Abstract
This paper focuses on a cost effective and environment friendly technique for green synthesis of cerium oxide (CeO2) nano particles from cerium (III) nitrate hexahydrate solution by co-precipitation method using the leaf extract of different species of Artemisia pallens which acts as reducing and capping agent. The prepared CeO2 nano particles were characterized by XRD, TEM, FTIR, UV–Vis spectroscopy. The Photocatalytic activity of the prepared ceria powders was determined by their ability to degrade Methylene blue solution under UV-light radiation. The photo degradation result observed with 10 mg/L ceria at pH = 11 for 180 min have highest output and pseudo 1st order rate constant was 0.983.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is an emerging field of physics, chemistry, biology, engineering and electronic application. It involves the creation and/or manipulation of materials at the nano meter scale which contains single groups of atoms or bulk materials. Nano dimension of a material induces significant change of its properties namely optical absorption, electrical conductivity, chemical reactivity, biocompatibility compared to the macro dimension of the materials. With reduction in particle size, the surface area increases outstandingly and the number of atoms situated on the surface of the particles is higher which grants a considerable change of morphology properties [1]. Generally nano particles have large surface to volume ratio when compared to their bulk form. In general, large numbers of surface atoms are present compared to bulk materials, which play a vital role in an energetic state making significant contribution to the total free energy. These factors can change the properties such as reactivity, mechanical strength and electrical characteristics. Presently nano particles are widely used in technological application like LEDs, sensors and solar cell etc. [2,3,4]. Semiconductor nano particels usually exhibit variable as well as often controllable properties. In general when the size of the particle decreases, there is a change of energy structure and hence enhances surface properties. This will enhances their optoelectronic properties. Cerium is a rare earth element, which exists in trivalent state; cerium also occurs in IV states and may alternate between these two in a redox reaction. Based on this results, it was theorized that cerium oxide nano particles prolong cellular longevity by scavenging free radicals generated during their lifetime. The distinct structure of ceria nano particles with regard to the valence, support cell longevity which is a benefit of its antioxidant properties. Antioxidant behaviour is strongly influenced by the co-existence of both Ce3+ and Ce4+ oxidation states in CeO2 nano particles. Cerium di oxide has wide band gap (3.18 eV) energy. It is a technological important material, which has wider application such as super capacitor, buffer layers in conductors, fuel cell battery, polishing material, UV blockers and optical storage devices. It is an important material that can be used in photo catalytic reaction [5,6,7,8,9,10]. Several methods have been engaged to prepare ceria powder [11,12,13,14,15,16] were employed on the past decade. This paper focuses on preparing ceria nano powder using green synthesis method. Many bioactive compounds are contained in Atemesia. It is known for its antimalarial activity and cytotoxicity against tumor cells [17]. These plants are also popular for the treatment of diseases such as hepatitis, cancer, inflammation and infections by fungi, bacteria and viruses [18]. It appears as a small and aromatic herbaceous plant and is native to the southern part of India. The leaves and flowers of this plant are highly valued for making floral decorations and oils. The plant consists of very small leaves, bluish green with yellow flowers. Traditional Ayurvedic medicinal formulations utilize this plant. The synthesized nano powder was characterized by x-ray powder diffraction,TEM and FTIR spectrum to study the structure and bonding nature. These samples were also studied for their photo catalytic activity using MB under UV light.
2 Experimental
The bio synthesis of ceria (CeO2) nano particles were done by green chemistry method using cerium (III) nitrate hexahydrate. In the first step of synthesis 5 g of cerium (III) nitrate hexahydrate was added to 25 ml distilled water and stirred thoroughly at 60 °C for 1 h to form a clear solution. In the second step 10 g of Artemisia pallens was added into 50 ml distilled water and heated at 60 °C for 1 h. This solution was filtered and this filtrates was taken in a burette. This clear solution in burette was added as drop by drop to cerium (III) nitrate hexahydrate solution under constant stirring condition to form a precursor. In the third step, two tablets of NaOH were dissolved in 50 ml of distilled water and then added drop by drop into a beaker containing the precursors. A small quantity of NaOH was used to increase the pH to alkaline condition. After few hours precipitation occurred. This precipitate was centrifuged at 6000 rpm and was then dried at 373 K. The X-Ray diffraction patterns of the CeO2 nano particles was recorded using panalytical’s X-Ray diffractometer for various calcined temperatures. The FTIR spectrums of the CeO2 nano particles were taken using Perkin Elmer spectrometer. The surface morphology and particle size of the CeO2 nano powder were analyzed by Transmission electron microscopy (TEM) images were obtained with JEOL JEM 2010HR operating at 200 kV. Absorption spectrums for the CeO2 nano particles were recorded by using Shimadasu UV visible spectrophotometer. The CeO2 nano particles calcined at different temperature in the range of 400–500 °C were employed to study the degradation of Methylene blue dye under UV radiation from a 12 W UV lamp. Five centimeter distance was maintained between the photoreaction vessel and the lamp source. About 5 mg of CeO2 photo catalyst was added to 10 mg/L of methylene blue solution (100 ml) in a typical run. At regular intervals of 60 min, 3 ml of the above solution was centrifuged to separate the photo catalyst particles, The concentration of methylene blue in this solution was evaluated from the absorption spectrum obtained with the help of Hitachi UV–VIS–NIR spectrophotometer.
