Abstract
The study aims to evaluate the association between glycaemic variability and the risk of chronic kidney disease (CKD) progression in patients with diabetes and comorbid kidney disease. A comprehensive search was conducted of three databases from their inception to March 2022: Medline, Embase, and CINHAL. Publications were screened for eligibility and the quality of studies included was appraised using the Newcastle–Ottawa Scale. Extracted data were tabulated and reported in a narrative synthesis. Fourteen studies were included in the review providing data on 62,498 participants. Eight studies reported that greater glycaemic variability was associated with an increased incidence of CKD. Three studies reported an increased likelihood of CKD progression in those with high glycaemic variability, although the rate and risk of progression varied across the studies. Three studies reported an increased risk of progression to end-stage kidney disease (ESKD) with higher glycaemic variability. One study found that high glycaemic variability was associated with a decreased risk of progression to ESKD. Greater glycaemic variability was associated with the onset and progression of CKD. More research is required to verify whether glycaemic variability increases the risk of progression to ESKD in patients with diabetes and mild/moderate comorbid CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is the leading cause of chronic kidney disease (CKD) and end-stage kidney failure [1, 2]. The challenge for clinicians who care for patients with diabetes and comorbid CKD is the lack of evidence and guidance for optimal glycaemic targets [3]. Glycated haemoglobin (HbA1c) is the most commonly used marker for determining glycaemic control as it correlates strongly with the risk of diabetes complications [4,5,6,7]. Unfortunately, patients with a history of diabetes-related CKD were excluded from the major studies from which current glycaemic targets are derived, the Diabetes Control and Complications Trial (DCCT) [8] and The United Kingdom Prospective Diabetes Study (UKPDS) [9].
More recent trials of glycaemic intensification in people with type 2 have provided some data on the impact of tightening glycaemic control on kidney outcomes, although findings have been equivocal. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [10] reported that lowering glycaemia did not reduce the incidence of end-stage kidney disease (ESKD) or lead to a decrease in the decline of estimated glomerular filtration rate (eGFR). Similarly, the Action in Diabetes and Vascular Disease (ADVANCE) trial [11] reported that intensive glucose control did not reduce the need for renal replacement therapy or renal-related mortality. In the Veterans Administration Diabetes Trial (VADT) [12], whilst decline in eGFR was similar in the intervention and control arms, higher levels of albumin excretion and progression to macroalbuminuria were observed in the control group.
It has also been recognised that, along with the level of glycaemic control, the stability of the HbA1c over time is important [4, 13]. Two recent meta-analyses have suggested that glycaemic variability (fluctuations and changes in the glycaemic level) may be associated with an increased risk of diabetes complications and mortality [13, 14]. Gorst et al. [14] assessed the association between glycaemic variability and microvascular complications and mortality in patients with type 1 and type 2 diabetes mellitus. Their results showed that, amongst patients with type 1, glycaemic variability was associated with diabetes-related nephropathy (HR 1.56, 95% CI 1.08–2.25), increased cardiovascular events (HR 1.98, 95% CI 1.39–2.82), and retinopathy (HR 2.11, 95% CI 1.54–2.89). Their findings for participants with type 2 were similar, with higher glycaemic variability also being associated with diabetic nephropathy (HR 1.34, 95% CI 1.15–1.57) and cardiovascular events (HR 1.27, 95% CI 1.15–1.40). In a meta-analysis by Cheng et al. [15], high glycaemic variability was also associated with a change in kidney status in patients with both type 1 and type 2 diabetes mellitus. These authors reported that high glycaemic variability was significantly associated with the progression of CKD amongst people with type 1 (HR 1.70, 95% CI 1.41–2.05) and type 2 (HR 1.20, 95% CI 1.12–1.28). However, a limitation of these analyses was in the pooling of heterogenous studies with divergent methods for characterising glycaemic variability with varying ranges in the number of readings required for the analysis and in the time intervals between these measures. In addition, these reviews concentrated on patients with diabetes who had no previous history of CKD, and hence did not consider how the variability might impact on the progression of CKD. To address the limitations of these previous reviews, we present an updated systematic review of empirical research with a more analytical perspective on the methods and findings of studies addressing the question of glycaemic variability and CKD status, together with the findings from some additional more recent studies, in undertaking the review aimed to assess the role of glycaemic variability in the progression of CKD in people with diabetes.
