Abstract
This review summarizes published findings of the beneficial and harmful effects on the heart, lungs, immune system, kidney, liver, and central nervous system of 47 drugs that have been proposed to treat COVID-19. Many of the repurposed drugs were chosen for their benefits to the pulmonary system, as well as immunosuppressive and anti-inflammatory effects. However, these drugs have mixed effects on the heart, liver, kidney, and central nervous system. Drug treatments are critical in the fight against COVID-19, along with vaccines and public health protocols. Drug treatments are particularly needed as variants of the SARS-Cov-2 virus emerge with some mutations that could diminish the efficacy of the vaccines. Patients with comorbidities are more likely to require hospitalization and greater interventions. The combination of treating severe COVID-19 symptoms in the presence of comorbidities underscores the importance of understanding the effects of potential COVID-19 treatments on other organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
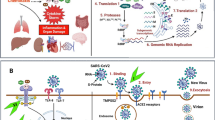
Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped RNA virus and the cause of the coronavirus (CoV) disease 2019 (COVID-19) pandemic, manifests in a range of symptoms, predominantly fever, dry cough, and breathing difficulties, as well as fatigue, pneumonia, dizziness, and gastrointestinal tract (GI) symptoms [1, 2]. There is an overrepresentation of patients with severe COVID-19 illness who have underlying medical conditions, such as hypertension, cardiovascular disease, and diabetes [3,4,5]. COVID-19 can lead to acute respiratory distress syndrome (ARDS), acute respiratory failure, and significant rise in cytokine and chemokine levels, including cytokine storm syndrome [6], septic shock, and multiple organ dysfunction (MOD) [7,8,9,10,11,12]. These conditions are involved in the higher mortality of the most severe cases of COVID-19 and this increased risk of mortality is greater in older patients and patients with comorbidities, i.e., hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, and cerebrovascular disease [7, 8, 13,14,15].
Beyond pneumonia, there are heart, liver, kidney, and neurological complications [1, 16]. With many patients, COVID-19 manifests functional damage to several organs, including acute kidney injury, cardiac and myocardial injury, liver dysfunction, and neurological disorders [7, 17]. Patients with pre-existing kidney, heart, or liver disease are more likely to suffer severe kidney, cardiac, or liver symptoms and complications. The involvement of these organs in COVID-19 is multifaceted, related to direct effects of the virus [17, 18] or to host immune response (inflammatory infiltrates), and in particular with the liver where there is also the potential of drug -induced liver injury [19].
The rapid growth of COVID-19 into an extraordinary pandemic has forced physicians and other health care providers to improvise in treating the most severe symptoms. This has included the off-label use of many drugs. While much is being learned about the unexpected effects of COVID-19, including uncommon effects, there has been the real time need to treat patients. These efforts have led to decreased morbidity in those presenting with COVID-19. Yet, as the pandemic continues, there is need for a review of the available drugs and their beneficial and adverse effects on systems in the body.
Additionally, we examine some of the effects of remdesivir (RDV) and chloroquine (CQ) on cardiac response in induced pluripotent stem cell (iPSC)–derived human heart organoids. Similarly, a recent study assessed the cardiotoxicity and QT prolongation of CQ-treated and RDV-treated human iPSC-derived cardiomyocytes [20] and found that RDV is associated with both cardiotoxicity and arrhythmogenic risk.
SARS-CoV-2 Susceptibility and Disease Manifestation
Viral Susceptibility
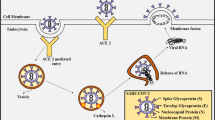
To assess the viral susceptibility of the various organs, researchers have measured the expression levels of the SARS-CoV-2, ACE2, and type II transmembrane serine protease 2 (TMPRSS2). SARS-CoV-2 spike (S) protein binds angiotensin-converting enzyme 2 (ACE2), which is an enzyme attached to the cell membranes of many cell types, and along with TMPRSS2, which primes the S protein by proteolytic cleavage of the S protein, promotes entry of the virus into host cells [21]. ACE2 and TMPRSS2 are expressed at varying degrees in numerous tissues, including the lungs, kidney, liver, heart, and brain [22,23,24,25,26] (https://www.proteinatlas.org/). Interestingly, ACE2 and TMPRSS2 have lower expression in airways of children as compared to adults [27, 28], while they are upregulated in cardiomyocytes of older adults [29]. This could contribute to the milder manifestation of the disease in children, but nonetheless the children show complement-mediated thrombotic microangiopathy levels indicative of blood vessel damage [30]. ACE2 is abundantly expressed in the lungs, small intestines [31], kidney, liver, and brain-endothelium and brain-vascular smooth muscle cells [31, 32]. ACE2 and TMPRSS2 are co-expressed in the respiratory tract (oral cavity and lungs) and highly co-expressed in the GI tract [27, 33,34,35,36]. They are also both expressed in the kidney [37] and were found in kidneys of patients with COVID-19 [38, 39]. ACE2 and TMPRSS2 levels were found to be expressed in liver progenitors cells with a cholangiocyte fate bias [40], which has been posited to compromise the regenerative capabilities of the liver [41]. While ACE2 is highly expressed in human heart, TMPRSS2 is less expressed there, although other proteases (e.g., cathepsin L and furin) are more highly expressed in the heart [42].
Viral Infection
SARS-CoV-2 infection has been reported in the lungs [43,44,45,46] and other organs, including the kidneys, heart, liver, and brain [38, 39, 45, 47,48,49]. The viral load detected in the respiratory tract of COVID-19 patients has been positively associated with severity of the lung disease [50]. This is less clear with the other organs. SARS-CoV-2 has been found to directly infect engineered human kidney organoids, human-induced pluripotent stem cell–derived cardiomyocytes, cholangiocytes in human liver ductal organoids, and neuronal cells in human brain organoids (with a concomitant increase in cell death) [51,52,53,54,55]. However, the mechanism of viral entry is unclear.
