightly. At the end of the experiment (800 h), clogging substance DW was 6.07–11.74 mg/cm2, while DW of MW was still less than that of other water. DW of MW reached 6.07 mg/cm2 at the end of the experiment, in which the DW of MW was 48.34% less than that of YR and 35.03% less than that of BW.
In addition, in growing adaptive phase, the clogging substance DW of 0.40 Pa was 78.53% higher than that of 0.05 Pa, 10.76% higher than that of 0.20 Pa, and 76.90% higher than that of 0.70 Pa. In rapid growing period and dynamic stable period, clogging substance DW of 0.20 Pa was 108.85% higher than that of 0.05 Pa, 21.68% higher than that of 0.40 Pa, and 69.71% higher than that of 0.70 Pa.
3.2 Inorganic mineral deposits
X-ray diffraction and its Topas software were used to analyze the inorganic mineral compositions of clogging substance. Figure 6 shows the results of quantitative analysis of mineral components. It could be deduced that clogging substance contains 10 major types of inorganic mineral components including quartz, alkali feldspar, aragonite, calcite, siderite, hydrotalcite, dolomite, anorthoclase, magnesite, and inorganic salt. Based on the elemental composition, these substances can be categorized into three different deposits: Si deposits (quartz, alkali feldspar, and anorthoclase), Ca/Mg deposits (aragonite, calcite, hydrotalcite, dolomite, and magnesite), and other deposits. The ratios between different deposits in clogging substances were changed as the system operation. The content of Si deposits and Ca/Mg deposits was 27.76–75.37% and 11.00–64.22%, respectively. Clogging substance in condition of YR was mainly Si deposits with the content of 75.37%, and clogging substance in condition of BW was mainly Ca/Mg deposits with the content of 64.22%. Great changes of deposits distribution occurred after mixing Yellow River water and brackish water. The content of Si deposits for MW was 60.72%, which was 19.44% less than those for YR. The content of Ca/Mg deposits for MW was 21.79%, which was 66.08% less than those for BW.
As for different hydrodynamic shear force (τ), the contents of Si deposits and other deposits for 0.40 Pa were both higher than those for other hydrodynamic shear force. The content of Si deposits for 0.40 Pa was 5.85%, 16.35%, 25.87% higher than those for 0.05 Pa, 0.20 Pa, 0.70 Pa, respectively. The content of other deposits for 0.40 Pa was 39.16%, 40.15%,21.46% higher than those for 0.05 Pa, 0.20 Pa, 0.70 Pa, respectively.
3.3 Correlation between different deposits and DW
Table 4 shows the results of correlation between dry weights (DW) and different deposits including Si deposits, Ca/Mg deposits, and other deposits. There was a positive correlation between all kinds of deposits and DW, especially the correlation of Si deposits and DW, and the correlation of other deposits and DW were higher than that of Ca/Mg deposits and DW. In the condition of YR, the effect of Si deposits on clogging substance was extremely significant, the effect of other deposits on clogging substance was significant, and the effect of Ca/Mg deposits on clogging substance was insignificant. In the condition of BW, the effect of Ca/Mg deposits on clogging substance was extremely significant, the effect of Si deposits on clogging substance was extremely significant only in τ of 0.05 Pa and 0.20 Pa, and the effect of other deposits on clogging substance was significant only in τ of 0.40 Pa. Mixing Yellow River water and brackish water not only changed deposits distribution of clogging substance but also decreased the correlation of different deposits and DW. For example, in the condition of MW, the effect of other deposits on clogging substance was extremely significant only in τ of 0.40 Pa and 0.70 Pa, and generally significant in τ of 0.05 Pa and 0.20 Pa, and the effect of Si deposits on clogging substance was generally significant only in τ of 0.20 Pa. Moreover, Ca/Mg deposits had no correlation with clogging substance in MW.
