Abstract
A sensitive, accurate and precise method was developed and validated for the trace level determination of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods using inductively coupled plasma mass spectrometry. The selectivity and linearity, limit of detection (LOD) and limit of quantification (LOQ), recovery study, repeatability and within-laboratory reproducibility, Horwitz ratio (HORRAT), measurement uncertainty and fitness of purpose of the method were determined. The LOD for 75As, 111Cd, 201Hg, and 208Pb were found to be 14.68, 12.70, 5.13, and 2.74 µg/kg respectively whereas, the LOQ obtained were 48.94, 42.33, 17.11 and 9.13 µg/kg respectively. The relative standard deviation for the repeatability and within-laboratory reproducibility for all determined trace elements were below 3 and 16%, respectively. The HORRATr and HORRATR values of repeatability and reproducibility were lower than 2. Furthermore, the recovery values of the spiked samples ranged from 81.79 to 101.67%. These results complied with the performance criteria established by Council Regulation (EC) 333/2007 and the clause 5.4.5.2, ISO/IEC 17025:2005. Therefore, the procedure is implemented for the routine analysis to determine trace level quantification of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Trace elements are present in every ecosystem in the world [1]. These elements can be soluble in water and react with organic matter forming complexes and chelates, which increase its solubility, availability, and dispersal [2, 3]. The metal species can be mobilized from the environment, and accumulated in the biota in water, and are subsequently transferred to humans through the food chain [4]. Metals, such as arsenic, mercury, lead, and cadmium are toxic, even in trace amounts [5]. Though, the increase of these metals and metalloids in marine systems has become a risk to human health due to their toxic effects [6]. To protect the public health at present, many countries enforce regulations on contamination level of toxic metals in fish and fish products. However, only three metals, cadmium, mercury, and, lead have been included in the regulations of the European Union for hazardous metals [7], while the Food Safety and Standards Authority of India [8] and United States Food and Drug Administration (USFDA) has included arsenic in the list [9]. These regulations require precise and accurate analytical method for the determination of heavy metals contaminant. For this reason, there is a growing global interest in performing validation procedures of the analytical method for legislation implementation to show that the method produces reliable results and provide accurate and reproducible results.
A number of analytical techniques have been used for validating method for monitoring heavy metals in fish and fish products like flame atomic absorption spectroscopy (FAAS), graphite furnace atomic absorption spectroscopy (GFAAS), inductively coupled plasma-atomic emission spectrometry (ICP-AES) but inductively coupled plasma mass spectrometry (ICP-MS) is being the most sophisticated and reliable technique [10,11,12,13]. However, it is also an important point of consideration that the presence of spectral interferences (undesirable signals interfering with the element signal) and non-spectral interferences (physical and chemical interferences, also called matrix interferences) may result in false-positive analytical results [14]. Therefore, in order to eliminate or control effects of these interferences, it is necessary to use an optimized and validated ICP-MS method which permits to obtain the quantification of elements with the accuracy and precision. However, to the best of our knowledge, no accredited methods have been reported in the literature concerning the simultaneous determination of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods samples. The aim of the current study was to develop a rapid, low-cost analysis, accurate, and precise method for the determination of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods by inductively coupled plasma mass spectrometry (ICP-MS) according to the Council Regulation (EC) 333/2007 [16] and clause 5.4.5.2, ISO/IEC 17025: 2005 [15].
2 Materials and methods
2.1 Instrumentation
A standard Agilent 7800 ICP-MS, which includes High Matrix Introduction (HMI) system with SPS 4 autosampler, was used for the analysis. The ICP-MS was configured with the standard sample introduction system consisting of a micromist glass concentric nebulizer, a standard double-pass peltier-cooled spray chamber cooled at 2 °C, quartz spray chamber, and quartz torch with 2.5 mm id injector. Interface consisted of a nickel-plated copper sampling cone and a nickel skimmer cone. The operating conditions of instrument mentioned in Table 1 which has been selected on the basis to minimize interferences.
Tuning of the instrument was done by using 1 ppb (7Li, 24Mg, 59Co, 89Y, 140Ce, 205TI) tuning solution in normal mode (no-gas mode) and helium mode to ensured optimal performances in terms of sensitivity, oxide ratio, and doubly charged ratio and to minimize interferences. In normal mode acquired element were 111Cd, 201Hg, and 208Pb whereas, 75As was acquired in helium (He) collision mode. The digestions were performed on a multiwave Go (Anton Paar).
2.2 Reagents and chemicals
Ultrapure water (18.2 MΩ cm at 25 °C) from a Millipore Direct-Q-UV water purifier (Millipore) was used to prepare all standards and samples. Hydrogen peroxide (30%) (Merck India), Nitric acid (69.0–70.0%) and ACS Reagent grade hydrochloric acid (36.5–38.0%) were of JT Baker. The purity of argon plasma gas and helium collision gas were 99.999%.