There is evidence of direct viral infection of endothelial cells, including viral particles in the endothelial cells of the glomerular capillary loops of kidney tissues [18]; however, it is unclear whether SARS-CoV-2 infection of the kidneys leads to acute kidney injury (AKI) [56]. Nonetheless, AKI is observed frequently in COVID-19 patients and is associated with respiratory failure and poor prognosis [57]. A high prevalence of kidney disease on admission and development of AKI in hospitalized patients with COVID-19 were associated with in-hospital mortality [58].
The SAR-CoV-2 virus has been isolated from mycocardial and liver tissues [38, 49, 59], and SAR-CoV-2 infection has been detected in endomyocardial biopsies (EMBs) from a few patients [60]. Cardiac injury is a common condition among hospitalized COVID-19 patients and underlying cardiovascular disease (CVD) with myocardial injury is linked to increase mortality with COVID-19 [61, 62]. Whether viral entry into the heart leads to the cardiac complications and dysfunction observed in COVID-19 is actively being studied. A study of iPSC-derived cardiac cells exposed to SARS-CoV-2 virus led to cytopathic changes in the cells supporting that viral infection could lead to cardiac damage [63].
Likewise with the liver, hypoalbuminemia and abnormal liver biochemistries are associated with higher rates of complications and mortality and worse recovery [64, 65]. It also has been suggested that injury to the liver could be due more to the immune response than virus itself [66]. However, an ultrastructural analysis of livers of COVID-19 patients with abnormal liver enzymes suggests that the SARS-CoV-2 infection contributes to cytopathic lesions, lending support that infection in the liver contributes to hepatic impairment [67].
Understanding viral infection of the brain by SARS-CoV-2 is actively being investigated. Meningitis, encephalitis, encephalopathy, loss of smell, altered taste, headache, and dizziness are all suggestive of potential neural involvement and have been reported in COVID-19 patients [68,69,70]. The SARS-CoV-2 virus has been detected in neural and capillary endothelial cells in the frontal lobe tissue [71] and in the cerebrospinal fluid (CSF) of some COVID-19 patients [69], albeit not necessarily in the cases with severe neurological complications [72]. Evidence of neuroinvasion was found with SARS-COV and MERS-CoV lending support to a potential of viral entry into the brain in contributing the neurological manifestations of COVID-19 [73]. Along these lines, a study found that the spike protein of SARS-CoV-2 can cross the blood brain barrier [74]. Given that the loss of taste and smell is one of the common first symptoms of COVID-19, it is unsurprising that SARS-CoV-2 has been found to enter the CNS via the olfactory system, which is likely due to the proximity of neurons to the thin submucosal lining [75]. Indeed, a recent study found evidence of neuroinvasion of SARS-CoV-2 in brains of humans and mice and administering ACE2 antibody mitigated neuronal infection [76].
Role of Inflammation
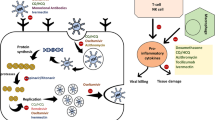
In addition to viral infection, host inflammatory responses contribute to the disease manifestation of COVID-19. Accumulation of inflammatory cells has been found in the lungs, heart, kidneys, and liver [18, 39]. The lungs are targeted by SARS-CoV-2, with both immediate and potentially lasting consequences. COVID-19 is characterized by respiratory symptoms that can lead to respiratory failure [77]. ARDS is a common cause of death in critically ill COVID-19 patients [78]. ARDS, sepsis, and MOD all contribute to lung complications in COVID-19 patients. SARS-CoV-2 infection could have both direct cardiovascular and indirect cardiovascular consequences, including myocardial injury, acute coronary syndromes, cardiomyopathy, arrhythmias, and inflammation [17, 79]. Additionally, inflammation is a potential contributor to myocarditis in COVID-19 [80, 81]. Patients with COVID-19 are known to have increased inflammatory responses [82]. In the heart, this increase in cytokines modifies K+ channels, which can disrupt cardiac action potentials, resulting in lethal cardiac arrhythmias [79, 83]. Older patients with cardiovascular comorbidities and diabetes that contract COVID-19 are at higher risk of myocardial injury and mortality [61, 62]. Athletes are not immune to cardiovascular manifestation of COVID-19, with evidence by reports of myocardial injury (including myocarditis) [84]. Additionally, previously infected individuals can have ongoing myocardial inflammation after recovering from COVID-19 [85]. It has not been conclusively determined if kidney injury in COVID-19 patients are from direct viral involvement in the tissue or the accumulation of inflammatory cells and the host inflammatory response (cytokine storm). Factors that contribute to AKI include systemic hypoxia, infiltration of inflammatory cells, abnormal coagulation, and possible drug effects [39]. Similarly, it remains unclear if liver damage is caused by direct viral infection or immune system activation and the resulting cytokine storm or drug effects [86]. The effects on liver observed in patients include elevated enzyme levels, hepatocellular necrosis, and moderate microvesicular steatosis [86, 87]. Additionally, in a retrospective patient study, therapeutic use of lopinavir/ritonavir for COVID-19 was associated with liver damage [87]. Increases in systemic cytokine levels due to SARS-CoV-2 infection could contribute to central nervous system (CNS) dysfunction [88, 89]. Neurological damage resulting from COVID-19 infection includes anosmia, encephalopathy, inflammatory CNS syndromes, ischemic strokes, and peripheral neurological disorders. In particular, there was a high incidence of acute disseminated encephalomyelitis with hemorrhagic change, which was not associated with severity of the disease [88, 90]. Therefore, both the viral infection and host inflammatory responses could contribute to the lung-, heart-, liver-, and kidney-related complications in COVID-19.