3 Result and discussion
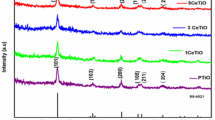
X-Ray diffraction pattern of cerium oxide nano particles obtained in the range of 28° to 95° is shown in Fig. 1. XRD exhibits the following peaks (111), (220), (311), (400), (331), (422) and (511) corresponding to single phase cubic structure (JCPDS NO: 431002). The broad peaks show the nano size crystallites. Crystallite size was calculated using 2θ = 28°48′ (111) peak with the Scherrer formula
where λ is the wavelength of X-Ray, θ is the Bragg diffraction angle and β is the full width half maximum (FWHM) of the XRD peak appearing at the diffraction angle θ. The average crystalline size and dislocation density are tabulated in Table 1. Generally size of nanoparticles increases with increase of temperature due to the aggregation of individual particles. In this case as the calcinations temperature increases recrystallization takes place along with grain aggregation and hence an increase in grain size.
Figure 2 Shows the Infrared spectrum of raw CeO2 powder and various calcinations temperature of the samples. FTIR spectrophotometer between 450 and 4000 cm−1. FTIR studies have been used to confirm the formation of metal oxide. The bands at 528 cm−1 and 719 cm−1 are due to the Ce–O stretching vibration whereas the bands at 1379 cm−1 and 1383 cm−1 are due to C-O stretching vibration and the band at 3395 cm−1 is due to O–H vibration of water absorbed from the moisture respectively [16].
Ultrafine nanoparticles of CeO2 with average particle size in the range of 4.0 nm to 16.0 nm is observed in the TEM images (Fig. 3a, b) for the nanoparticles calcined at different temperature, Nearly spherical particles are observed. Thos results agrees well with the aize calculated using Scherrrer’s equation from XRD data. A fairly uniform distribution of particles is observed. The average fringe distance between two adjacent fringes in the HRTEM pattern (Fig. 3c) is 0.31 nm. The selected area diffraction (SAED) pattern of the CeO2 nanoparticles calcined at 450 °C (Fig. 3d) indicates the mulilayered pattern of the particles which is suggestive of a polycrystalline nature of the particles as is supported by the mixed fringes pattern observed in HRTEM result.
The EDS spectrum shows the presence of Ce and O element no other element were observed in this spectrum (Fig. 4). Figure 5 shows the transmission spectra of prepared CeO2 nano particles in the range of 100–1000 nm. The energy band gap of CeO2 materials were calculated using optical transmission spectrum. The formula is given by
where λ is the wavelength (nm) and Ebg is the optical band gap energy. The optical band gap of CeO2 is 3.45 eV that is quite large compared to reported value (3.22 eV) of bulk CeO2 [19, 20]. The enhancement in optical band gap of CeO2 nano particles could be due to the quantum size effect and it is consistent with literature report for CeO2 nanoparticles [21].
3.1 Photocatalytic activity studies
In analytical chemistry MB is widely used as a redox indicator. MB is a dark green powder that yields a blue solution in water. In blue bottle experiment, MB is an oxidizing environment and turn colourless when exposed to a reducing agent. In biological field, MB is used to examine RNA or DNA under a microscope or in a gel. Photocatalytic activities of the CeO2 nano particles was studied by decolourization of methylene blue (MB) aqueous solution under stimulated UV radiation.