Methods
The review was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement [16].
Data Source and Searches
A comprehensive literature search was conducted using three electronic databases (Medline, Embase, and CINHAL) in September 2020, with an updated search conducted in March 2022. The search used both Medical Subject Headings (MeSH) and free-text synonyms for the following terms: diabetes mellitus, glycaemic variability, and chronic kidney disease. The searches were limited to materials available in English and studies that involved human subjects. No time restrictions were placed on the search so that all potentially relevant studies could be considered. The search strategy for Medline and Embase is provided as online supplementary material (Appendix A). Secondary citations were also used to identify potential studies. Retrieved articles were screened by two independent researchers for their relevance (H. H.-A., A. F.).
Study Selection
Inclusion criteria were adults (18 + years) with a diagnosis of diabetes mellitus and participants with CKD stages 1–3 at baseline or without CKD. Studies were eligible if they reported glycaemic variability and at least one of the following outcomes: onset of CKD, a decline in kidney function, or end-stage kidney disease. Publications were excluded if the study population included children (age < 18 years) or those with gestational diabetes. Reviews, case reports, and conference abstracts were excluded.
Data Extraction
One author (H. H.-A.) performed data extraction using a predefined data extraction template, and all extractions were verified by other authors (D. W., A. F.). Extracted information included study characteristics (author, year of publication, country, and number of participants), study objectives and participant characteristics (age, gender, type, and duration of diabetes), methods used to assess glycaemic variability, and kidney outcomes.
Quality Assessment
The methodological quality of the included articles was evaluated using the Newcastle–Ottawa Scale (NOS) quality assessment tool. The scores of the NOS system range from 0 to 9 and consist of three subscales: selection of studies (0–4 points), comparability (0–2 points), and exposure (0–3 points). There were three levels of evidence: low risk of bias (a score 7–9), moderate risk of bias (a score of 5–6), and high risk of bias (score < 5) [17]. A study was excluded if it scored poor. The quality assessments were performed independently by two authors (H. H.-A., A. F.) with any disagreement between reviewers resolved through discussion. Summary of critical appraisal of the included studies using the Newcastle–Ottawa Scale is presented in Table 1 of the supplementary file 2.
Data Synthesis
Study characteristics, methods, and results were tabulated. Details of the study results of interest were identified from the table result column and used to inform a synthesis of the evidence. Due to heterogeneity in the studies’ glycaemic assessment methods and outcome measurements, meta-analyses were not appropriate (I2 = 78.5%, indicating considerable heterogeneity); hence, the extracted data were tabulated to provide an overview of the findings from the identified studies.
Results
Search Results
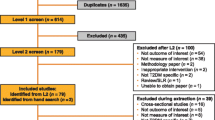
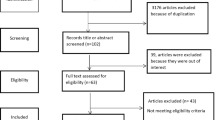
In total, 116 citations were identified from the three electronic databases (Fig. 1). Thirty-six duplicates were subsequently removed, and the remaining 80 articles were screened based on information in their titles and abstracts. Following the screening, a further 60 articles were excluded as they did not meet the inclusion criteria. The remaining 20 full-text articles were then assessed for their eligibility in accordance with selection criteria, of which 15 met the inclusion criteria. One article was subsequently excluded due to a poor-quality score, and the remaining 14 articles were included in the review.
Study and Participant Characteristics
All 14 studies were observational cohort studies: seven were prospective, six were retrospective in design, and one was a cross-sessional study. The characteristics of the studies, the sample size, and the methods used to assess glycaemic variability and CKD progression data are presented in Table 1. Nine of the included studies were conducted in Asia (China, Japan, and Taiwan) and four in Europe (UK, Spain, Italy, and Finland), with one study from the USA. A total of 62,498 subjects were included, 59% (n = 36,873) of whom were male. The mean age of the participants in those studies reporting age (n = 14) was 60.06 (± 11.40). Of the identified studies, nine reported progressions of CKD in patients with type 2 diabetes mellitus (n = 58,950) and two in patients with type 1 diabetes mellitus (n = 3548). Six studies reported duration of diabetes, with a mean duration of 9.80 years (SD ± 6.21) [21,22,23]. Six studies reported the median duration of diabetes, which ranged from 1 to 23 years [24,25,26,27,28,29]. The ethnic composition of the subjects was detailed in nine studies [21, 22, 25,26,27,28,29,30,31]. The period of observation ranged from 0.5 to 10 years. There were variations in the definitions used for renal endpoints between the included studies. Some studies used the new-onset (i.e., incidence of CKD (n = 9)), others the change in kidney function assessed by albuminuria status or eGFR (n = 3), or progression to either end-stage renal disease (ESKD) or dialysis (n = 3). As a result of this variation, our findings are organised into three subheadings: onset of CKD, progression of CKD, and developing ESKD.