2.3 Sample collection, preparation, and digestion
The Cephalopods samples were collected from the fish market, Andheri West, Mumbai, Maharashtra 400053, India and transported to the laboratory in the refrigerated condition. Samples were milled and homogenized in a homogenizer (Deimos, Astori Tecnica, Italy) as they arrived in the laboratory with a stainless steel cutter to obtain a representative homogenous subsample. The homogenized samples were labeled with a unique code and stored at − 20 ± 2 °C till analysis. All necessary precaution has been taken to avoid secondary contaminants while preparing homogeneous samples.
The digestion of samples was performed as following 5 ml of concentrated HNO3, 1 mL of HCl and 1.0 ml of concentrated H2O2 were added to 1.0 g of test sample accurately weighed in the digestion vessel. After a few minutes, the vessels were closed and then placed in the microwave digestion system. The digestion was performed as per the temperature control programme summarized in Table 2.
The digested samples were left to cool, quantitatively transferred to 50 ml volumetric flasks, diluted to the mark with Milli-Q water and stored in a refrigerator prior to ICPMS analysis, which was performed within 24 h after the digestion. All of the decomposed sample solutions were clear, without visible residues. The digestion vessels were cleaned after each run using the same reagents and program that was applied for sample digestion, followed by rinsing with Milli-Q water.
2.4 Range of the linearity and calibration curve
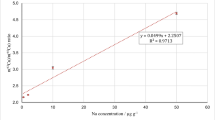
National Institute of Standards and Technology (NIST) traceable standards solutions of As, Cd, Hg, and Pb, 1000 mg/l in 2% HNO3 were procured from Merck India. The standard blank solution was prepared using 5% HNO3 and 0.5% HCl. The calibration range selected from 50 to 150% in relation to the expected result of analysis. The linearity of the calibration curve considered acceptable when the coefficient of regression (r2) was at levels equal to at least 0.99.
2.5 Experimental set up of the method validation
Different experiments were set up in order to investigate the performance characteristics of the method in terms of specificity, linearity, limit of detection (LOD) and limit of quantification (LOQ), measuring range, repeatability, within-laboratory reproducibility, recovery, as per Commission Regulation 333/2007/EC [16]. The method validation was performed by spiking blank matrix at 0.5, 1.0 and 1.5 times the maximum level as per Commission Regulation (EC) 1881/2006 for cadmium, lead, and mercury [17]. However, for arsenic 0.5, 1.0 and 1.5 times of 1 ppm was considered. The dilution factor 50 was considered for the calculation of analyte concentration. In order to check cross contaminations during the validation study procedural blank was prepared along with the samples.
The analytical sensitivity of ICP-MS was assessed by determining the values of limit of detection (LOD) and limit of quantification (LOQ). The values of LOD and LOQ were calculated as 3.3 SD/b and 10SD/b respectively, where SD is the standard deviation of the intercept and b is the slope of the obtained calibration curve. According to Commission Regulation (EC) 333/2007, the LOD should be equal to three-tenths of LOQ. Whereas, LOQ for Hg, Cd, and Pb should be less than one-fifth of the maximum level.
To check the accuracy of the analytical method, the recovery studies were performed in order to confirm no loss or contamination occurred during sample preparation and matrix interferences throughout the measurement step. Certified reference materials or control samples were not available, thus, the recovery studies were performed by spiking with the known amount of concentration prepared from NIST traceable standard in the blank sample according to the Table 3. The recovery was calculated as recovery % = 100C/spiked concentration where C is the element concentration found.
2.6 Precision
The analytical precision was determined by assessing the repeatability and within-laboratory reproducibility of instrument response to analyte according to the Commission Regulation (EC) 657/2002 [18]. To assess the repeatability the analysis was done for three different concentrations levels according to Table 3, and in each level, the analysis was performed with six replicates. To assess the within-laboratory reproducibility the steps were repeated on two other occasions by a different analyst. The repeatability and within-laboratory reproducibility were calculated as the relative standard deviation.
The acceptance of repeatability and within-lab reproducibility Horwitz approach described in EURACHEM [19] and Thompson et al. [20] was used. This approach involves the Horwitz equation to calculate the relative standard deviation and the value was used in HORRAT ratio which was compared with acceptance values to finally evaluate acceptance of repeatability and within-lab-reproducibility.
The Horwitz equation used to calculate predicted relative standard deviation is, PRSDR = 2C−0.15, where, C is the concentration ratio. Mean concentration, standard deviation, and relative standard deviations were calculated from results generated under repeatability (RSDr) and reproducibility (RSDR) conditions. HORRAT ratio was computed from the data of calculated RSDr, RSDR, PRSDr and PRSDR as HORRATr = RSDr (%)/PRSDr (%) and HORRATR = RSDR (%)/PRSDR (%). According to Commission Regulation (EC) 836/2011 [16], the HORRATr and HORRATR values should be less than two.