3.2 Degradation of MB catalysed by cerium oxide
The degradation efficiency of MB (10 mg/L) versus time in the absence and presence of CeO2 nanoparticle (10 mg/L) at a pH of 11 are shown in Fig. 6 In the absence of any photocatalyst the degradation of the MB molecule occurs under UV irradiation at a slow rate. This degradation efficiency with irradiation increases in the presence CeO2 nano particles, since the onset of absorption is lower than 420 nm wavelength which is suitable for transfer of electron from the valence band to conduction band [22]). This leads to formation of holes and electrons in the valence band and conduction band respectively. The holes in valance band react with water which leads to degradation of the dye by the interaction of holes and OH species. [23] The highest photocatalytic activity is observed for the samples calcined at 500 °C. Photo catalytic activity is affected by particles size, crystallinity and surface properties. Preparation method can control the above factor along with the catalytic loading. Nanomaterials have unique adsorption properties in addition to high specific surface area and its disordered surface region [24]. Though the as synthesized and powders calcined at temperatures below 500 °C possess high surface area compared to the powder calcined at 500 °C, the highest photocatalytic activity of the high temperature calcined powder can be explained as a result of the improved crystallinity of these powders. The degradation efficiency of MB (10 mg/L) at different photocatalyst dosages ranging from (5 to 40 mg/L) at pH 11 have been studied. Figure 7 shows the photodegradation efficiency of MB which increases as the amount of photocatalyst was increased up to 5 mg/L and thereafter decreases with increase in photocatalyst loading. The availability of actives sites and thus an increase in the number of dye molecule adsorbed on the surface of the catalyst as well as an increase in the density of particle in the illumination area is the reason for the increase in decolourization rate [25, 26]. At the higher catalyst loading of 40 mg/L, the dye degradation rate decreases due to agglomeration of the photocatalyst. This results in decrease of the radiation penetration depth and causes radiation scattering at higher photo catalyst concentrations. Therefore it is desirable to add the optimum amount of CeO2 to avoid the presence of excess catalyst and thus ensure the total absorption of light photons for efficient photodegradation.
The pH of the dye plays an important role in dye degradation, since, the adsorption capacity of dyes on the photo catalyst is an important factor. An increase in the rate of molecule decomposition is the result of an increase in the number of target molecules adsorbed onto a catalyst [27]. In this study, the effect of pH on the photocatalytic activity of CeO2 nano particles (10 mg/L) during the MB degradation process with an initial concentration of 5 mg/L over a pH ranges 1–12 was studied. The pH of the dye solution was adjusted by adding suitable amounts of HCL for pH range up to 6, beyond which NaOH (concentrations of 10 mg/L). The degradation yield in the different pH solution is shown in Fig. 8. The results show that the maximum degradation efficiency was observed at a pH of 11. This is due to the fact that, higher the pH of the solution, the higher will be the concentration of hydroxyl ions for reaction with the holes and for the formation of hydroxyl radicals. Due to the negative charge of the catalyst surface, adsorption of positively charged dye molecules occurred in the basic solution. Inhibition of dye degradation occurs at a pH higher than 11, since, under these conditions, the hydroxyl ions compete with dye molecules in the adsorption on the catalysts surface [28, 29]. Figure 9 shows the effect of initial concentration of MB dye (5–40 mg/L) on the rate of degradation of the dye. A decrease in the rate of degradation is observed for an increase in the initial MB concentration. There are two factors, that are responsible for the decrease in degradation efficiency with an increase in the initial dye concentration, viz., an increase in the number of dye molecules adsorbed on the surface of the catalyst leads to a decrease in the number of active sites that generate hydroxyl radicals, and an increases in the light absorbed by the dye molecules leads to a decrease in the number of photons that reach the catalyst surface [30].
The influence of many factors and their mutual effects heterogeneous photocatalysis reactions are complicated processes. In this study, MB photo degradation followed a pseudo first order kinetic expression as shown in Fig. 10). Langmuir–Hinshelwood kinetic expression is followed in this study similar to earlier reports [31].
In Eqs. 3 and 4, [dye]o is the initial concentration of MB (mg/L), kdye is the Langmuir–Hinshelwood adsorption equilibrium constant (L/mg),k is the rate constant of the surface reaction (mg/L min) and kapp is the pseudo first order rate constant. According to Eq. 4, a plot of 1/kapp versus [dye] is a straight line and in this case the rate constant for the surface reaction is k = 0.188 mg/(L min) and the adsorption equilibrium constant kdye = 0.102 for the powder calcined at 500 °C (Fig. 10). The obtained regression coefficient R is 0.983, which suggests that the photo degradation of MB catalyzed by CeO2 fits the Langmuir–Hinshelwood kinetic model, and also leads to the conclusion that the CeO2 powders calcined at different temperature in the present work can be an efficient photocatalyst for the degradation of Methylene blue (Table 2).