Drug Repositioning
Although innovations in care have improved survival rates of severe cases, there remains no definitive treatment or cure for COVID-19. Given the urgency of this pandemic, multiple pharmaceutical treatment modalities are being pursued in the form of vaccine and repurposing of existing drug therapies. Given the lengthy process involved in vaccine development, drug therapies are vital in mitigating the more severe responses of COVID-19, thereby necessitating viable strategies to search for new classes of medicines. Repositioning or repurposing FDA-approved drugs is a feasible approach to discover new therapies to respond quickly to COVID-19 than de novo drug development which takes many years. Drug repositioning offers shorter route to the clinic and have well-known safety concerns since they have been through several stages of clinical development [91]. The range of drugs that have been proposed for treating COVID-19 includes (1) anti-virals that interfere with viral entry, synthesis, or replication (including protease inhibitors), (2) immunomodulatory agents that affect cytokine levels and host inflammatory responses (e.g., antibiotics, antihistamines, steroids, and monoclonal antibodies) that could affect respiratory distress syndrome or cytokine storm, (3) antiparasitic drugs (antihelmintic), and (4) vasodilators. Table 1 summarizes this range of drugs and Supplementary Table 1 provides their classification. Most of the drugs are beneficial to the immune system, reducing inflammation and attenuating viral infection in the lungs, while their effects across the other organs (heart, kidneys, liver, and CNS) are not uniformly beneficial.
The Effects of Drugs on Various Organs
In light of the manifestation of COVID-19 on various organs, it is important that the drugs used to treat COVID-19 patients do not exacerbate symptoms associated with the disease. This suggested the benefit of gathering a list of drugs that are being used off-label or in clinical trials for COVID-19 and searched for potential beneficial and harmful effects of these drugs on the immune system, lungs, heart, kidneys, liver, and CNS, when used to treat specific conditions or diseases. Search of PubMed, Google Scholar, Google, bioRxiv, and medRxiv, using the organ and name of the drug(s) and disease(s) as keywords, revealed multiple aspects of the drug interactions with various organs and systems. The goal is to provide an extensive depiction of how the drugs could have differential effects on the various organs, beneficial to some while harmful to other organs. To a lesser extent, this analysis could suggest situations in which specific drugs could be counter-indicated under certain conditions. The goal was to identify any positive or negative effects on these organs from the treatment of these drugs for any disease, including COVID-19, and potentially future infectious diseases. Table 1 provides a summary of each drug’s effects on these organs. Supplementary Tables 2-7 show in greater detail the effects of the drugs on the (1) immune system, (2) lungs, (3) heart, (4) kidneys, (5) liver, and (6) CNS. Several observations are apparent. First, many of the repositioned drugs are beneficial to the immune system and the lungs, but have mixed effects across the other organs. This is unsurprising as the drugs are predominantly immunomodulators or anti-virals and breathing difficulty and cytokine storm are major manifestations of COVID-19 that are targeted by the drugs. Second, few of these drugs are beneficial across all vital organs. Of the drugs evaluated in this study, APN01, a recombinant human ACE2, appears to have positive effects reported across the organs evaluated in this study. Third, patients with comorbidities are often more vulnerable to COVID-19 infection. These patients are most likely to show severe symptoms requiring hospitalization and treatment, underscoring the importance on being aware of the effect that the repositioned drugs have on various organs. For each of the organs, a summary of the findings is depicted in Fig. 1. For the immune system, most of the drugs have a beneficial effect. The drugs are generally immunosuppressive and function by reducing the cytokines released by both the innate and adaptive immune systems that contribute to COVID-19 pathology. These immune systems play an integral part in combatting cytokine release syndrome (CRS) of COVID-19. The few drugs with negative effects on the immune system were typically immunostimulatory, and adverse effects were most often observed in individuals with impaired immune systems (Supplementary Table 2). For the lungs, most of the drugs in this study had beneficial effects, nonetheless about a third of the drugs reviewed reported both positive and negative effects on the lungs. Anti-inflammatories, vasodilators, and serine protease inhibitors generally were helpful. In contrast, a couple of the nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) primarily affected the lungs negatively (Supplementary Table 3). For the heart, several drugs had reported favorable effects on the cardiac system. Most of these benefits related to improving the cardiovascular system or indicate limited risk of cardiac injury for COVID-19 patients. For example, statins reduced the progression of atherosclerosis, and in hospitalized COVID-19 patients, statins did not increase risk of cardiac injury. However, a majority of the drugs reported both positive and negative effects or primarily negative effects on the heart. In fact, many of the drugs have not been thoroughly investigated for their cardiovascular effects. Of these drugs, some of the more common adverse effects were hypertension, congestive heart failure (CHF), myocarditis, and QT interval prolongation. For example, AZM was associated with myocarditis and QT interval prolongation (Supplementary Table 4). For the kidneys, the drugs that report both positive and negative impact on the kidney or predominantly negative effects tend to appear in patients with autoimmune conditions such as HIV, hepatitis, or rheumatoid arthritis (RA). In healthy patients, these drugs may be less deleterious (Supplementary Table 5). For the liver, most of the drugs reported negative or both positive and negative effects due to its role in drug metabolism. Considering the frequency of liver dysfunction in COVID-19 patients, attributing hepatotoxicity to the drug vs. the underlying disease can be difficult. Most adverse effects were not severe, and resolved upon ceasing treatment. The most common negative effects were increases in liver enzymes and drug-induced liver injury (Supplementary Table 6). For the CNS, of the drugs studied, most had a positive effect on the CNS, particularly the immunosuppressant drugs and cytokine inhibitors. The only drugs as a class that were primarily negative to the CNS were NRTIs. The most common neurological side effects were headaches, dizziness, and fatigue, which in most cases were not severe enough to discontinue treatment (Supplementary Table 7).