2.7 Measurement uncertainty
The combined standard measurement uncertainty (Uc) was calculated based on the Eurachem/Citac Guidelines [19], from the summary squared of several independent parameters such as (a) the mass uncertainty; (b) the dilution volume uncertainty; (c) the calibration uncertainty; (d) the bias uncertainty, as estimated by the recovery tests; and (e) the precision uncertainty, as estimated by the RSDR% values for the three different concentration levels under reproducibility conditions. The choice of factor k is 2 based on the confidence level of 95%.
Methods suitable for official control must produce results with combined standard measurement uncertainties (Uc) less than the maximum standard measurement uncertainty (Uf) calculated using the formula: \(U_{f} = \sqrt {(LOD/2)^{2} + ( \propto {\complement })^{2} }\) where, LOD is the limit of detection of the method (µg/kg), C is the concentration of interest (µg/kg), α is a numeric factor to be used depending on the value of C [16].
3 Result and discussion
In this study, an ICP-MS method for quantitative analysis of, 75As, 111Cd, 201Hg, and 208Pb in Cephalopods was developed and validated.
3.1 Selectivity and linearity
Before, proceeding with the validation procedure the analytical method selectivity towards naturally present substances (metabolites, endogenous substances, matrix constituents, etc.) has ensured. To verify the selectivity calibration curves using aqueous standard solutions and matrix matching calibration curves were compared. The result showed that slope of the calibration curve from aqueous standards is almost similar to the slope of the curve plotted by matrix matching calibration curves indicating no matrix interferences. Therefore, aqueous calibration curves were used for calibration in all the experiments.
Table 4 shows the equation of a straight line and linear regression coefficient (r2) of a calibration curve (Fig. 1) of the determined elements. Results indicated that the linear regression model was acceptable for the selected analyte in the defined range with a satisfactory correlation coefficient (r2 ≥ 0.99).
3.2 Limit of detection (LOD) and limit of quantification (LOQ)
The LOD for 75As, 111Cd, 201Hg, and 208Pb was found to be 14.68, 12.70, 5.13, and 2.74 µg/kg, whereas, LOQ obtained was 48.94, 42.33, 17.11 and 9.13 µg/kg respectively (Table 5). The value of LOD and LOQ comply with the Commission Regulation (EC) 333/2007, laying down the methods of sampling and analysis for the official control of the levels of trace elements and processing contaminants in foodstuffs. Furthermore, measuring range was found from 10 to 2500 µg/kg, which shows that developed method is sensitive and can quantify elements at trace level for the assessment of Cephalopods.
3.3 Recovery study
The recovery values for accuracy studies spiked with different levels of 75As, 111Cd, 201Hg, and 208Pb are presented in Table 6. In the present study, the mean recovery ranged between 81.79–101.67% at all three spiked levels. The obtained results are within ± 20% of the target value thus the developed method was considered as ‘‘fit for purpose’’.
3.4 Repeatability and within-laboratory reproducibility
The data in Table 6 shows the repeatability, within-laboratory reproducibility expressed with the relative standard deviation and HORRAT. The RSDr % values obtained between 0.55 and 2.99 however, RSDR % values ranging between 1.83 and 15.86. According to Horwitz, as cited from Gonzalez and Herrador [21] the maximum RSD value acceptable for the analyte at the level of 100 µg/kg and 1 mg/kg is 22.6 and 16% respectively. The values of HORRATr and HORRATR for repeatability and reproducibility was lower than 2 at all the concentration levels which in accordance to the performance criteria for methods of analysis as per Commission Regulation (EC) 836/2011 [16]. Therefore, it can be stated that the developed method exhibited a good precision.
3.5 Quality control programs
Internal quality control charts (IQCs) were prepared to monitor whether results are reliable enough to be reported. The goal of IQCs is the extension of method validation continuously checking the accuracy of analytical data obtained from routine analysis in the laboratory. The analytical system is under control if no more than 5% of the measured values exceed the warning limits and none of them the action or control limits [22]. The external quality assurance program was maintained through the participation in proficiency programs organized by Export Inspection Agency -Kolkata, India. The results of proficiency test were within ± 2 Z scores.
3.6 Measurement uncertainty and fitness for purpose
The data of combined standard measurement uncertainty (Uc) and maximum standard measurement uncertainty (Uf) are summarized in Table 7. The results show that the method is suitable for the official control of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods samples because it produced results with combined standard measurement uncertainties less than the maximum standard measurement uncertainty.