Quality Appraisal
The quality scores ranged from 4 to 9. Pereira et al. [18] was excluded as it was considered to be of poor quality for the following reasons (quality score 4): a small sample size (n = 221); the exclusion of participants with additional comorbidities, which may have a biased sampling from the population of people with diabetes; inadequate follow-up; high attrition bias (221/538); and a failure to adjust for potential confounders (covariates such as age and diabetes duration). No robust statistical analyses were conducted to compare the association glycaemic variability and renal outcome. Overall, most of the remaining 14 included studies were judged to be of good quality (see Table 1 of the supplementary file 2).
Measurement of Glycaemic Variability in the Included Studies
Glycaemic variability was assessed using HbA1c results in all the studies, although the definition of glycaemic variability varied between them. The majority of studies used the intrapersonal mean and standard deviation (SD) of HbA1c [19,20,21,22,23,24,25,26], two studies [27,28,29,30] used the coefficient of variation (CV) of HbA1c, and Yan et al. [29] used glycaemic change score and area under the curve. Cumming et al. [31] used three different metrics based on the area under the curve (AUC) for three different models related to a threshold value of an HbA1c of 7% (based on a minimum of 10 readings), as follows: (1) AUC of HbA1c values > 7%; (2) AUC of HbA1c values above and below 7% combined; and (3) the difference in AUC of values > and < 7% (i.e., AUC for HbA1c > 7% minus the AUC for HbA1c < 7%). One study [29] also used clinically significant changes in HbA1c (> 0.5%, 5.5 mmol/mol) to calculate a variability score, in addition to SD, AUC, and CV.
Association Between Glycaemic Variability and Onset of CKD
Eight studies assessed the association between glycaemic variability and the onset of CKD. Table 2 presents the reported relationships identified between glycaemic variability and the onset of CKD. There was variation in the criteria used for defining onset of CKD: four studies used the increase in albumin excretion rate (AER) values(≥ 3.4 mmol/mg), whereas Luk et al. [22], Lin et al. [28], and Chiu et al. [26] used eGFR < 60 and Penno et al. [24] used both AER and eGFR. Irrespective of the outcome measures used, all eight studies demonstrated that those with high glycaemic variability had a higher incidence of CKD onset.
Four studies [28, 31, 32] investigated the risk of onset of CKD by comparing stratified groups of participants based on the SD or CV of their HbA1c readings. Lin et al. [21] reported that participants with higher glycaemic variability (top tertile) had a two-fold increased risk of developing microalbuminuria (HR 2.22, 95% CI 1.67–2.93), whilst those in the second tertile of HbA1c-CV had a 36% increased risk of developing microalbuminuria with the lowest tertile as the reference. Hsu et al. [32] also found that the increased risk of microalbuminuria accrued for each quartile of SD of HbA1c at 48%, 9%, and 3%, respectively, for the fourth, third, and second quartiles with the lowest quartile as the reference. However, the relationship between the second and third quartiles was not statistically significant. Chiu et al. [26] examined the effect of glycaemic variability on decline in the eGFR rate in people with type 2 diabetes. They found that patients who are in the highest HbA1c-CV group at baseline (3 years prior to assessment) had a greater risk of decline in kidney function (HR − 2.53, 95% CI − 3.28, − 1.78), compared to the second (HR − 1.73, 95% CI − 2.38, − 1.08) and third highest (HR − 0.99, 95% CI,− 1.67, − 0.31) variability groups, with the lowest group as the reference. Similarly, Yan et al. [29] explored glycaemic variability and the risk of diabetes kidney disease. They found that higher glycaemic variability defined using three methods (HbA1c-SD, HbA1c-AUC, and a HbA1c variability score) were significantly associated with the development of microalbuminuria. In relation to eGFR progression, the result showed that eGFR progression from stage 1 was higher in the quartiles with the highest variability quartiles for HbA1c-SD, and HbA1c-CV compared to the reference group. However, there was no association between glycaemic variability and eGFR progression in those in the second or third highest quartiles, with the lowest as a reference.