4 Discussion
In order to facilitate the utilization of Yellow River water and brackish water in drip irrigation systems, three different types water including the Yellow River water, brackish water, mixed water of Yellow River water and brackish water with the mix ratios of 1:1 were used to cultivate the emitter’s clogging substance in this study. And the shear force index was chosen to represent the location point where most of clogging substance was attached. In order to find the control threshold for the influence of water shear force on clogging substance growth process, the process of clogging substance formation was given in detail. The results showed that the growth curve of the substance was the shape of “S,” which included the phases of growth adaptive phase, rapid growing phase, and dynamic balance phase, which was consistent with the findings of Li et al. [29] and Zhou et al. [30]. There were a lot of microorganisms, nutrients, organic, cations/anions, and biofilm attached on the surface of sediment particles in water. Insoluble deposit formed by reacting chemically of cations/anions, sediment particles, microorganisms, and organic could be bonded by extracellular polymers secreted by microbes. The clogging substance containing insoluble deposit, sediment particles, microorganisms, and organic could be attached on surface of PE sample by process of collision, flocculation, bio-adhesion. Meantime, the water flow on slice’s surface could not only transport the substrate to support the formation of clogging substance [22] but also make clogging substance fall off by hydrodynamic shear force [31]. The phase of slow growth can be explained by logging substance need to adapt to the hydraulic conditions at the beginning. With the development of chemical reaction and the increasing of microorganisms in clogging substance, more and more deposits and organic aggregates attached on surface of PE sample, which increased wall roughness. Large roughness exacerbated the attachment and deposition of particles and microbes as large roughness had changed collision characteristics of sediment particles and wall [32].
The result comparing different irrigation water showed that the content of Si deposits for MW was 19.44% less than those for YR, and the content of Ca/Mg deposits for MW was 66.08% less than those for BW. There was no deposit that had significant effect on the clogging substance in MW though Si deposit had extremely significant effect on the clogging substance YR, and Ca/Mg deposit had extremely significant effect on the clogging substance in BW. This is mainly because there was a large amount of sediment particles with content of 36.5 kg/m3 containing 41.39–67.85% SiO2 in Yellow River water (YR) [33]; a lot of SiO2 attached on surface of PE sample because of biofilm on surface of particles. There were a lot of ions including Mg2+, Ca2+, HCO3−, and CO32− (Table 3) and CaCO3 deposited easily in condition of pH > 8 (pH = 8.27 in this experiment) as increasing electrolysis of OH− ions in irrigation water was beneficial to form carbonate deposit [12, 34]. Contents of sediment and ions were reduced significantly after mixing Yellow River water and brackish water. For example, the content of Mg2+ in MW was 18.12% less than those in BW, the content of Ca2+ in MW was 12.96% less than those in BW, the content of HCO3− in MW was 33.33% less than those in BW, and the content of CO32− in MW was 33.33% less than those in BW. The values of pH, Ec, and TDS were also reduced because of mixing (Table 3). Besides, mixing Yellow River water and brackish water with ratio 1:1 could not only dilute Ca/Mg deposits in MW but also inhibit the formation of Ca/Mg deposits in MW because the content of Ca/Mg deposits in clogging substance for MW was over 50% (66.08%) less than those for BW. The reasons listed above could explain why the clogging substance DW in MW was 47.38– 65.71% less than those in YR and 26.53–47.40% less than those in BW. The result of this study could be proved that mixing Yellow River water and brackish water can relieve emitter’s clogging effectively.
In addition, it was easier to form clogging substance in emitter’s flow path in condition of hydrodynamic shear force (τ) of 0.20–0.40 Pa because clogging substance DW was the highest in 0.20–0.40 Pa. This can be explained in the following two aspects: (1) Clogging substance was composed of sticky fine particles as the particles flocculated into flocculation; then different flocculation developed and grew into substance with porous structure. In condition of low hydrodynamic shear force, it is difficult for sticky fine particles to collide and bond with each other [35], and it is not conducive to produce chemical deposits as anions and cations update difficultly [36]. Small particles are bonded with each other into large particles by extracellular polymers (EPS containing extracellular polysaccharide and extracellular protein), which is main secretion of microorganisms in biofilm attached on the surface of particles. The structure of clogging substance becomes porous and loose because the secretion of extracellular polysaccharide decreased when shear force is low [37]. And thus clogging substance attaches on the surface of PE samples difficultly. (2) Increasing hydrodynamic shear force has significant effect on shedding of clogging substance [31, 38]. Rittmann [19] found that there was a linear relationship between shedding rate of attachment and τ0.58 on the surface of pipe wall, and Bakke et al. [39] found that there was a linear relationship between shedding rate of attachment and τ on the surface of pipe wall, because large shear force would impede formation of flocculation and mud flocs and destroy the mud flocs that have been formed [35, 39]. Thus, on the one hand, clogging substance would increase with increase in hydrodynamic shear force within a certain range. On the other hand, increase in hydrodynamic shear force would also strengthen the shearing effect on clogging substance and accelerate shedding of clogging substance. The maximum amount of clogging substance accumulation would be reached when the advantage and disadvantage are balanced. Those were the reasons why clogging substance DW was the highest in 0.20–0.40 Pa. In other words, there is sufficient evidence to prove that there is a control threshold for the influence of water shear force on clogging substance growth process: [0.20, 0.40] Pa and that clogging can be effectively inhibited when the shear force is higher or below this shear force range.