Long-term Effects of the Drugs
Studies on the long-term outcomes of the 2003 SARS indicate that there are consequences of the infection and of the drugs used to treat them, ranging from metabolic to pulmonary fibrosis to femoral head necrosis to neurological [92,93,94,95,96,97,98]. Although it is too early to know whether some of the symptoms that persist in recovered COVID-19 patients will be long lasting, it does raise concerns on the potential of not only the virus itself but of the treatments, including certain drugs currently being used off-label or in clinical trials to treat COVID-19, that could contribute to exacerbating these symptoms [99]. For example, the use of corticosteroid could help suppress lung inflammation but there is a concern that it could inhibit host immune response. In a 15-year follow-up of SARS patients treated with high-dose steroid pulse therapy, the femoral head necrosis was found not to be progressive and to show some recovery [94]. The existing evidence based on past experiences with SARS and MERS suggest that the use of steroids is inconclusive and caution is recommended as a routine treatment [100, 101]. Nonetheless, in cases of hyperinflammation, immunosuppression (including interleukin antagonists) could be beneficial without increased adverse effects [6]. As with any drug, there is the potential for drug-induced liver injury [102]. Drugs that could increase the risk of liver injury based on the medical condition of the individual [103,104,105,106]. Long-term damage of the viral infection could lead to chronic illness that impact not only the lungs but also the heart, immune system, brain (mental), and other organs. Therefore, we search in the literature to find if any of the drugs on our list (Table 1) have been associated with inducing chronic effects or have negative effects upon long-term administration of the drug (Table 2). The most common persistent effects after COVID-19 are fatigue and dyspnea [99, 130, 131]. Therefore, we searched in the literature to identify drugs that affect chronic fatigue syndrome (CFS) and indicate possible drugs that could either exacerbate or mitigate CFS (Table 3).
Clinical Trial Results
Many of these drugs have undergone or are undergoing clinical trials. Some drugs have few published results. Clinical trials of each drug for treatment of COVID19 listed clinicaltrials.gov included available, not yet recruiting, recruiting or enrolling by invitation, active-not yet recruiting, recruiting, suspended, terminated, withdrawn, and completed for the drugs listed in Table 1 as of January 11, 2021. A search for trials with publications of the results was performed. For each peer-reviewed publication, we searched for the NCT number and confirmed the publication contains controlled and randomized results from the trial. Table 4 collates the current results of COVID-19 clinical trials that are completed for drugs that report results in peer-reviewed publication.
Emergency Use Authorization for HCQ/CQ and RDV
Early in the pandemic, hydroxychloroquine (HCQ) was given Emergency Use Authorization (EUA) for COVID-19 in April 2020 [195] (https://www.fda.gov/media/136537/download) which was subsequently revoked due to serious adverse cardiac events (https://www.fda.gov/media/136784/download) (https://www.fda.gov/media/138945/download). Indeed, both chloroquine (CQ) and HCQ, still undergoing clinical trials for COVID-19, had some studies that assessed for adverse cardiovascular complications or cardiac toxicity by monitoring QT interval prolongation or torsades de pointes. Subsequently, RDV was approved for EUA for COVID-19 in May 2020 (https://www.fda.gov/media/137564/download). Based on studies that RDV lowers viral load in animals [196, 197] and inhibits SARS-CoV-2 infection in cells [197], and a clinical trial (NCT04280705) found RDV was superior to placebo in shortening recovery time of hospitalized adult COVID-19 patients [189], there is ample support for the use of RDV for COVID-19. Nonetheless, another clinical trial (NCT04257656) found hospital patients with severe COVID-19 treated with RDV was not associated with statistically significant clinical benefits [190]. In addition, search for the effect of these drugs on the various organs identified an instance of torsades de pointes in a COVID-19 patient treated with RDV and requiring resuscitation [198], although it is unclear if this was due directly to RDV. Many clinical trials use QT interval threshold as part of the exclusion criteria, and while a number of drugs (e.g., AZM, baricitinib, CM, colchicine, corticosteroid, HCQ, ivermectin, LPV/RTV, sarilumab, TCZ) monitor for QT prolongation or cardiac arrhythmia as a secondary measure (NCT04381936, NCT04366206, NCT04382625, NCT04374019), trials with RDV to date do not appear to. In light of the comorbidities of COVID-19 patients, it would be beneficial to measure QT interval or other cardiac function as part of the measures and outcomes in the clinical trials of drugs for COVID-19.
The Effect of CQ and RDV on 3D Human Heart Organoids
A retrospective study that analyzed electrocardiograms from 524 COVID-19 patients showed approximately 20% showed QT prolongation [199]. Therefore, it is critical that the drugs used to treat COVID-19 do not enhance the potential of QT prolongation. Although it is known that CQ has a potential for QT interval prolongation and was found to prolong QT interval in COVID-19 patients [200], less is known about RDV. Of the 78 clinical trials in Clinicaltrial.gov with RDV, none are explicitly monitoring for QT prolongation or torsades des pointes. While we were performing this study, another group assessed for cardiotoxicity and QT prolongation of CQ-treated and RDV-treated human iPSC-derived cardiomyocytes [20] and found that RDV is associated with both cardiotoxicity and arrhythmogenic risk. Although iPSC-derived cardiomyocytes constitute an excellent model for human cardiotoxicity studies [201,202,203], recent advances in stem cell technologies have facilitated the emergence of human stem cell–derived organ-like model systems (organoids) which allow for higher degree of precision and physiological relevance [204, 205]. Organoids recapitulate many organ properties, structure, and physiology to a significant extent, thus arguably constituting a better model than traditional 2D cell cultures containing a single cell type and no microenvironment [206]. In contrast, organoids have multiple cell types that interact with cardiomyocytes, along with matrix native to the heart, providing physicochemical properties that are better able to mimic the heart in vivo. Organoids are particularly useful to study unapproachable disease states in humans and have been used to model a wide range of tissues and disease conditions with great success [206, 207], including SARS-CoV-2 infection of human lungs and intestine [51, 205, 208]. Using a recently developed protocol for the generation of highly sophisticated human heart organoids (hHOs) from hiPSCs [209], the cardiac effects of CQ and RDV were explored with hHOs. Given the higher complexity in the organoids, including different cell types, morphology, and extracellular matrix, we expected the organoids will be more robust than cardiac monolayer cultures.
Experiments were performed on hHOs derived from human iPSCs treated with CQ (known to have adverse cardiac effects) or RDV at two different concentrations to assess a potential of RDV for adverse cardiac effect. CQ and RDV were prepared as described in the methods (see supplementary file). At 96-h post-treatment with control media, CQ-containing media, or RDV-containing media, all organoids treated with control media or 10 μM of CQ were beating while 25% of the hHOs treated with 10 μM of RDV were beating. None of the heart organoids treated at the higher concentration (100 μM) of either CQ or RDV were beating (Fig. 2).