Similarly, Sugawara et al. [19] observed that participants with higher glycaemic variability had a 20% increased risk of developing microalbuminuria (HR 1.20, 95% CI 1.03–1.39). This pattern was also found by Kilpatrick et al. [21], who reported that those with greater glycaemic variability had at least a two-fold increased risk of developing microalbuminuria (HR 1.86, 95% CI 1.41–2.47). Likewise, Luk et al. [22] conducted a prospective cohort study showing that patients with higher glycaemic variability had a 16% increased risk of developing CKD (HR 1.16, 95% CI 1.10–1.22).
Penno et al. [24] reported that those with higher HbA1c-SD variability were 31% more likely to develop microalbuminuria (odds ratio (OR) 1.31, 95% CI 1.10–1.56), were 41% more likely to develop macroalbuminuria (OR 1.41, 95% CI 1.03–1.93), and 24% more likely to develop a reduced eGFR (OR 1.24, 95% CI 1.02–1.51) compared to those in quartile one (low variability). In addition, high HbA1c-SD was associated with a 30% increased risk of developing CKD stages 1 and 2 and 46% higher risk of developing CKD stage 3–5 albuminuria (OR 1.46, 95% CI 1.10–1.96). However, they also found that HbA1c-SD was not associated with non-albuminuric CKD. This may be because their study did not assess longitudinal changes in AER due to the cross-sectional design employed.
Association Between Glycaemic Variability and Progression of CKD
A total of three cohort studies examined the relationship between glycaemic variability and the progression of chronic kidney disease amongst patients with diabetes who had comorbid CKD [23, 31, 33]. Table 3 presents the reported relationships identified between glycaemic variability and the progression of CKD. Studies consistently reported the increased likelihood of CKD progression in those with high glycaemic variability, although the rate and risk of progression varied in the included studies.
Rodriguez et al. [23] found that even when adjusting for a broad range of demographic and clinical variables, there was a 37% higher risk of increased albumin excretion in patients with type 2 diabetes mellitus with greater HbA1c variability. Incremental increase in CV was associated with a significantly increased risk of progression in CKD. Similar findings based on concurrent follow-up of HbA1c and AER have been reported by Waden et al. [33], where SD of HbA1c was associated with a nearly twofold increase in progression of renal disease (HR 1.98, 95% CI 1.39–2.47). Cummings et al. [31] also reported a significant worsening of CKD with an average excess HbA1c > 7% (OR 1.173, 95% CI 1.031–1.335). However, their models such as AUC of HbA1c values above and below 7% combined and the difference in AUC of values > and < 7% (i.e., AUC for HbA1c > 7% minus the AUC for HbA1c < 7%) did not show a significant effect on CKD progression.
Association of Glycaemic Variability and Progression to ESKD
Four cohort studies evaluated the association of HbA1c variability with the development of ESKD in diabetes patients [22, 25,26,27]. Table 4 presents the reported relationships identified between glycaemic variability and the development of ESKD. Studies describing the role of glycaemic variability in progression to ESKD produced mixed results. Three studies [22, 26, 27] found that higher glycaemic CV-HbA1c and SD-HbA1c were associated with increased risk of progressing to ESKD, respectively. All the three studies reported an increased progression risk with increasing CV-HbA1c or SD-HbA1c variability. In contrast, a study by Lee et al. [25] assessed the association of HbA1c variability with progression of CKD in patients with diabetes who had mild to moderate CKD (stages 3–5) and found that participants with CKD stages 3 and 4 and HbA1c ≥ 7% in the higher HbA1c tertile had decreased risk of progression to dialysis. This is contrary to current evidence. This finding may be partly due to the fact that the study used 3 HbA1c measures over the study period to determine glycaemic variability, which may be inadequate to characterise the pattern of glycaemic variability over an extended period.
Discussion
This systematic review aimed to identify and evaluate the available published studies on the relationship between glycaemic variability and onset of CKD or worsening of kidney disease or the development of ESKD in people with diabetes. Most studies were of good methodological quality and provided a reasonable collective sample size to examine the association between glycaemic variability and the progression of CKD. Although it is important to emphasise that the methods employed to assess glycaemic variability in some of the studies were inadequate.