Photocatalytic degradation of an organic dye can be understood as follows. As the first step, MB dye adsorbs onto the nano CeO2 surface. When the CeO2 is irradiated with UV light, electron- hole pairs are created. While the photo generated electrons in the conduction band of CeO2 interact with the oxygen molecules adsorbed on the CeO2 to form the superoxide anion radicals (O ·−2 ), the holes generated in the valence band of CeO2 react with the surface nydroxyl groups to form highly reactive hydroxyl radicals (OH·). These two highly reactive radicals react with the MB dye adsorbed on the photocatalysrt resulting in the decolouration and hence degradation of the dye [32, 33]. The two important factors for a good catalyst to be used in practical applications are its recyclability and stability [34]. The degradation/regeneration capacity and hence the structural stability during the entire process was evaluated by studying the reusability of the photocatalyst for 5 cycles. Initially the rate of decrease of the degradation efficiency is fast and after the 4th cycle, the rate of decrase is slow compared to the first three cycles. There is 5% decline in the degradation efficiency after 5 cycles (Fig. 11). This stands testimony to the excellent photocatalytic activity of the CeO2 nanopowder synthesized in this work.
4 Conclusion
Ceria nano particle were prepared using cerium (III) nitrate hexahydrate and NaOH as precursors in the absence of any capping agent using the extract of Artemisia pallens. The photo generated holes are the major active species of Cerium oxide in the photo catalytic degradation of MB. The results revealed that the smaller size CeO2 nanoparticles are potential photo catalyst for the degradation of methylene blue. The MB degradation shows the highest photocatalytic reactivity at pH 11 for CeO2 calcinated at 500 °C. In the presence of OH and superoxide radicals considerable reduction is observed in the colour of MB. This shows that the photo generated holes and electrons in the catalyst upon irradiation plays an important role in photo degradation. The photocatalyst could be reused for 5 cycles with a decline of only 5% efficiency in the degree of degradation. The excellent reusability if the synthesized CeO2 nano powders indicates the efficient utilization of these materials in environmental remediation.
References
Pop OL, Diaconeasa Z, Mesaroş A, Vodnar DC, Cuibus L, Ciontea L, Socaciu C (2015) FT-IR studies of doped cerium oxide nanoparticles and natural zeolite. Mater Bull UASVM Food Sci Technol 72:50–55
Zhang Y, Andersson S, Muhammed M (1995) Synthesis of doped cerium oxides as oxygen storage promoters. Appl Chem B Environ 337:6325–6337
Lira-Cantu M, Norrman K, Andreasen JW, Krebs FC (2006) Oxygen release and exchange in niobium oxide MEHPPV hybrid solar cel. Chem Mater 18:5684–5690
Abecassis-Wolfovich M, Jothiramalingam R, Landau MV, Herskowitz M, Viswanathan B, Varadarajan TK (2005) Cerium incorporated ordered manganese oxide OMS-2 materials-improved catalysts for wet oxidation of phenol compounds. Appl Catal B Environ 59:91–98
Matijevic E, Hsu WP (1987) Preparation and properties of monodispersed colloidal particle of lanthanide compounds I Gadolinium europium terbium samarium CeO2. J Colloid Interface Sci 118:506–510
Chen PL, Chen IW (1993) A theoretical model and experiments for the pop-out phenomena via Nanoindentation test of diamond films. J Am Ceram Soc 76:1577–1581
Li T, Ikegami JG, Wang YR, Mori T (2002) Synthesis of Graphene-scattered Nano Li2Fe SiO4 for lithium ion battery application. J Am Ceram Soc 85:2376–2382
Tsunekawa S, Sivamohan R, Ohsuna T, Kasuya A, Takasashi H, Tohji K (1999) Indirect exchange coupling of magnetic moments in Rare earth metals. Rare Earths 439:315–317
Djuricic B, Pickering S (1999) Nanocrystallization and phase transformation in monodispersed ultrafine Zirconia particle from various homogeneous precipitation method. J Eur Ceram Soc 19:1925–1932
Zhon XD, Hucbner W, Anderson HU (2002) Vaporization thermodynamics MgB2 and MgB4. Appl Phys Lett 80:2892–2894