It is important to note that, within the context of drip irrigation, the current study comprised some inherent limitations, instead mixing Yellow River water and brackish water can reduce emitter’s clogging substance. The current study did not include analyses of other organic components and microorganisms, and thus only indicative conclusions of solid particles and inorganic components in clogging substance. Future work should concentrate on elaborating the forming mechanism of clogging substance comprehensively and deeply because complex irrigation water makes emitter’s clogging to develop a compound clogging including physical clogging, chemical clogging, and bio-clogging. Additionally, future work is required to add field experiment in order to facilitate the utilization of Yellow River water and brackish water in drip irrigation systems and make further recommendations pertaining to safe and reliable use of the alternative water.
5 Conclusions
This study is the first to examine the effect of mixing Yellow River water and brackish water on the emitter’s clogging in drip irrigation. Accordingly, results may be used to provide a theoretical reference for using Yellow River water and brackish water in agriculture irrigation. Three different types water were examined using clogging substance cultivation system designed by our group. The principal findings were:
-
1.
The growth curve of the substance was the shape of “S,” which included the phases of slow growth (0–320 h), rapid growth (320–640 h), and dynamic balance growth (640–800 h). The dry weight (DW) of clogging substance was 1.59–15.60 mg cm−2 at the ending of running.
-
2.
Mixing Yellow River water and brackish water can mitigate the formation of the clogging substance effectively as the dry weight (DW) on mixed water (MW) was 0.45–6.07 mg cm−2, which was 47.38–65.71% less than the DW on Yellow River water (YR) and 26.53–47.40% less than brackish water (BW).
-
3.
The Si deposit had extremely significant effect on the clogging substance in the condition of YR, and the content of Si deposit was over 75.37%, the Ca/Mg deposit had extremely significant effect on the clogging substance in the condition of BW, and the content of Ca/Mg deposit was over 64.22%. However, there was no deposit that had significant effect on the clogging substance in the mixed water. Besides, the Si deposit content in MW was 19.44% less than those in YR and the Ca/Mg content was 66.08% less than those in BW.
-
4.
Clogging substance DW was the highest in 0.20–0.40 Pa, which was 12.38–15.60 mg cm−2. The average DW in 0.20–0.40 Pa was 101.6% higher than those in 0.05 Pa and 68.4% higher than those in 0.70 Pa
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Jiang RQ, Cao HW (2012) Study on sediment in irrigated area of yellow river. China Water&Power Press, Beijing
Wang WN, Xu FP, Zhou B et al (2014) Structural and fractal characteristics of biofilm attached on surfaces of sediments in Yellow River for drip irrigation. J Drain Irrig Mach Eng 10(32):914–919 (in Chinese with English abstract)
Zhou B, Li YK, Pei YT et al (2013) Quantitative relationship between biofilms components and emitter clogging under reclaimed water drip irrigation. Irrig Sci 31(6):1251–1263
Chen SF, He XL, Wang ZF et al (2010) Development of research on drip irrigation of saline water. Water Sav Irrig 02:6–9 (in Chinese with English abstract)
Karlberg L, Rockstrom J, Annandale JG et al (2007) Low-cost drip irrigation-a suitable technology for southern Africa: an example with tomatoes using saline irrigation water. Agric Water Manag 89:59–70
Wan SQ, Kang YH, Wang D et al (2007) Effect of drip irrigation with saline water on tomato (Lycopersicon esculentun Mill) yield and water use in semi-humid area. Agr Water Manag 90:63–74
Zhang YL, Lu WL, Zhang W et al (2006) Effects of long term brackish water irrigation on characteristics of agrarian soil. J Agro Environ Sci 25(4):969–973 (in Chinese with English abstract)
Wang QJ, Xu YM, Wang JD et al (2002) Application of saline and slight saline water for farmland irrigation. Irrig Drain 04:73–77 (in Chinese with English abstract)
Bucks DA, Nakayama FR (1979) Trickle irrigation water quality and preventive maintenance. Agric Water Manag 2(2):149–162
Nakayama FR, Bucks DA (1991) Water quality in drip/trickle irrigation: a review. Irrig Sci 12(4):187–192
Zhangzhong LL, Yang PL, Ren SM et al (2016) Chemical clogging of emitters and evaluation of their suitability for saline water drip irrigation. Irrig Drain 65(4):439–450