Overall, the results of these studies suggest that there may be a link between higher glycaemic variability and the risk of the development or the progression of renal complications in people with diabetes. The results of most of the included studies (n = 13/14) showed that patients with greater glycaemic variability have an increased risk of developing CKD, progression of CKD, or development of ESKD. An exception was the study by Lee et al. [30], which evaluated the association of HbA1c variability with progression of CKD in patients with diabetes and CKD (stages 3–5). This was one of the two studies that assessed glycaemic variability amongst patients with established CKD (baseline CKD stage 3). However, the study was comparatively small with 388 participants and only had three HbA1c measures over the study period to determine glycaemic variability. In addition, the study follow-up ranged from 0.5 to 10 years limiting the amount of potential progression in those with a shorter follow-up. In addition, the Cox regression analysis does not consider the competing risk of death; therefore, there was a risk of overestimating the effect [34]. Chiu et al. [26] examined the contribution of glycaemic variability to progression to macroalbuminuria in a cohort of 110 people with diabetes with and without microalbuminuria at the baseline. Chui et al. used the Kaplan–Meier method to compare time to overt macroalbuminuria of different groups based on glycaemic variability and CKD status. They reported that patients with microalbuminuria and higher levels of HbA1c SD were more likely to progress to macroalbuminuria in the 6-year observation period, but they could not quantify the difference in risk for each group. The authors also acknowledged that their sample is small and did not adjust for any covariates in their analysis. Hence, whilst the collective data indicates some association between glycaemic variability and the development of CKD, there are currently no high-quality studies addressing whether such variability affects progression (increase in albuminuria and/or decline in eGFR) in those who have established CKD. Therefore, additional longitudinal studies with adequate adjustment and robust measures of glycaemic variability are needed to explore the role of glycaemic variability in people with diabetes in the progression of CKD, particularly from mild/moderate to severe renal impairment.
Our findings are consistent with those of previous systematic reviews on glycaemic variability whilst providing some additional insights and considerations of bias. Cheng et al. [15] was the only previous systematic review that addressed the question of whether glycaemic variability was associated with the progression of kidney disease. This review contained eight of the studies included in our review, and in addition, they presented a meta-analysis, which estimated that glycaemic variability increased the risk of nephropathy with a pooled HR of 1.43 (95% CI 1.24–1.64). However, the validity of their meta-analysis is somewhat questionable given the heterogeneity in the glycaemic variability metrics included in the primary studies. The systematic review by Cheng et al. [15] had a number of significant limitations, including the exclusion of studies that did not report hazard ratios; despite providing relevant findings, they did not address the problems in the measurement of HbA1c (frequency of measurement and duration of measurement) that are considered in this review to be a source of potential bias. Further, the reported results were pooled against different renal outcomes (for example, some studies used the change of albuminuria whilst others reported change in eGFR) which may not be comparable. Therefore, the findings of their meta-analysis have a very high risk of bias and error, and again, they focused on studies assessing the development or incidence of CKD, rather than progression.
An important observation from this review is how to measure or assess glycaemic variability, as this was a major weakness in many of the studies. At present, there is no standardised metric for measuring glycaemic variability or consensus over how long glycaemic variability should be measured. The studies included in this review measured glycaemic variability using a wide range of different methods such as SD or CoV (SD divided by the mean) over different time periods ranging from 1 to 10 years. Most of the studies adjusted for the potential impact of the number of HbA1c measurements on standard deviation exception of Waden and Lee’s studies. Some of the included studies calculate a coefficient of variability to correct for large standard deviations due to the higher absolute value of HbA1c. Only in one study was the clinical magnitude of the changes contribution to variability considered although the findings of this analysis were equivocal.
SD was used in most of the included studies, indicating dispersion of results rather than an estimation of glycaemic variability based on clinically relevant changes in HbA1c values, with exception of one study. As such these findings are limited in providing an indication of what this observed dispersion indicates, as it will include changes in values that are potentially declining, increasing, and fluctuating. Overall, the adopted measures in these studies lacked any clinical specificity in terms of directionality or levels of variation which could inform clinical practice.