Chloroquine (CQ) and remdesivir (RDV) influence human heart organoid beat rate. Beat rate (per minute) was assessed following 96 h of treatment. Heart organoids were treated with either a CQ (n = 3, control; n = 4, 10 μM and 100 μM) or b RDV (n = 4 for all conditions) at concentrations of 10 μM or 100 μM for 96 h. Beats per min (BPM) in the treatment conditions were normalized to BPM in the pre-treatment condition for each individual organoid in each condition. (Value = mean ± s.d., two-way ANOVA multiple comparison test; *p = 0.0571, **p < 0.01, ****p < 0.0001)
To assess for QT interval abnormalities in the hHOs, an in-house microelectrode array (MEA) system was used to record electrical activity of individual organoids. The QT interval in the control condition was 306 ± 4.70 ms. The QT intervals were heightened for both the 10 μM CQ and 10 μM RDV-treated hHOs, albeit non-significantly (444 ± 30.5 ms and 344 ± 24.2 ms, respectively) (Fig. 3). Notably, both 100 μM CQ and 100 μM RDV induced significant QT prolongation in the hHOs (527 ± 35.2 ms and 501 ± 52.5 ms, respectively). CQ was shown to exhibit an increased effect on QT prolongation as compared to RDV. Thus, the electrophysiological abnormalities arising from CQ and RDV treatment indicate a cardiotoxic mechanism in both CQ and RDV.
CQ and RDV induce QT interval prolongation in human heart organoids.Using the electrophysiological data obtained with the MEA system, QT intervals were measured in organoids with or without treatment of a CQ or b RDV. (value = mean ± SEM, one-way ANOVA multiple comparison test, compared to control; *p < 0.05, ** p < 0.005)
CQ and RDV both exhibit a pathological phenotype in the treatment of hHOs. While CQ induced cessation of visible beating at 100 μM, RDV exerted a similar effect at both 10 μM and 100 μM, suggestive of cardiotoxicity. These data suggest that further studies are needed to determine the safety and efficacy of RDV on the heart in the treatment of COVID-19.
Limitations of Study
Therapies not covered in this review include stem cell therapy [210, 211], transfusion of convalescent plasma [212], and engineered decoys for neutralizing pathogens (including hACE2 for SARS-CoV-2) [213]. Although pilot studies [214,215,216,217] suggest that convalescent plasma could be beneficial, a randomized controlled study and an open-label, multicenter, randomized clinical trial did not find it to be associated with a reduction in the progression to severe COVID-19 or result in a statistically significant reduction in time to clinical improvement [218, 219], while early results of a clinical trial suggests it is safe and efficacious [220, 221]. This present study does not evaluate pharmacogenetics or how the genetic makeup of an individual contributes to their differential immune response to SARS-CoV-2 [222] or their differential response to a drug [223], which could aid in predicting which drugs affect an individual adversely while being beneficial to another.
Conclusion
Even as vaccines are becoming available for COVID-19, variants of the SARS-Cov-2 virus are proliferating, leading to concerns that some mutations may reduce the efficacy of the vaccines to stem the broader pandemic [224, 225]. Therefore, treatments will continue to be an essential aspect in the fight against COVID-19 and its variants. Innovative repurposing of drugs, such as recent reports of the use of sarilumab and TCZ, are showing promise in the treatment of patients with severe COVID-19 [191]. Still, clinical trials are needed to assess the effects of these drugs on non-targeted organs and systems, such as the cardiovascular system. This study has compiled an extensive report of the many drugs proposed to treat COVID-19 and improve lung performance, along with examination of the effects of these drugs on other systems. Furthermore, the findings of this study are relevant to diseases other than COVID-19 by providing indications of how these drugs affect various organs. This could assist in guiding the implementation of these drugs in their repositioning for established and emerging diseases.
Data Availability
Available upon request.
Abbreviations
- AA:
-
Ascorbic acid
- AZM:
-
Azithromycin
- CM:
-
Camostat mesilate
- CQ:
-
Chloroquine
- DEX:
-
Dexamethasone
- DS:
-
Disulfiram
- DSTAT:
-
Dociparstat sodium aka 2-0, 3-0 Desulfated Heparin (ODSH)
- EVT:
-
Entecavir
- EPO:
-
Epoprostenol
- HCQ:
-
Hydroxychloroquine
- LPV:
-
Lopinavir
- MP:
-
Methylprednisolone
- NM:
-
Nafamostat
- NTZ:
-
Nitazoxanide
- NO:
-
Nitric oxide
- rhACE2:
-
Recombinant human angiotensin-converting enzyme 2
- RDV:
-
Remdesivir
- RTV:
-
Ritonavir
- RUX:
-
Ruxolitinib
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
- TET:
-
Tetrandrine
- THD:
-
Thalidomide
- TCZ:
-
Tocilizumab
- AZT:
-
Zidovudine
- ARDS:
-
Acute respiratory distress syndrome
- ANG:
-
Angiotensin
- ANP:
-
Atrial natriuretic peptide
- CCCR#:
-
C-C chemokine receptor #
- CRP:
-
C-reactive protein
- CKD:
-
Chronic kidney disease
- CLL:
-
Chronic lymphocytic leukemia
- COPD:
-
Chronic obstructive pulmonary
- CHF:
-
Congestive heart failure
- CRS/CSS:
-
Cytokine release syndrome/cytokine storm syndrome
- CMV:
-
Cytomegalovirus
- ER:
-
Endoplasmic reticulum
- eNOS:
-
Endothelial nitric oxide synthase
- EMT:
-
Epithelial-mesenchymal transition
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HDL:
-
High-density lipoproteins
- HMGB1:
-
High-mobility group box 1
- HIV:
-
Human immunodeficiency virus
- iPSC:
-
Induced pluripotent stem cell
- iNOS:
-
Inducible nitric oxide synthase
- IFN:
-
Interferon
- IRF:
-
Interferon regulatory factor
- IL#:
-
Interleukin #
- JAK:
-
Janus kinase
- LPD:
-
Lipopolysaccharide
- LDL:
-
Low-density lipoproteins
- mTOR:
-
Mammalian target of rapamycin
- MAP 2:
-
Microtubule-associated protein 2
- MNC:
-
Mononuclear cells
- MS:
-
Multiple sclerosis
- MF:
-
Myelofibrosis
- NF-κB:
-
Nuclear factor κB
- NTP:
-
Nucleoside triphosphate
- NRTIs:
-
Nucleoside/nucleotide reverse transcriptase inhibitors
- PGI2:
-
Prostacyclin
- ROS:
-
Reactive oxygen species
- RA:
-
Rheumatoid arthritis
- SARS:
-
Severe acute respiratory syndrome
- SBECD:
-
Sulfobutylether-β-cyclodextrin
- SLE:
-
Systemic lupus erythematosus
- TLRs:
-
Toll-like receptors
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
References
Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Vaduganathan M, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–9.