Deluga GA, Salge JR, Schmidt LD, Verykios XE (2004) Renewable hydrogen from ethanol by autothermal reforming. Science 203:993–997
Hu J, Li Y, Zhou X, Cai M (2007) Preparation and characterization of Ceria nanoparticle using crystalline hydrate cerium propionate as precursor. Mater Lett 61:4989–4992
Wang H, Zhu JJ, Zhu JM, Liao XH, Zu S, Ding T, Chen HY (2002) Preparation of nano crystalline ceria particle by sono chemical and microwave assisted heating methods. Phys Chem Chem Phys 4:3794–3799
Shin G, Wang Q, Wang Z, Chen Y (2011) Full ALD Al2O3/ZrO2/SiO2/Al2O3 stacks for high performance MIM capacitors. Mater Lett 65:1211–1214
Ketzid JJ, Nesara AS (2011) Synthesis of CeO2 nanoparticles by chemical precipitation and the effect of a surfactant on the distribution of particle size. J Ceramic Process Res 12(1):74–79
Prabaharan DM, Sadaiyandi K, Mahendran M, Sagadevan S (2016) Structural optical morphological and dielectric properties of cerium oxide nano particles. Mater Bull 19:478–482
Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M (2003) Artemisinins target the SERCA of plasemodium falciparum. Nature 424:957–961
Cavaletti G, Bogliun G, Zincone A, Marzorati L, Melzi P, Frattola L, Marzola M, Bonazzi C, Cantù MG, Chiari S, Galli A (1998) Neuro and ototoxicity of high dose carboplatin treatment in poor prognosis ovarian cancer patients. Anticancer Res 18:3797–3802
Araujo VD, Avansi W, de Carvalho AH, Moreira ML, Longo E, Ribeiro C, Bernardi MI (2012) CeO2 nano particle synthesized by a microwave assisted hydrothermal method evolution from nanospheres to nanorods. CrystEngComm 14:1150–1154
Phoka S, Laokul P, Swatsitang E, Promarak V, Seraphin S, Maensiri S (2009) Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater Chem Phys 115:423–428
Chen HI, Chang HY (2005) Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram Int 31:795–802
Brunaner S, Emmett PH, Jeller E (1938) Coordination compounds of palladous chloride. J AM Chem Soc 60:882–884
Culily BD (1978) Elements of X-ray diffraction reading. Addison Wesley, Boston
Hu CG, Zhang ZW, Liu H, Gao PX, Wang ZL (2006) Direct synthesis and structure of characteristics of ultrafine CeO2 nanoparyicles. Nanotechnology 17:5983–5987
Xu J, Li G, Li L (2008) CeO2 nanocrystals: seed-mediated synthesis and size control. Mater Res Bull 43:990–995
Height MJ, Pratsinis SE, Mekasuwandumrong O (2006) Praserthdam P Ag-ZnO catalysts for UV-photodegradation of methylene blue. Appl Catal B 63:305–312
Alkaim AF, Aljeboree AM, Alrazaq NA, Baqir SJ, Hussein FH, Lilo AJ (2014) Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J Chem 26:8444–8448
Ranjit KT, Willner I, Bossmann SH, Braun AM (2001) Photo degradation of organic pollutant using sol gel method. Environ Sci Technol 35:544–551
Pouretedal HR, Eskandrri H, Keshaverz MH, Semnani A (2009) Photodegradation of organic dyes using nanoparticle of cadmium sulphide doped with manganese, nickel and copper as nanophotocatalyst. J Acta Chem Solv 56:353–359
Pouretedal HR, Norozi A, Keshavarz MH, Semnani A (2009) Nanoparticles of zinc sulphide doped with manganese, nickel and copper as nano photocatalyst in the degradation of organic dyes. J Hazard Mater 162:674–681
Kumat PV, Meisel D (2002) Nano particle in advanced oxidation process. Curr Opin Colloid Interface Sci 7:282–290
Al-Ekabi H, Serpone N (1988) Kinetics studies in heterogeneous photo catalysis. J Phys Chem 92:5726–5731
Suna JH, Wang YK, Sun RX, Dong SY (2009) CeO2 nanoparticle catalysis of methylene blue photodegradation: kinetics and mechanism. Mater Chem Phys 115:303
Li K, Yang C, Ying D, Wang Y, Jia J (2013) Effect of Inorganic anions on Rhoda mine β removal under visible light irradiation using BiO2/Ti rotating disk reactor. J Chem Eng 211:208–213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanathan, G., Rathan, S.V. & Murali, K.R. Photocatalytic activity of biosynthesized CeO2 nano particles. SN Appl. Sci. 1, 116 (2019). https://doi.org/10.1007/s42452-018-0103-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0103-y