Following these limitations, we recommend the development of a standard definition for glycaemic variability for future studies, such as a glycaemic variability score, which considers exposure to clinically significant changes in glycaemic control. This can be calculated using HbA1c results and counting the number of times successive readings differ by 0.5% or 5.5 mmol/mol or more, divided by the number of comparisons and multiplying by 100. This method would help to assess the direction of change and overall variability and incorporates a magnitude of change in HbA1c which is clinically important and reduces the effect of random changes. This approach was used by Forbes et al. [35] when examining the relationship between glycaemic variability and mortality risk in older adults with diabetes in a study with 6 years of follow-up (n = 54,803). They also validated this method against different estimates of SD using total, year-to-year, and accumulative estimates; whilst the overall SD estimates were broadly comparable with their clinical change method, they found that the latter approach increased both the granularity and definition of the associations observed.
The included studies also used a range of different outcome measures for assessing kidney disease with differences in length of follow-up. The use of albuminuria may be particularly problematic for estimating the progression of kidney disease. Radcliff et al. [30] argued that albuminuria as a marker for progression is controversial as not all patients with diabetes who develop a decline in GFR experience significant albuminuria. Therefore, the results of the studies by Waden et al. [33] and Rodrigues-Segade et al. [23] may have underestimated the progression of CKD in their study population.
It is also important to recognise that these studies are limited to observed associations between glycaemic variability and CKD progression, and as such, they do not consider potential underlying explanatory factors that may contribute to the associations observed. Glycaemic variability can be driven by multiple factors, with changes in the person’s metabolic function and the use of glucose-lowering therapies being primary considerations. Whilst the mechanisms for the association between glycaemic variability and diabetes complications are unknown, it has been shown that variations in day-to-day glucose levels can increase oxidation stress and thereby accelerate the pathogenesis of some diabetes complications (Epidemiology of Diabetes Interventions and Complication (EDIC)), although others have disputed this hypothesis [36]. The contribution of glycaemic variability may be related to excess treatment effects of glucose-lowering therapies causing hypoglycaemia or general shifts in glucose levels leading to increased physiological stress as the body moves from higher to lower glucose states. In addition, glycaemic variability can be a marker for underlying changes in the health status of an individual, for example, in the case of an older person who may have reduced energy intake. Wilmot et al. [37] argue that glycaemic variability needs an increased recognition as an important therapeutic target in addition to HbA1c. Hence, in studying the association between glycaemic variability and CKD progression, it is also important to consider interactions between different diabetes treatments and general physical parameters (such as age and BMI) when estimating the association.
Limitations of the Review
Thus, as discussed, there are several limitations to this review that must be considered. First, the studies included were heterogeneous in terms of outcomes, definitions of glycaemic variability, and adjusted covariates. An additional limitation includes short follow-up periods in some of the studies. Most of the studies were observational in nature, which limits their findings to determining associations, rather than establishing cause-and-effect relationships. Although the included studies are mostly from Asia and North America, only a few studies reported ethnic composite of the study participants. Given that some ethnic groups have a much higher prevalence of diabetes nephropathy, we suggest that future studies should consider whether there is an interaction between ethnicity, glycaemia variability, and risk of diabetes nephropathy. It may also be useful if future studies were prospective so that they could better assess the association between glycaemic variability and time to the development of nephropathy.
Conclusion
This narrative review found that greater HbA1c variability is associated with increased risk of onset and progression of CKD as well as the development of ESKD in diabetes patients irrespective of the definition used for glycaemic variability or kidney outcomes. The review identified the lack of literature describing the role of glycaemic variability in diabetes patients with mild/moderate to severe kidney disease. Notably, the evidence that was found which described glycaemic variability in this group of patients (CKD stage 3 at baseline) was published more recently [30], which suggests that this is an emerging area of research. Further research is required to verify whether glycaemic variability increases the risk of progression to ESKD in patients with diabetes and baseline CKD stage 3. In addition, longitudinal studies are required to confirm the relationship between glycaemic variability and progression of CKD in non-albuminuric CKD patients with comorbid diabetes.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Limkunakul C, et al. The association of glycated hemoglobin with mortality and ESKD among persons with diabetes and chronic kidney disease. J Diabet Complicat. 2019;33(4):296–301.
Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am. 2013;97(1):1–18.