Sommerstein R, et al. Coronavirus disease 2019 (COVID-19): Do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9(7):e016509.
Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323(18):1769–70.
Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4.
Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Du Y, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–9.
Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763.
Feng Y, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–8.
Yang J, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5.
Guan WJ, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5).
Du RH, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5).
Ammirati E, D.W. W. SARS-CoV-2 inflames the heart. The importance of awareness of myocardial injury in COVID-19 patients. Int J Cardiol. 2020;311:122–3.
Gupta A, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–32.
Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8.
Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Choi SW, et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res. 2020;184:104955.
Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8.
Li MY, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45.
Zou X, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–92.
Dong M, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678.
Ubuka T, et al. Identification of transmembrane protease serine 2 and forkhead box A1 AS THE POTENTIAL BISPHENOL A RESPONSIVE GENES IN THE NEONATAL MALE RAT BRAIN. Front Endocrinol (Lausanne). 2018;9:139.
Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107(6):1482–94.
Lingappan K, et al. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol. 2020;319(1):L39–44.
Saheb Sharif-Askari N, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 Is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1–6.
Robinson EL, et al. Genes encoding ACE2, TMPRSS2 and related proteins mediating SARS-CoV-2 viral entry are upregulated with age in human cardiomyocytes. J Mol Cell Cardiol. 2020;147:88–91.
Diorio C, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4(23):6051–63.
Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7.
Qi F, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135–40.
Xu H, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8.
Zang R, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020:5(47).
Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut Immunity. 2020:1–9.
Wu CZM. Single-cell RNA expression profiling shows that ACE2, the putative receptor of COVID-2019, has significant expression in nasal and mouth tissue, and is co-expressed with TMPRSS2 and not co-expressed with SLC6A19 in the tissues. medRxiv. 2020.
Pan XW, et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–6.
Puelles VG, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–2.
Su H, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–27.
Seow JJW, Pai R, Mishra A, Shepherdson E, Kiat T, Lim H, et al. scRNA-seq reveals ACE2 and TMPRSS2 expression in TROP2+ liver progenitor cells: implications in covid-19 associated liver dysfunction. bioRxiv. 2020.
Kumar P, et al. Pathogenesis of liver injury in coronavirus disease 2019. J Clin Exp Hepatol. 2020.
Liu H, et al. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc Res. 2020;116(10):1733–41.
Zhu N, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Lu R, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Wolfel R, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.
Schaefer IM, et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020;33(11):2104–14.
Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8.
Ding Y, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–30.
Tavazzi G, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–5.
Liu Y, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–74.
Monteil V, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–13 e7.
Sharma A, et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1(4):100052.
Zhao B, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–5.
Ramani A, et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39(20):e106230.
Zhang BZ, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30(10):928–31.
Wang L, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan. China. Am J Nephrol. 2020;51(5):343–8.
Hirsch JS, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18.
Cheng Y, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38.
Wichmann D, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–77.
Escher F, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020.
Guo T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–8.
Shi S, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10.
Perez-Bermejo JA, et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. bioRxiv. 2020.
Fu Y, et al. Clinical features of COVID-19-infected patients with elevated liver biochemistries: a multicenter, retrospective study. Hepatology. 2020.
Huang J, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020.
Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–30.
Wang Y, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–16.
Moriguchi T, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8.
Ellul MA, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–83.
Baig AM, Sanders EC. Potential neuroinvasive pathways of SARS-CoV-2: deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19). J Med Virol. 2020;92(10):1845–57.
Paniz-Mondolfi A, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699–702.
Helms J, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–70.
Cheng Q, Yang Y, Gao J. Infectivity of human coronavirus in the brain. EBioMedicine. 2020;56:102799.
Rhea EM, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2020.
Meinhardt J, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2020.
Song E, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021:218(3).
Carsana L, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–40.
Li X, X M. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198.
Kochi AN, et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–8.
Siripanthong B, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–71.
Rajpal S, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020.
Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–62.
Capecchi PL, et al. Autoimmune and inflammatory K(+) channelopathies in cardiac arrhythmias: clinical evidence and molecular mechanisms. Heart Rhythm. 2019;16(8):1273–80.
Kim JH, et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2020.
Puntmann VO, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020.
Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United European Gastroenterol J. 2020;8(5):509–19.
Fan Z, et al. Clinical Features of COVID-19-Related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–6.
Aghagoli G, et al. Neurological involvement in COVID-19 and potential mechanisms: A REVIEW. Neurocrit Care. 2020.
Bodro M, et al. Increased CSF levels of IL-1beta, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020:7(5).
Paterson RW, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020.
Ashburn TT, K.B. T. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–83.
Wu Q, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):9110.
Xiong TY, et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–800.
Zhang P, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8.
Cheng VC, et al. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100(2):407–19.
Rogers JP, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–27.
Lam MH, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–7.
Guo L, Han Y, Li J, Chen Q, Ren Y, Wu Q, et al. Long-term outcomes in patients with severe acute respiratory syndrome treated with oseltamivir: a 12-year longitudinal study. Int J Clin Exp Med. 2019;12(10):12464–71.
Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020.
Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–5.
Shang L, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–4.
Fix OK, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72(1):287–304.