Hills DJ, Nawar FM, Waller PM (1989) Effects of chemical clogging on drip-tape irrigation uniformity. Trans asae 32(4):1202–1206
Marzio P, Enrico N, Thomas J (2002) DNS study of turbulent transport at low Prandtl numbers in a channel flow. J Fluid Mech 458:419–441
Zhang J, Zhao WH, Wei ZY et al (2007) Numerical and experimental study on hydraulic performance of emitters with arc labyrinth channels. Comput Electron Agric 56:120–129
Saur T, Morin E, Habouzit F et al (2017) Impact of wall shear stress on initial bacterial adhesion in rotating annular reactor. PLoS ONE 12(2):e0172113
Characklis WG, Wilderer PA (1989) Structure and function of biofilms. Wiley, New York, pp 6–9
Characklis G, Marshall KC (1990) Biofilms. Wiley, New York
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36(7):1653–1665
Rittmann BE (1982) The effect of shear stress on biofilm loss rate. Biotechnol Bioeng 24(2):501–506
Stoodley P, Lewandowski Z, Boyle D, Lappin-Scott HM (1999) Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng 65(1):83–92
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. Mc-Graw Hill, New York
Van Loosdrecht MCM, Eikelboom D, Gjaltema A et al (1995) Biofilm structures. Water Sci Technol 32(8):35–43
Ochoa JC, Coufort C, Escudié R et al (2007) Influence of non-uniform distribution of shear stress on aerobic biofilms. Chem Eng Sci 62(14):3672–3684
Li YK, Zhou B, Liu YZ et al (2013) Preliminary surface topographical characteristics of biofilms attached on drip irrigation emitters using reclaimed water. Irrig Sci 31(4):557–574
Wei F (2002) Water and exhausted water monitoring analysis method. China Environmental Science Press. ISBN 7-80163-400-4
Zhangzhong LL, Yang PL, Ren SM et al (2015) Flow characteristics and pressure-compensation mechanism of non-pressure-compensation drip irrigation emitters. Irrig Drain 64(5):637–646
Park A, Jeong H-H, Lee J, Kim KP, Lee C-S (2011) Effect of shear stress on the formation of bacterial biofilm in a microfluidic channel. BioChip J 5:236–241
Zhu KQ, Xu CX (2009) Viscous fluid mechanics. Higher Education Press, Beijing
Li YK, Liu YZ, Li GB et al (2012) Surface topographic characteristics of suspended particulates in reclaimed wastewater and effects on clogging in labyrinth drip irrigation emitters. Irrig Sci 30(1):43–56
Zhou B, Li YK, Song P et al (2016) Growth kinetics model of biofilms inside drip irrigation emitters when reusing reclaimed water in agriculture. Agric Water Manag 168:23–34
Chang HT, Rittmann BE, Amar DR et al (1991) Biofilm detachment mechanisms in a liquid fluidized bed. Biotechnol Bioeng 38(5):499–506
Shafagh J (1986) Plaque accumulation of cast gold complete crown. Prosthet Dent 55:339–342
Gao J, Ding D, Tian S et al (2011) Silicon isotope compositions of suspended matter in the yellow river, china, and its significance in geological environment. Acta Geol Sin 85(10):1613–1628 (in Chinese with English abstract)
Aali KA, Liaghat A, Dehghanisanij H (2009) The effect of acidification and magnetic field on emitter clogging under saline water application. J Agric Sci (Toronto) 1(1):132–141
Bagnold RA (1954) Experiments on the gravity-free dispersion of large solid spheres in a newtonian fluid under shear. Proc R Soc A 225(1160):49–63
Xavier JDB, Picioreanu C, Loosdrecht MCMV (2005) A general description of detachment for multidimensional modelling of biofilms. Biotechnol Bioeng 91(6):651–669
Laspidou CS, Rittmann BE (2004) Modeling the development of biofilm density including active bacteria, inert biomass, and extracellular polymeric substances. Water Res 38(14–15):3349–3361
Etienne P, Juan CO, Yoan P et al (2012) Effect of shear stress and growth conditions on detachment and physical properties of biofilms. Water Res 46(17):5499–5508
Bakke R, Trulear MG, Robinson JA et al (1984) Activity of pseudomonas aeruginosa in steady-state biofilms. Biotechnol Bioeng 26(11):1418–1424
Acknowledgements
We are grateful for the financial support from the National Natural Science Fund of China (Nos. 51321001, 51179190, 51339007), Public Welfare Project of PRC Ministry of Water Resources (No. 201401078).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Guo, Z. & Kuo, CT. Effects of mixing Yellow River water with brackish water on the emitter’s clogging substance and solid particles in drip irrigation. SN Appl. Sci. 1, 1269 (2019). https://doi.org/10.1007/s42452-019-1287-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1287-5