ABCD. Managing hyperglycaemia in patients with diabetes and diabetic nephropathy-chronic kidney disease. UK: ABCD; 2018.
Hirsch IB. Glycemic variability: it’s not just about A1C anymore! Diabetes Technol Ther. 2005;7(5):780–3.
Hanefeld M. Postprandial hyperglycaemia: noxious effects on the vessel wall. Int J Clin Pract Suppl. 2002;129:45–50.
Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
ABCD. Clinical practice guidelines for management of lipids in adults with diabetic kidney disease. UK: ABCD; 2021.
DCCT. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1993;44(8):968–83.
UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–13.
Ismail-Beigi F, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet (London, England). 2010;376(9739):419–30.
Patel A, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Duckworth W, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12(4):288–98.
Gorst C, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–69.
Cheng D, et al. HbA1C variability and the risk of renal status progression in diabetes mellitus: a meta-analysis. PLOS ONE. 2014;9(12):e115509.
Moher D, et al. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
McPheeters ML, et al. Closing the quality gap: revisiting the state of the science (quality improvement interventions to address health disparities). Evid Rep/Technol Assess. 2012;3(208.3):1–475.
Pereira S, et al. The impact of glycemic variability in the progression of renal disease in diabetic patients treated with insulin therapy. Rev Med Investig. 2016;4(1):15–9.
Sugawara A, et al. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55(8):2128–31.
Waden J, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58(11):2649–55.
Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabet Care. 2008;31(11):2198–202.
Luk AOY, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong diabetes registry. Diabet Metab Res Rev. 2013;29(5):384–90.
Rodriguez-Segade S, et al. Intrapersonal HbA(1c) variability and the risk of progression of nephropathy in patients with type 2 diabetes. Diabet Med. 2012;29(12):1562–6.
Penno G, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes. Cardiovasc Diabetol. 2013;12(1):2301–2310. https://doi.org/10.2337/dc12-2264.
Lee M-Y, et al. Association of HbA1C variability and renal progression in patients with type 2 diabetes with chronic kidney disease stages 3–4. Int J Mol Sci. 2018;19(12):4116.
Chiu WC, et al. HbA1C variability is strongly associated with development of macroalbuminuria in normal or microalbuminuria in patients with type 2 diabetes mellitus: a six-year follow-up study. Biomed Res Int. 2020;2020:7462158.
Yang Y-F, et al. Visit-to-visit glucose variability predicts the development of end-stage renal disease in type 2 diabetes: 10-year follow-up of Taiwan diabetes study. Medicine. 2015;94(44):e1804.
Lin C-C, et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med. 2013;126(11):1017.e1-1017.e10.
Yan Y, et al. Predictive ability of visit-to-visit variability of HbA1c measurements for the development of diabetic kidney disease: a retrospective longitudinal observational study. J Diabetes Res. 2022;2022:6934188.
Lee CL, et al. The variability of glycated hemoglobin is associated with renal function decline in patients with type 2 diabetes. Ther Adv Chronic Dis. 2020;11:2040622319898370.
Cummings DM, et al. Glycemic control patterns and kidney disease progression among primary care patients with diabetes mellitus. J Am Board Fam Med. 2011;24(4):391–8.
Hsu CC, et al. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55(12):3163–72.
Waden J, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58(11):2649–55.
Dalrymple LS, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26(4):379–85.
Forbes A, et al. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabet Endocrinol. 2018;6(6):476–86.
Nathan DM, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
Wilmot EG, et al. Glycaemic variability: the under-recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21(12):2599–608.
Author information
Authors and Affiliations
Contributions
Hellena Hailu Habte-Asres: reviewed background literature, conceived the review question, designed and executed the search strategy, extracted and tabulated data, synthesised results, and prepared the manuscript.
David Wheeler: supported the identification of background literature, provided expert input to the synthesis of review data, and assisted in revisions of the paper.
Angus Forbes: guarantor of the work who supervised the review process; validated the search strategy, the extraction of data, and the synthesis of results; and guided the preparation of the paper and assisted in its revisions.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All three authors have approved the final article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habte-Asres, H., Wheeler, D. & Forbes, A. The Association Between Glycaemic Variability and Progression of Chronic Kidney Disease: a Systematic Review. SN Compr. Clin. Med. 4, 102 (2022). https://doi.org/10.1007/s42399-022-01182-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01182-5