Teoh NC, G.C. F. Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin Liver Dis. 2003;7(2):401–13.
Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169–83.
Fowler R, Imrie K. Thalidomide-associated hepatitis: a case report. Am J Hematol. 2001;66(4):300–2.
Tarantino G, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37(6):410–5.
Lacy SA, et al. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol Sci. 1998;44(2):97–106.
Gupta AP, et al. Pancreastatin inhibitor activates AMPK pathway via GRP78 and ameliorates dexamethasone induced fatty liver disease in C57BL/6 mice. Biomed Pharmacother. 2019;116:108959.
Letteron P, et al. Glucocorticoids inhibit mitochondrial matrix acyl-CoA dehydrogenases and fatty acid beta-oxidation. Am J Physiol. 1997;272(5 Pt 1):G1141–50.
Yeh TF, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics. 1998;101(5):E7.
Filosto M, et al. Disulfiram neuropathy: two cases of distal axonopathy. Clin Toxicol (Phila). 2008;46(4):314–6.
Song JH, et al. A case of severe peripheral polyneuropathy occurring after entecavir treatment in a hepatitis B patient. Korean J Gastroenterol. 2016;67(4):216–9.
McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–82.
Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–60.
Mauthe M, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14(8):1435–55.
Frustaci A, et al. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol. 2012;157(1):117–9.
Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110(6):481–9.
Zorzon M, et al. Long-term effects of intravenous high dose methylprednisolone pulses on bone mineral density in patients with multiple sclerosis. Eur J Neurol. 2005;12(7):550–6.
Miyawaki H, et al. Long-term Effects of the Janus Kinase 1/2 Inhibitor Ruxolitinib On Pulmonary Hypertension And The Cardiac Function In A Patient With Myelofibrosis. Intern Med. 2020;59(2):229–33.
Novaro GM, et al. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104(18):2205–9.
Ubels FL, et al. Effects of initial and long-term lipid-lowering therapy on vascular wall characteristics. Atherosclerosis. 2001;154(1):155–61.
Schweinsburg BC, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11(4):356–64.
Tosi P, et al. Neurological toxicity of long-term (>1 yr) thalidomide therapy in patients with multiple myeloma. Eur J Haematol. 2005;74(3):212–6.
Wulff CH, et al. Development of polyneuropathy during thalidomide therapy. Br J Dermatol. 1985;112(4):475–80.
Clemmensen OJ, Olsen PZ, Andersen KE. Thalidomide neurotoxicity. Arch Dermatol. 1984;120(3):338–41.
Dalakas MC, et al. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322(16):1098–105.
Volberding PA, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–63.
Rachlis A, Fanning MM. Zidovudine toxicity. Clinical features and management. Drug Saf. 1993;8(4):312–20.
Sundar K, et al. Zidovudine-induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature. Crit Care Med. 1997;25(8):1425–30.
Weerahandi H, et al. Post-discharge health status and symptoms in patients with severe COVID-19. medRxiv. 2020.
Carfi A, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5.
Roerink ME, et al. Cytokine signatures in chronic fatigue syndrome patients: a case control study and the effect of anakinra treatment. J Transl Med. 2017;15(1):267.
Suh SY, et al. Intravenous vitamin C administration reduces fatigue in office workers: a double-blind randomized controlled trial. Nutr J. 2012;11:7.
Vermeulen RC, H.R. S. Azithromycin in chronic fatigue syndrome (CFS), an analysis of clinical data. J Transl Med. 2006;4:34.
Michaud K, et al. Relative impact of pain and fatigue on work productivity in patients with rheumatoid arthritis from the RA-BEAM baricitinib trial. Rheumatol Ther. 2019;6(3):409–19.
Lo YC, et al. Fatigue as the only clinical manifestation of colchicine induced myopathy. Acta Neurol Taiwan. 2010;19(3):184–8.
Kakumanu SS, et al. Effect of topical nasal corticosteroids on patients with chronic fatigue syndrome and rhinitis. J Am Osteopath Assoc. 2003;103(9):423–7.
Yennurajalingam S, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–82.
Palacios R, et al. Minor emtricitabine intolerance in treatment-stable patients switched from tenofovir/lamivudine to a fixed-dose combination of tenofovir/emtricitabine (Truvada). J Antimicrob Chemother. 2008;61(2):462–3.
Rich S, McLaughlin VV. The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J Am Coll Cardiol. 1999;34(4):1184–7.
Barst RJ, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301.
Janowitz T, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69(9):1592–7.
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020.
Wang SQ, et al. Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):186.
Giloteaux L, et al. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):30.
Visser J, et al. CD4 T lymphocytes from patients with chronic fatigue syndrome have decreased interferon-gamma production and increased sensitivity to dexamethasone. J Infect Dis. 1998;177(2):451–4.
Russell A, et al. Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology. 2019;100:276–85.
See DM, Tilles JG. alpha-Interferon treatment of patients with chronic fatigue syndrome. Immunol Invest. 1996;25(1-2):153–64.
Chandler RE. Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98(2):382–8.
Hardy M, et al. The safety of combined triple drug therapy with ivermectin, diethylcarbamazine and albendazole in the neglected tropical diseases co-endemic setting of Fiji: A cluster randomised trial. PLoS Negl Trop Dis. 2020;14(3):e0008106.
Li L, et al. Humanized PA14 (a monoclonal CCR5 antibody) for treatment of people with HIV infection. Cochrane Database Syst Rev. 2014;7:CD008439.
Sprinz E, et al. Substitution with lopinavir/ritonavir improves patient-reported outcomes including quality of life in patients who were intolerant to their antiretroviral therapy. HIV Clin Trials. 2006;7(6):291–308.
Rossignol JF, et al. Effect of nitazoxanide in persistent diarrhea and enteritis associated with blastocystis hominis. Clin Gastroenterol Hepatol. 2005;3(10):987–91.
Suarez A, et al. Nitric oxide metabolite production during exercise in chronic fatigue syndrome: a case-control study. J Womens Health (Larchmt). 2010;19(6):1073–7.