4 Conclusions
The current work describes development and validation of a method for the trace level analysis of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods as per Council Regulation (EC) 333/2007 and ISO/IEC 17025:2005 using inductively coupled plasma mass spectrometry (ICP-MS). The validation results have been presented and organized in the tables to provide an overview of the method’s performance. The proposed method has met all performance criteria for a method of analysis defined in 333/2007/EC for LOD, LOQ, accuracy, precision, and considered as “fit for purpose”. The method was accredited and implemented in the routine analysis and will also be helpful for monitoring trace elements for ensuring compliance in Cephalopods.
References
Kojadinovic J, Potier M, Le Corre M, Cosson R, Bustamante P (2007) Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ Pollut 146(2):548–566
Agency for Toxic Substance and Disease Registry (2013) substance priority list. Department of Health and Humans Services, Public Health Service, Centres for Diseases Control, Atlanta
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19(2–3):125–140. https://doi.org/10.1016/j.jtemb.2005.02.010
Heier LS, Lien IB, Strømseng AE, Ljønes M, Rosseland BO, Tollefsen K-E, Salbu B (2009) Speciation of lead, copper, zinc and antimony in water draining a shooting range—time dependant metal accumulation and biomarker responses in brown trout (Salmo trutta L.). Sci Total Environ 407(13):4047–4055
Olmedo P, Pla A, Hernández AF, Barbier F, Ayouni L, Gil F (2013) Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ Int 59:63–72
Klaassen CD (2015) Casarett and Doull’s toxicology: the basic science of poisons. In: Erik JT et al (eds) Toxic effects of metals, vol 8. McGraw-Hill, New York (Ch 23)
Official journal of European communities. Commission regulation No. 466/2001(2001). https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32001R0466&from=EN. Accessed 10 July 2017
Food Safety and Standard (Food products standards and food additives) regulation (2011). http://www.fssai.gov.in/home/fss-legislation/fss-regulations.html. Accessed 15 Oct 2017
US FDA: Guidance Document for Arsenic in Shellfish, United States Food and Drug Administration (1993). http://www.speciation.net/Database/Links/US-FDA-Guidance-Document-for-Arsenic-in-Shellfish-;i762. Accessed 18 Nov 2017
Hwang BG, Lee SJ, Gil K (2016) In-vivo analysis of the uptake process of heavy metals through maize roots by using synchrotron X-ray fluorescence spectroscopy. J Korean Phys Soc 69(12):1824–1829
Aydin FA, Soylak M (2010) Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. J Hazard Mater 173(1–3):669–674. https://doi.org/10.1016/j.jhazmat.2009.08.137
Panahi HA, Kalal HS, Moniri E, Nezhati MN, Mahmoudi F (2009) Amberlite XAD-4 functionalized with m-phenylendiamine: synthesis, characterization and applications as extractant for preconcentration and determination of rhodium (III) in water samples by Inductive Couple Plasma Atomic Emission Spectroscopy (ICP-AES). Microchem J 93(1):49–54
Shubina NA, Kolesov GM (2002) Determination of heavy metals as environmental pollutants: use of instrumental neutron activation analysis. J Anal Chem 57(10):912–919
Velasco S, Ortiz LT, Rodríguez ML, Rebolé A, Treviño Benito JT, Gómez-Pinilla I, López-Andrés S (2017) Validation of an inductively coupled plasma-optical emission spectrometry method for the determination of major elements in farmed rainbow trout (Oncorhynchus mykiss). J Anal Chem 72(9):966–971. https://doi.org/10.1134/S106193481709012X
ISO/IEC 17025 General requirements for the competence of testing and calibration Laboratories (2005)
Commission regulation (EC) No. 333/2007 (2007), Official journal of European Union, L 88/29-L 88/38
Commission regulation (EC) No. 1881/2006 (2006), Official journal of European Union L 364/5-L 364/24
Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off Journal of the European Communities. 2002/657/EC, L 221/8–L 221/36
Ellison SLR, Williams A (2012) Quantifying uncertainty in analytical measurement, 3rd edn. EURACHEM/CITAC Guide CG 4. https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf. Accessed 25 Oct 2017
Michael T, Stephen LRE, Roger W (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem 74(5):835–855
Gonzalez AG, Herrador MA (2007) A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends in Anal 26(3):227–238
Taverniers Isabel, De Loose Marc, Van Bockstaele Erik (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal Chem 23(8):535–552
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, P.C., Elanchezhiyan, A., Shukla, S.C. et al. Development, validation, and accreditation of a method for the determination of 75As, 111Cd, 201Hg, and 208Pb in Cephalopods using inductively coupled plasma mass spectrometry (ICP-MS). SN Appl. Sci. 1, 304 (2019). https://doi.org/10.1007/s42452-019-0294-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0294-x