Meeus M, et al. Nitric oxide concentrations are normal and unrelated to activity level in chronic fatigue syndrome: a case-control study. In Vivo. 2010;24(6):865–9.
Verstovsek S, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–27.
Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86(12):1188–91.
Strand V, et al. Sarilumab improves patient-reported outcomes in rheumatoid arthritis patients with inadequate response/intolerance to tumour necrosis factor inhibitors. RMD Open. 2017;3(1):e000416.
Hammer J, et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study. Orphanet J Rare Dis. 2018;13(1):191.
Champion L, et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Ann Intern Med. 2006;144(7):505–9.
Bhatia HK, et al. Sofosbuvir: a novel treatment option for chronic hepatitis C infection. J Pharmacol Pharmacother. 2014;5(4):278–84.
Golomb BA, et al. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–2.
Maes M, et al. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30(4):470–6.
Petersen J, et al. Effectiveness and safety of tenofovir disoproxil fumarate in chronic hepatitis B: A 3-year prospective field practice study in Germany. Dig Dis Sci. 2016;61(10):3061–71.
Abdul Basit S, et al. Tenofovir alafenamide for the treatment of chronic hepatitis B virus infection. Expert Rev Clin Pharmacol. 2017;10(7):707–16.
Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003;1(3):194–205.
Offidani M, et al. Common and rare side-effects of low-dose thalidomide in multiple myeloma: focus on the dose-minimizing peripheral neuropathy. Eur J Haematol. 2004;72(6):403–9.
Corominas H, et al. Correlation of fatigue with other disease related and psychosocial factors in patients with rheumatoid arthritis treated with tocilizumab: ACT-AXIS study. Medicine (Baltimore). 2019;98(26):e15947.
Khan A, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234.
Furtado RHM, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–67.
Kalil AC, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020.
Deftereos SG, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3(6):e2013136.
Angus DC, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324(13):1317–29.
Group RC, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020.
Tomazini BM, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–16.
Cavalcanti AB, et al. Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–52.
Abella BS, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2020.
Boulware DR, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–25.
Self WH, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–76.
Hung IF, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704.
Gorial FI, Mashhadani S, Sayaly HM, Dakhil BD, AlMashhadani MM, Aljabory AM, et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (Pilot Trial). medRxiv. 2020.
Group RC. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020.
Li Y, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med (N Y). 2020;1(1):105–13 e4.
Salton F, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. open Forum. Infect Dis. 2020;7((10)):ofaa421.
Fadel R, et al. Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19. Clin Infect Dis. 2020;71(16):2114–20.
Jeronimo CMP, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020.
Goldman JD, et al. Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med. 2020;383(19):1827–37.
Spinner CD, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57.
Beigel JH, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–26.
Wang Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.
Investigators TR-C. Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv. 2021.
Furlow B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020;2(10):e592.
Salvarani C, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020.
Stone JH, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44.
Thomson K, Nachlis H. Emergency use authorizations during the COVID-19 pandemic: lessons from hydroxychloroquine for vaccine authorization and approval. JAMA. 2020;324(13):1282–3.
Williamson BN, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–6.
Pruijssers AJ, et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020;32(3):107940.
Zampino R, et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14(5):881–3.
Jain S, et al. Enhanced electrocardiographic monitoring of patients with coronavirus disease 2019. Heart Rhythm. 2020;17(9):1417–22.
van den Broek MPH, et al. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. 2020;28(7-8):406–9.
Panopoulos AD, et al. iPSCORE: A resource of 222 iPSC lines enabling functional characterization of genetic variation across a variety of cell types. Stem Cell Reports. 2017;8(4):1086–100.
Gintant G, et al. Use of human induced pluripotent stem cell-derived cardiomyocytes in preclinical cancer drug cardiotoxicity testing: a scientific statement from the American Heart Association. Circ Res. 2019;125(10):e75–92.
Rajasingh S, et al. Manipulation-free cultures of human iPSC-derived cardiomyocytes offer a novel screening method for cardiotoxicity. Acta Pharmacol Sin. 2018;39(10):1590–603.
Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364(6444):952–5.
Clevers H. COVID-19: organoids go viral. Nat Rev Mol Cell Biol. 2020;21(7):355–6.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–97.
Qian X, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–54.
Lamers MM, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–4.
Israeli Y, Gabalski M, Ball K, Wasserman A, Zou J, Ni G, et al. Generation of heart organoids modeling early human cardiac development under defined conditions. bioRxiv. 2020.
Tang L, et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–73.
Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16(3):427–33.
Yang L, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128.
Linsky TW, et al. De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2: Science; 2020.
Abolghasemi H, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59(5):102875.
Shen C, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–9.
Duan K, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–6.
Ye M, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan. China. J Med Virol. 2020.
Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
Li L, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–70.
Joyner MJ, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–7.
Joyner MJ, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 2020.
Pairo-Castineira E, et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2020.
Takahashi T, et al. Pharmacogenomics of COVID-19 therapies. NPJ Genom Med. 2020;5:35.
Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature. 2021;589:177–8.
Kozlov M, South African SARS-CoV-2 Variant alarms scientists, in The Scientist. 2021.
Code Availability
Not applicable.
Funding
This study was funded by the National Science Foundation (CBET 1802992, CBET 2029319) and the National Institute of Health (R21NS116496).
Author information
Authors and Affiliations
Contributions
All authors were involved in the analysis and writing of the manuscript
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Inform consent
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVID-19
Key Points
• Repositioned drugs aim to ameliorate the disease by attenuating the immune response and enhance lung function.
• They are not equally beneficial on the other organs (heart, kidney, liver, and central nervous system).
• As a first drug with emergency use authorization and approval for COVID-19, how remdesivir affects the heart is unclear and evidence exists of possible negative effects.
Supplementary Information
ESM 1
(PDF 678 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chan, C., Foster, S.T., Chan, K.G. et al. Repositioned Drugs for COVID-19—the Impact on Multiple Organs. SN Compr. Clin. Med. 3, 1484–1501 (2021). https://doi.org/10.1007/s42399-021-00874-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00874-8